Microsurgical Transplantation of Pedicled Muscles in an Isolation Chamber—A Novel Approach to Engineering Muscle Constructs via Perfusion-Decellularization
Abstract
:1. Introduction
2. Methods
2.1. Experimental Animals
2.2. Muscle Preparation
2.3. Muscle Stimulation
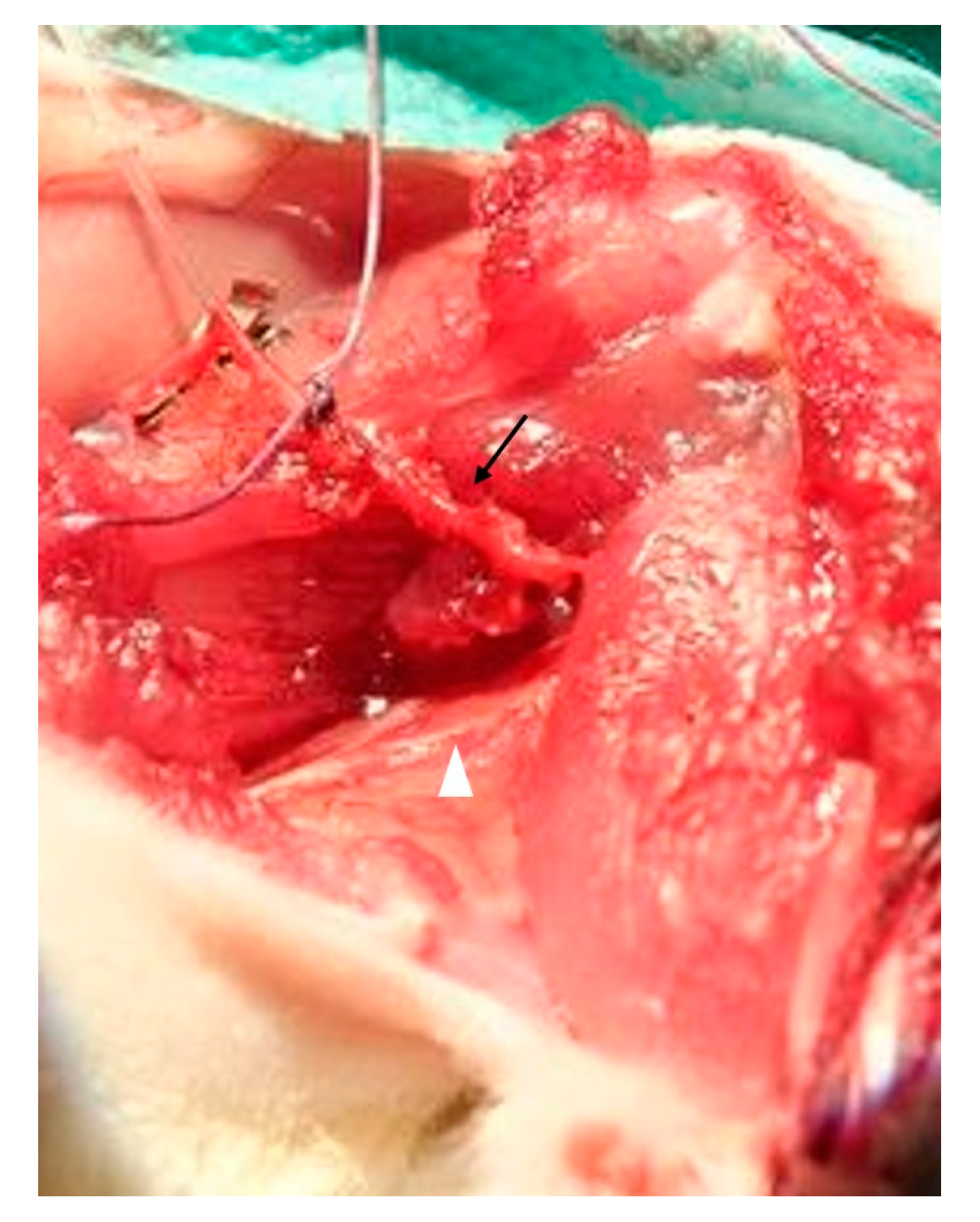
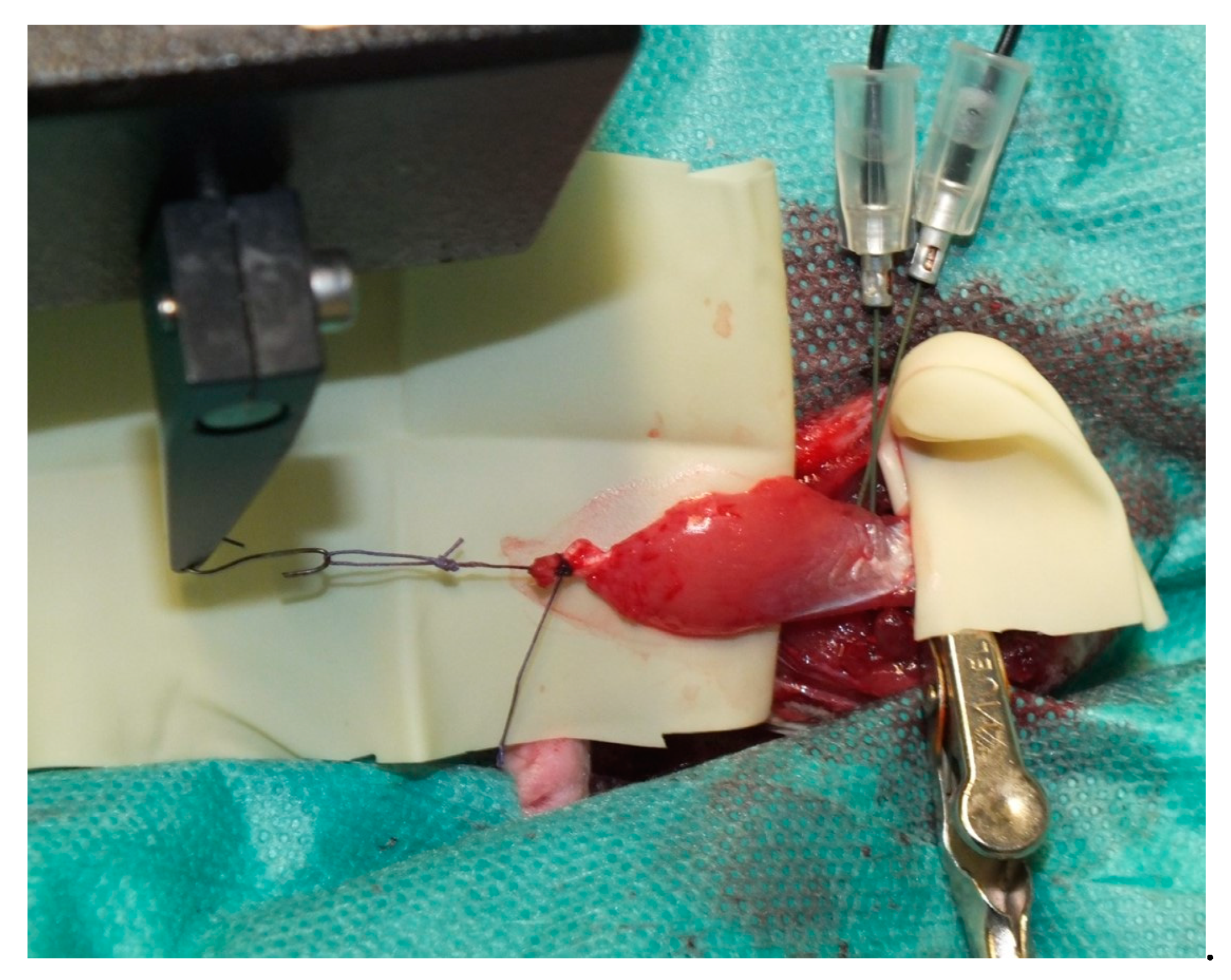
2.4. Muscle Transplantation
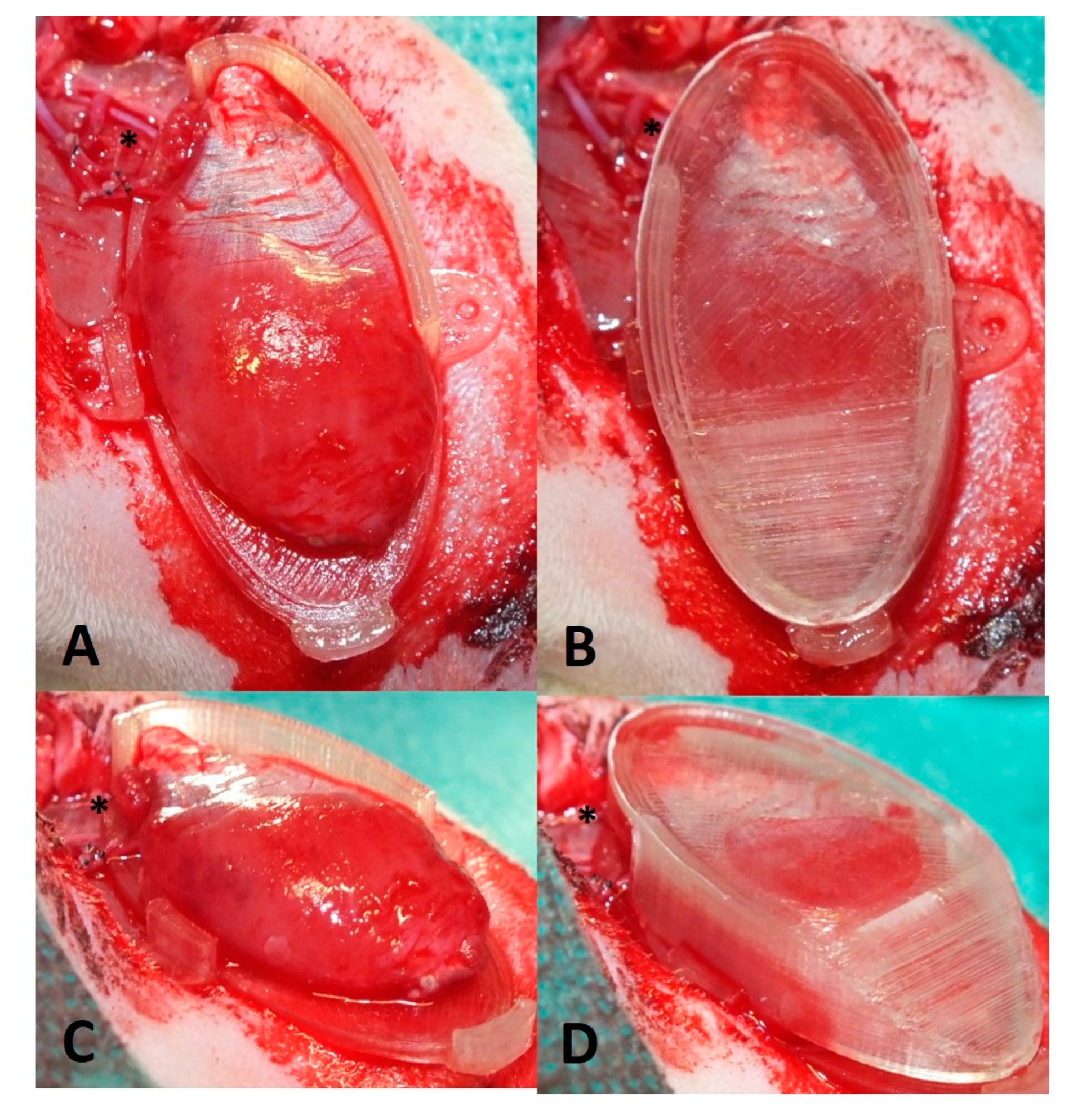
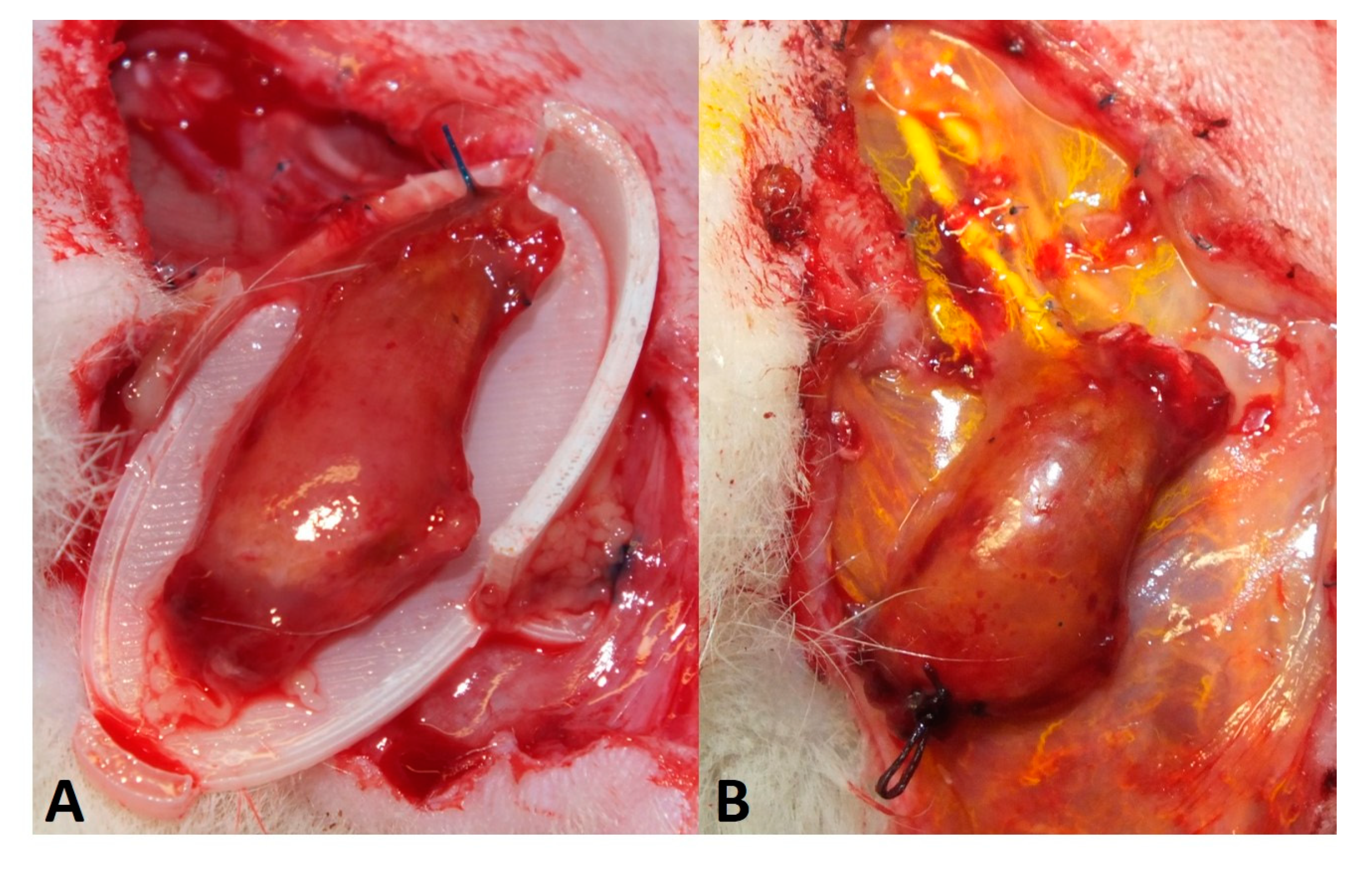
2.5. Perfusion and Explantation of Constructs
2.6. Micro-Computed Tomography
2.7. Immunohistochemical Analysis
2.8. Vessel Quantification
2.9. Statistical Analysis
3. Results
3.1. Surgery and Animals
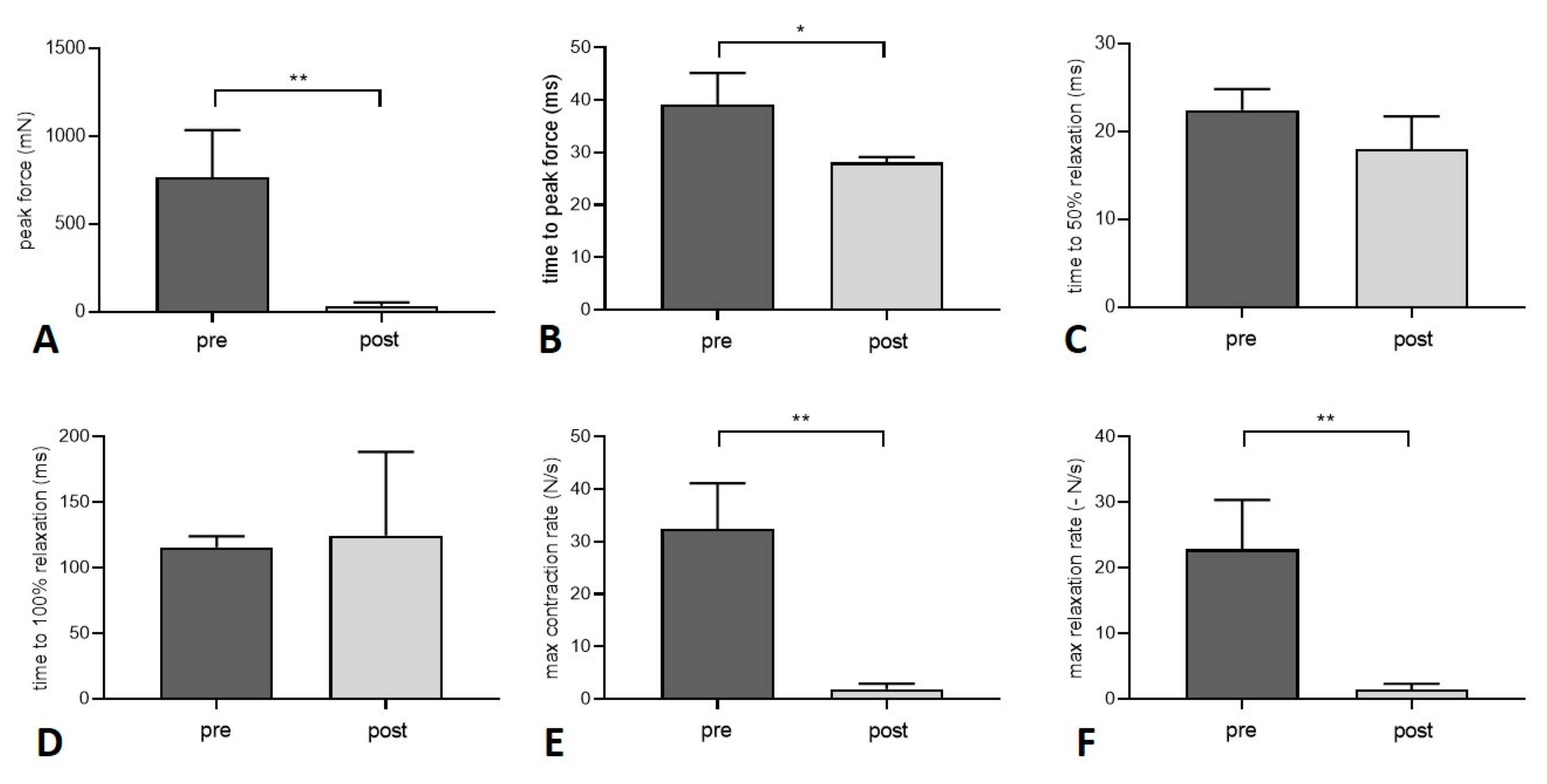
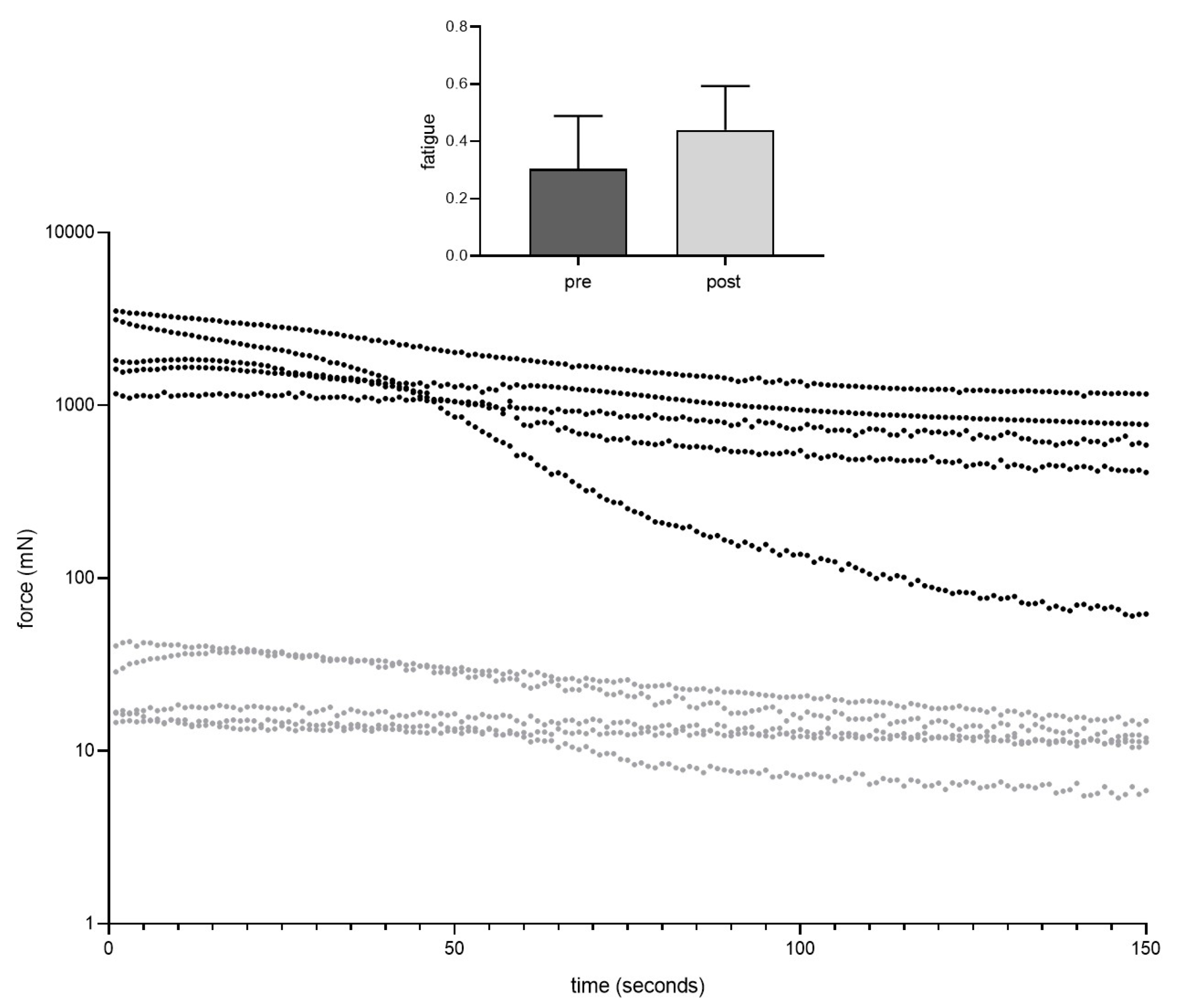
3.2. Muscle Function
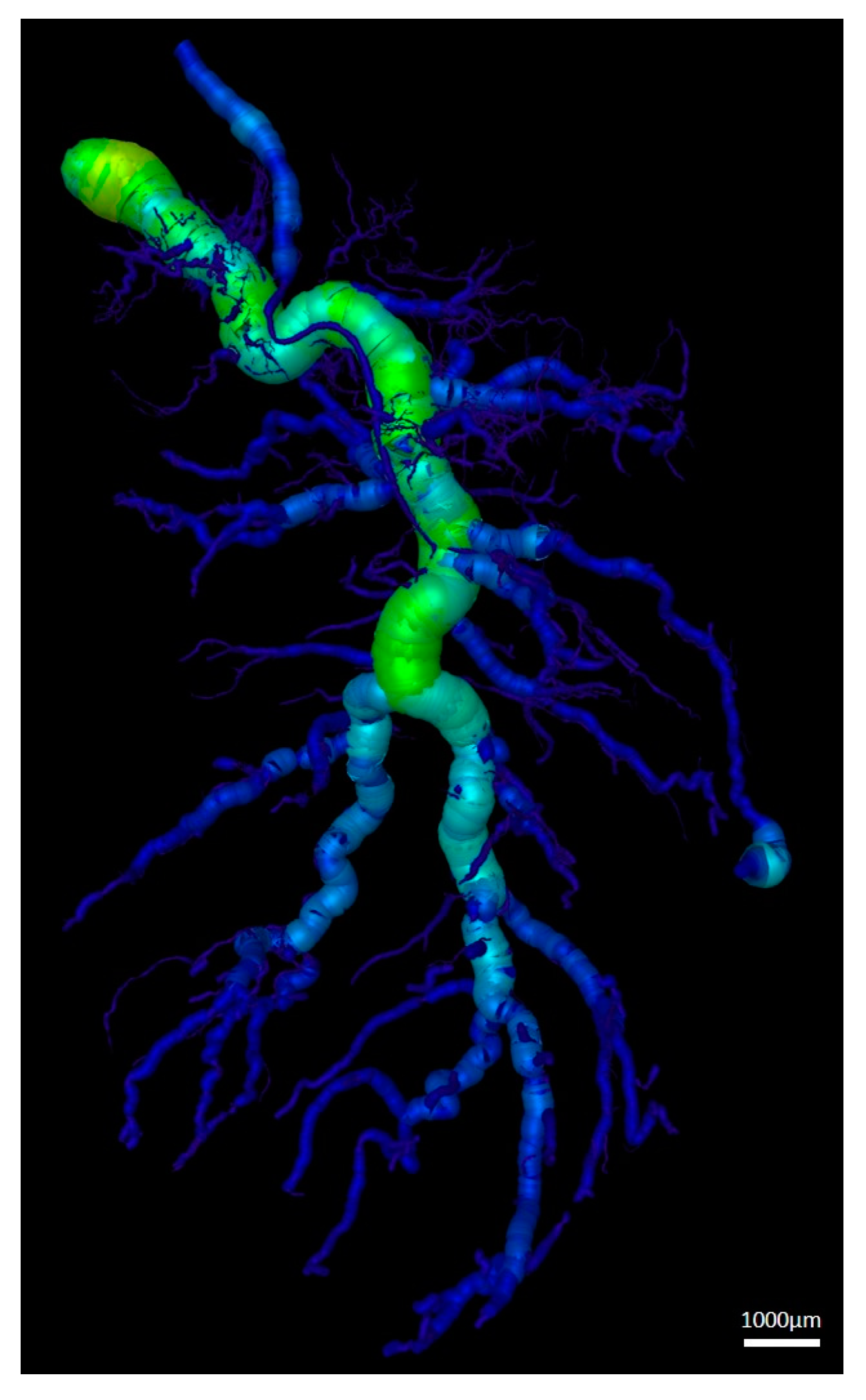
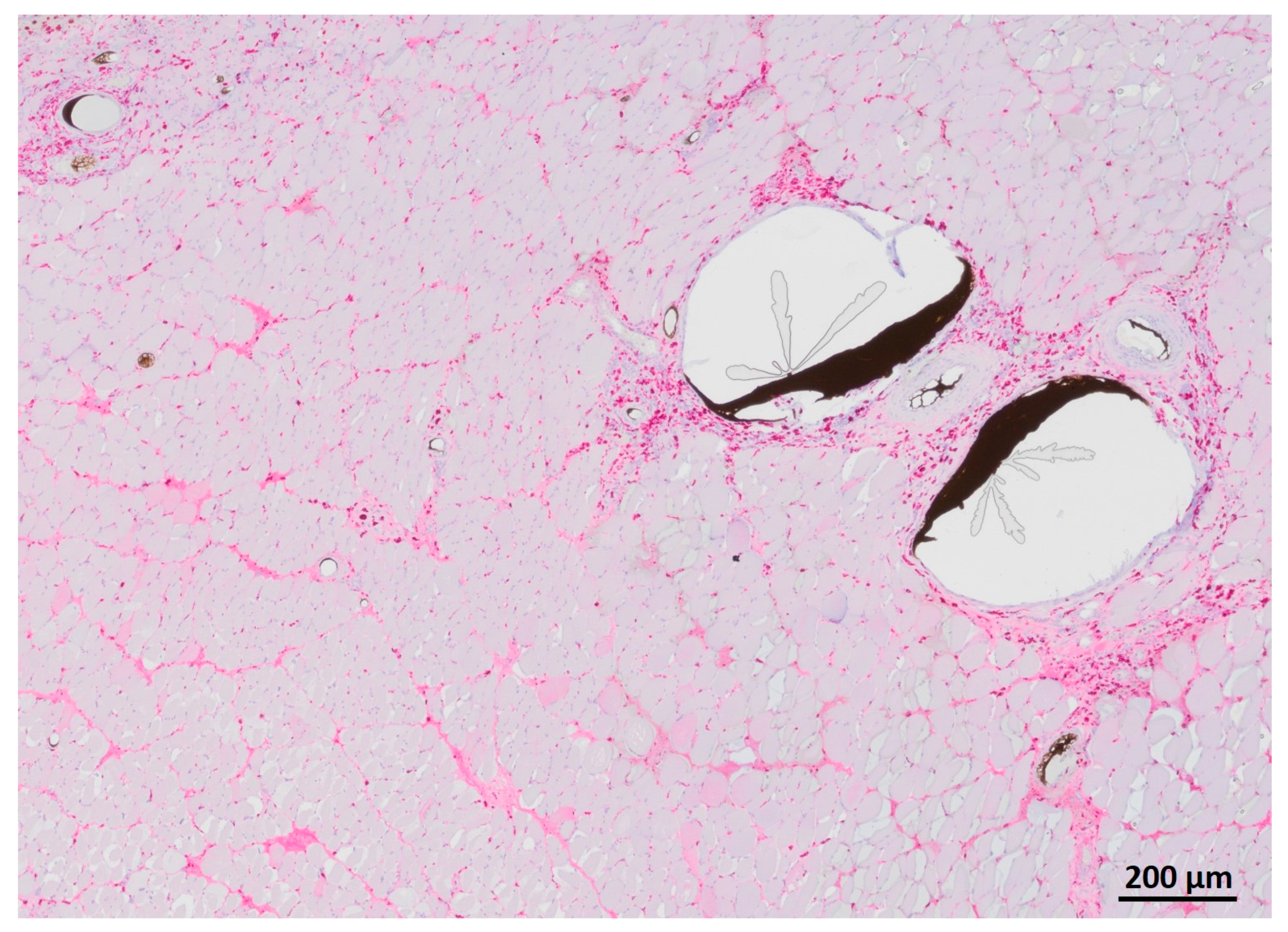
3.3. Vessel Quantification
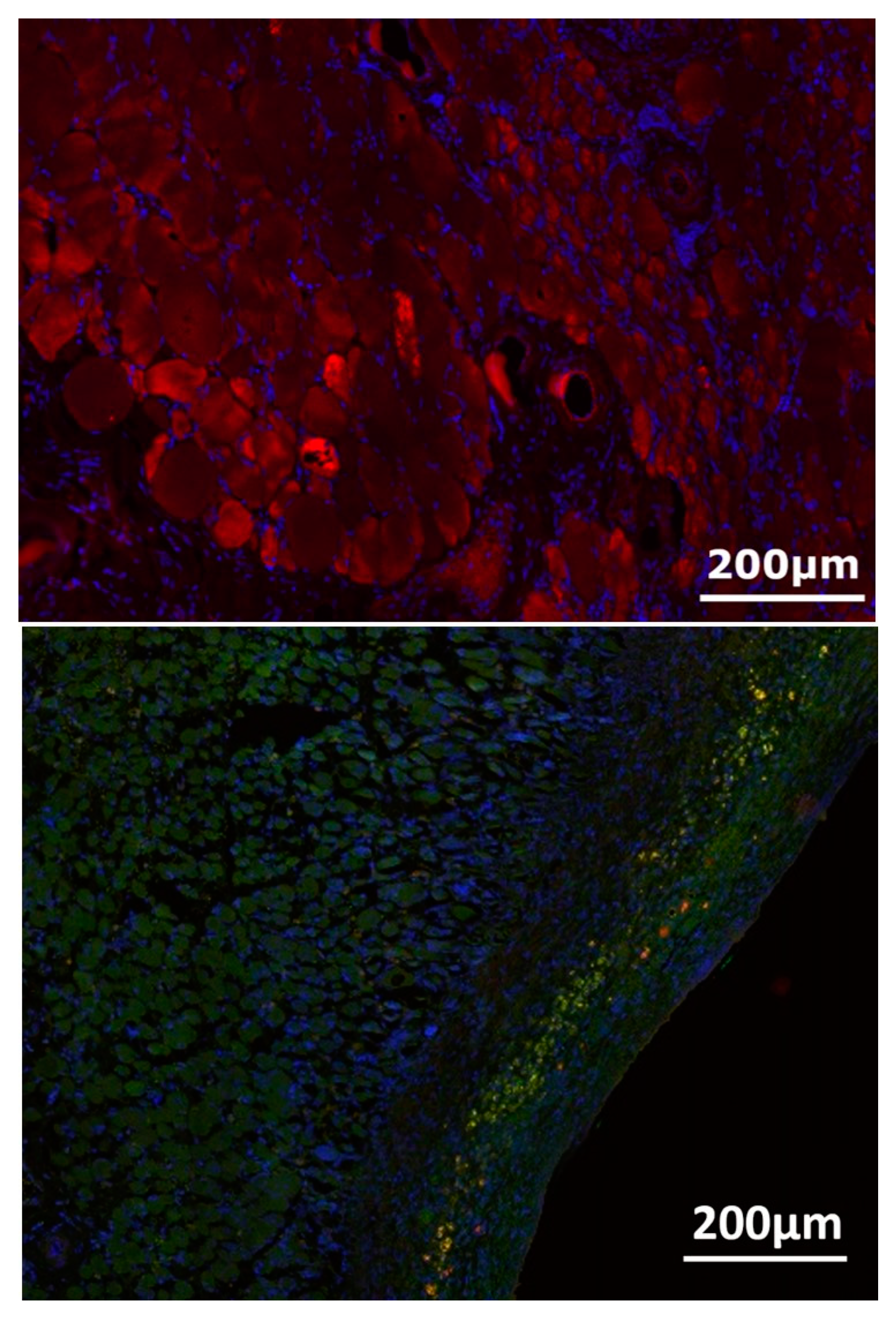
3.4. Nerve-Muscle-Junction and Myogenic Marker Expression
3.5. Myofiber Morphology and Macrophage Invasion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.T.; Mun, G.H. A systematic review of functional donor-site morbidity after latissimus dorsi muscle transfer. Plast. Reconstr. Surg. 2014, 134, 303–314. [Google Scholar] [CrossRef]
- Knox, A.D.C.; Ho, A.L.; Leung, L.; Tashakkor, A.Y.; Lennox, P.A.; Van Laeken, N.; Macadam, S.A. Comparison of Outcomes following Autologous Breast Reconstruction Using the DIEP and Pedicled TRAM Flaps: A 12-Year Clinical Retrospective Study and Literature Review. Plast. Reconstr. Surg. 2016, 138, 16–28. [Google Scholar] [CrossRef]
- Cai, A.; Hardt, M.; Schneider, P.; Schmid, R.; Lange, C.; Dippold, D.; Schubert, D.W.; Boos, A.M.; Weigand, A.; Arkudas, A.; et al. Myogenic differentiation of primary myoblasts and mesenchymal stromal cells under serum-free conditions on PCL-collagen I-nanoscaffolds. BMC Biotechnol. 2018, 18, 75. [Google Scholar] [CrossRef]
- Witt, R.; Weigand, A.; Boos, A.M.; Cai, A.; Dippold, D.; Boccaccini, A.R.; Schubert, D.W.; Hardt, M.; Lange, C.; Arkudas, A.; et al. Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering. BMC Cell Biol. 2017, 18, 15. [Google Scholar] [CrossRef] [Green Version]
- Dippold, D.; Cai, A.; Hardt, M.; Boccaccini, A.R.; Horch, R.; Beier, J.P.; Schubert, D.W. Novel approach towards aligned PCL-Collagen nanofibrous constructs from a benign solvent system. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 278–283. [Google Scholar] [CrossRef]
- Urciuolo, A.; Urbani, L.; Perin, S.; Maghsoudlou, P.; Scottoni, F.; Gjinovci, A.; Collins-Hooper, H.; Loukogeorgakis, S.; Tyraskis, A.; Torelli, S.; et al. Decellularised skeletal muscles allow functional muscle regeneration by promoting host cell migration. Sci. Rep. 2018, 8, 8398. [Google Scholar] [CrossRef] [Green Version]
- Smoak, M.M.; Han, A.; Watson, E.; Kishan, A.; Grande-Allen, K.J.; Cosgriff-Hernandez, E.; Mikos, A.G. Fabrication and Characterization of Electrospun Decellularized Muscle-Derived Scaffolds. Tissue Eng. Part C Methods 2019, 25, 276–287. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.Q.; Turner, N.J.; Teng, S.F.; Cheng, W.Y.; Zhou, H.Y.; Zhang, L.; Hu, H.W.; Wang, Q.; Badylak, S.F. Perfusion-decellularized skeletal muscle as a three-dimensional scaffold with a vascular network template. Biomaterials 2016, 89, 114–126. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Tayebi, L. Vascularization strategies in tissue engineering approaches for soft tissue repair. J. Tissue Eng. Regen. Med. 2021, 15, 747–762. [Google Scholar] [CrossRef]
- Weigand, A.; Beier, J.P.; Arkudas, A.; Al-Abboodi, M.; Polykandriotis, E.; Horch, R.E.; Boos, A.M. The Arteriovenous (AV) Loop in a Small Animal Model to Study Angiogenesis and Vascularized Tissue Engineering. J. Vis. Exp. 2016, 117, e54676. [Google Scholar] [CrossRef]
- Tamayo, T.; Eno, E.; Madrigal, C.; Heydemann, A.; Garcia, K.; Garcia, J. Functional in situ assessment of muscle contraction in wild-type and mdx mice. Muscle Nerve 2016, 53, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Hayhurst, J.W.; O’Brien, B.M. An experimental study of microvascular technique, patency rates and related factors. Br. J. Plast. Surg. 1975, 28, 128–132. [Google Scholar] [CrossRef]
- Arkudas, A.; Beier, J.P.; Pryymachuk, G.; Hoereth, T.; Bleiziffer, O.; Polykandriotis, E.; Hess, A.; Gulle, H.; Horch, R.E.; Kneser, U. Automatic quantitative micro-computed tomography evaluation of angiogenesis in an axially vascularized tissue-engineered bone construct. Tissue Eng. Part C Methods 2010, 16, 1503–1514. [Google Scholar] [CrossRef]
- Steiner, D.; Winkler, S.; Heltmann-Meyer, S.; Trossmann, V.T.; Fey, T.; Scheibel, T.; Horch, R.E.; Arkudas, A. Enhanced vascularization andde novotissue formation in hydrogels made of engineered RGD-tagged spider silk proteins in the arteriovenous loop model. Biofabrication 2021, 13, 045003. [Google Scholar] [CrossRef]
- Sarhaddi, D.; Poushanchi, B.; Merati, M.; Tchanque-Fossuo, C.; Donneys, A.; Baker, J.; Buchman, S.R. Validation of Histologic Bone Analysis Following Microfil Vessel Perfusion. J. Histotechnol. 2012, 35, 180–183. [Google Scholar] [CrossRef]
- Winkler, S.; Mutschall, H.; Biggemann, J.; Fey, T.; Greil, P.; Korner, C.; Weisbach, V.; Meyer-Lindenberg, A.; Arkudas, A.; Horch, R.E.; et al. Human Umbilical Vein Endothelial Cell Support Bone Formation of Adipose-Derived Stem Cell-Loaded and 3D-Printed Osteogenic Matrices in the Arteriovenous Loop Model. Tissue Eng. Part A 2021, 27, 413–423. [Google Scholar] [CrossRef]
- Conconi, M.T.; De Coppi, P.; Bellini, S.; Zara, G.; Sabatti, M.; Marzaro, M.; Zanon, G.F.; Gamba, P.G.; Parnigotto, P.P.; Nussdorfer, G.G. Homologous muscle acellular matrix seeded with autologous myoblasts as a tissue-engineering approach to abdominal wall-defect repair. Biomaterials 2005, 26, 2567–2574. [Google Scholar] [CrossRef]
- Chung, S.; Hazen, A.; Levine, J.P.; Baux, G.; Olivier, W.A.; Yee, H.T.; Margiotta, M.S.; Karp, N.S.; Gurtner, G.C. Vascularized acellular dermal matrix island flaps for the repair of abdominal muscle defects. Plast. Reconstr. Surg. 2003, 111, 225–232. [Google Scholar] [CrossRef]
- Zhang, F.; Kao, S.D.; Walker, R.; Lineaweaver, W.C. Pectoralis major muscle free flap in rat model. Microsurgery 1994, 15, 853–856. [Google Scholar] [CrossRef]
- Atabay, K.; Hong, C.; Bentz, M.L.; Hrach, C.J.; Futrell, J.W. Variations in the vascular pedicle of the rat gracilis muscle flap. J. Reconstr. Microsurg. 1995, 11, 265–269. [Google Scholar] [CrossRef]
- Dogan, T.; Kryger, Z.; Zhang, F.; Shi, D.Y.; Komorowska-Timek, E.; Lineaweaver, W.C.; Buncke, H.J. Quadriceps femoris muscle flap: Largest muscle flap model in the rat. J. Reconstr. Microsurg. 1999, 15, 433–437. [Google Scholar] [CrossRef]
- Tetreault, M.W.; Della Valle, C.J.; Hellman, M.D.; Wysocki, R.W. Medial gastrocnemius flap in the course of treatment for an infection at the site of a total knee arthroplasty. JBJS Essent Surg Tech. 2017, 7, e14. [Google Scholar] [CrossRef]
- Ritter, P.; Cai, A.; Reischl, B.; Fiedler, M.; Prol, G.; Frie, B.; Kretzschmar, E.; Michael, M.; Hartmann, K.; Lesko, C.; et al. MyoBio: An automated bioreactor system technology for standardized perfusion-decellularization of whole skeletal muscle. IEEE Trans. Biomed. Eng. 2022, 1. [Google Scholar] [CrossRef]
- Agata, N.; Sasai, N.; Inoue-Miyazu, M.; Kawakami, K.; Hayakawa, K.; Kobayashi, K.; Sokabe, M. Repetitive stretch suppresses denervation-induced atrophy of soleus muscle in rats. Muscle Nerve 2009, 39, 456–462. [Google Scholar] [CrossRef]
- Sakakima, H.; Yoshida, Y. Effects of short duration static stretching on the denervated and reinnervated soleus muscle morphology in the rat. Arch. Phys. Med. Rehabil. 2003, 84, 1339–1342. [Google Scholar] [CrossRef]
- Sakakima, H.; Yoshida, Y. The effect of short duration static stretching and reinnervation on the recovery of denervated soleus muscle in the rat. J. Jpn. Phys. Ther. Assoc. 2002, 5, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Finol, H.J.; Lewis, D.M. Proceedings: The effects of denervation on isometric contractions of rat skeletal muscle. J. Physiol. 1975, 248, 11P. [Google Scholar]
- Billington, L. Reinnervation and regeneration of denervated rat soleus muscles. Muscle Nerve 1997, 20, 744–746. [Google Scholar] [CrossRef]
- Kaariainen, M.; Kauhanen, S. Skeletal muscle injury and repair: The effect of disuse and denervation on muscle and clinical relevance in pedicled and free muscle flaps. J. Reconstr. Microsurg. 2012, 28, 581–587. [Google Scholar] [CrossRef]
- Bitto, F.F.; Klumpp, D.; Lange, C.; Boos, A.M.; Arkudas, A.; Bleiziffer, O.; Horch, R.E.; Kneser, U.; Beier, J.P. Myogenic differentiation of mesenchymal stem cells in a newly developed neurotised AV-loop model. Biomed. Res. Int. 2013, 2013, 935046. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, A.; Zhang, W.; Jiang, H.; Cai, Z. Correlation of contractile function recovery with acetylcholine receptor changes in a rat muscle flap model. Microsurgery 2010, 30, 307–313. [Google Scholar] [CrossRef]
- Cen, Y.; Liu, J.; Qin, Y.; Liu, R.; Wang, H.; Zhou, Y.; Wang, S.; Hu, Z. Denervation in Femoral Artery-Ligated Hindlimbs Diminishes Ischemic Recovery Primarily via Impaired Arteriogenesis. PLoS ONE 2016, 11, e0154941. [Google Scholar] [CrossRef]
- Hendrickse, P.; Degens, H. The role of the microcirculation in muscle function and plasticity. J. Muscle Res. Cell Motil. 2019, 40, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Huang, Z.; Ma, J.; Shao, L.; Wang, H.; Wang, Y. Perlecan and synaptophysin changes in denervated skeletal muscle. Neural Regen Res. 2012, 7, 1293–1298. [Google Scholar] [CrossRef]
- Miyamaru, S.; Kumai, Y.; Minoda, R.; Yumoto, E. Nerve-muscle pedicle implantation in the denervated thyroarytenoid muscle of aged rats. Acta Otolaryngol. 2012, 132, 210–217. [Google Scholar] [CrossRef]
- Mueller, M.; Leonhard, C.; Wacker, K.; Ringelstein, E.B.; Okabe, M.; Hickey, W.F.; Kiefer, R. Macrophage response to peripheral nerve injury: The quantitative contribution of resident and hematogenous macrophages. Lab. Investig. 2003, 83, 175–185. [Google Scholar] [CrossRef]



| Rat | Donor Rat Weight (g) | Gastrocnemius Muscle Weight (g) | Recipient Rat Weight (g) | Ischemia Time (min) |
|---|---|---|---|---|
| 1 | 285 | 1 | 410 | 120 |
| 2 | 240 | 0.87 | 440 | 100 |
| 3 | 260 | 0.94 | 400 | 85 |
| 4 | 290 | 1 | 370 | 90 |
| 5 | 320 | 1.03 | 410 | 90 |
| Pre Transplantation | Post Transplantation | |
|---|---|---|
| Peak twitch force (mN) | 768.5 ± 266.6 | 34.7 ± 19.7 (**) |
| Time to peak twitch force (ms) | 39.2 ± 6.0 | 28.1 ± 1.0 (*) |
| Time to 50% relaxation (twitch force) (ms) | 22.5 ± 2.4 | 18.0 ± 3.7 |
| Time to 100% relaxation (twitch force) (ms) | 115.6 ± 8.3 | 124.5 ± 63.9 |
| Maximum contraction rate (twitch) (N/s) | 32.5 ± 8.6 | 1.9 ± 1.1 (**) |
| Maximum relaxation rate (twitch) (-N/s) | 22.8 ± 7.5 | 1.4 ± 0.9 (**) |
| Maximum tetanic force (at 80 Hz) (mN) | 2771 ± 772.7 | 28.6 ± 14.7 (**) |
| Maximum contraction rate (tetanus at 80 Hz) (N/s) | 57.8 ± 14.7 | 1.0 ± 0.5 (**) |
| Time to peak force (tetanus at 80 Hz) (ms) | 33.7 ± 7.1 | 19.6 ± 2.2 (*) |
| Maximum tetanic force (at 100 Hz) (mN) | 2920 ± 981.9 | 32.1 ± 14.2 (**) |
| Maximum contraction rate (tetanus at 100 Hz) (N/s) | 59.1 ± 10.5 | 1.1 ± 0.5 (***) |
| Time to peak force (tetanus at 100 Hz) (ms) | 32.2 ± 4.6 | 19.7 ± 2.2 (**) |
| Maximum tetanic force (at 120 Hz) (mN) | 2970 ± 1172 | 34.5 ± 13.4 (**) |
| Maximum contraction rate (tetanus at 120 Hz) | 56.3 ± 19.7 | 1.2 ± 0.5 (**) |
| Time to peak force (tetanus at 120 Hz) (ms) | 32.5 ± 2.8 | 19.6 ± 1.9 (***) |
| Vessel Radius (µm) | Number of Vessels | Cumulative Length (mm) |
|---|---|---|
| 5–45 | 4168 ± 1110 | 595.4 ± 112.1 |
| 55–95 | 3007 ± 1858 | 266.2 ± 122.9 |
| 105–145 | 1017 ± 794.1 | 155.7 ± 111.2 |
| 155–195 | 444.2 ± 372.2 | 96.3 ± 88.1 |
| 205–245 | 243.2 ± 188.4 | 61.5 ± 51.5 |
| 255–295 | 157 ± 90.7 | 45.6 ± 33.5 |
| 305–335 | 68.8 ± 42.9 | 24.9 ± 16.8 |
| Vessel Radius (µm) | Number of Vessel Lumen |
|---|---|
| 0.1–20 | 165.8 ± 109.5 |
| 20.1–40 | 17.4 ± 2.7 |
| 40.1–60 | 3.4 ± 1.5 |
| 60.1–80 | 1.8 ± 1.6 |
| 80.1–100 | 1.4 ± 0.9 |
| 100.1–120 | 0.4 ± 0.5 |
| 120.1–140 | 0.2 ± 0.4 |
| 140.1–160 | 0.2 ± 0.4 |
| 180.1–200 | 0.4 ± 0.5 |
| 220.1–240 | 0.4 ± 0.9 |
| 240.1–260 | 0.2 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, A.; Zheng, Z.; Müller-Seubert, W.; Biggemann, J.; Fey, T.; Beier, J.P.; Horch, R.E.; Frieß, B.; Arkudas, A. Microsurgical Transplantation of Pedicled Muscles in an Isolation Chamber—A Novel Approach to Engineering Muscle Constructs via Perfusion-Decellularization. J. Pers. Med. 2022, 12, 442. https://doi.org/10.3390/jpm12030442
Cai A, Zheng Z, Müller-Seubert W, Biggemann J, Fey T, Beier JP, Horch RE, Frieß B, Arkudas A. Microsurgical Transplantation of Pedicled Muscles in an Isolation Chamber—A Novel Approach to Engineering Muscle Constructs via Perfusion-Decellularization. Journal of Personalized Medicine. 2022; 12(3):442. https://doi.org/10.3390/jpm12030442
Chicago/Turabian StyleCai, Aijia, Zengming Zheng, Wibke Müller-Seubert, Jonas Biggemann, Tobias Fey, Justus P. Beier, Raymund E. Horch, Benjamin Frieß, and Andreas Arkudas. 2022. "Microsurgical Transplantation of Pedicled Muscles in an Isolation Chamber—A Novel Approach to Engineering Muscle Constructs via Perfusion-Decellularization" Journal of Personalized Medicine 12, no. 3: 442. https://doi.org/10.3390/jpm12030442
APA StyleCai, A., Zheng, Z., Müller-Seubert, W., Biggemann, J., Fey, T., Beier, J. P., Horch, R. E., Frieß, B., & Arkudas, A. (2022). Microsurgical Transplantation of Pedicled Muscles in an Isolation Chamber—A Novel Approach to Engineering Muscle Constructs via Perfusion-Decellularization. Journal of Personalized Medicine, 12(3), 442. https://doi.org/10.3390/jpm12030442












