Abstract
Implementation of ultrasonography (USG), computed tomography (CT) and magnetic resonance imaging (MRI) into abdominal cavity diagnostics enabled early detection of cT1 graded renal cancers. According to European Association of Urology (EAU) and Polish urological Association (PUA) recommended method of treatment is sparing resection of renal parenchyma with tumour—nephron-sparing surgery (NSS). In selected cases other methods such as thermal ablation (TA) or cryoablation can be introduced /1/. Objectives: To evaluate the results of treatment of cT1 renal tumours with the use of NSS and TA methods. Material and methods: 140 patients with cT1 renal carcinoma were treated in 2nd Department of Urology of Medical University of Lodz between 2014 and 2017. Neuron-sparing surgery was performed in 56 cases (40%), while percutane-ous thermal ablation (TA) in 84 cases (60%). Demographic data, clinical data (lab results, Charlson index), nephrometry data (tumour size, location, R.E.N.A.L. score) post-operative data (Clavien-Dindo classifica-tion) were investigated. Histopathology results, Fuhrman malignancy grading, as total three-year survival of patients were evaluated. The following methods were used for statistical evaluation: Chi2, Fisher, W Shapiro-Wilk, U Mann-Whitney tests, Kaplan-Meier’s curve and Cox model. The results were displayed in a form of median and upper and lower quartile values (25–75%). Results: No statistical differences in gender nor left/right kidney location were observed. Patients, who underwent TA were at average 10 years older and had multiple comorbidities (median age for TA was 79, for NSS 68; median Charlson index for TA was 5 and for NSS was 3). TA patients had lesser haematological values (Hb, Ht). R.E.N.A.L. scoring demonstrated comparable nephrometry in both groups. NSS procedure was open laparotomy without temporary clamping of renal vessels. Surgical margins of resected tumours were negative. TA was performed with Cool-Tip Covidienequipment with the use of Cluster electrode and was ultraso-nography-guided. Post-treatment complications evaluated with the use of Clavien-Dindo classification were slightly more frequent for NSS method. Patients after NSS were discharged at average after 8.5 days and after TA after 3 days. Histopathological type and Fuhrman malignancy grading were comparable in both groups. TA treated patients’ death risk was 9-fold of that observed in NSS treated patients. There was 1 death for each group in perioperative period. Conclusion: 1. NSS was associated with slightly higher side effect rate but resulted in prolonged survival. 2. TA was applied to elderly patients with comorbidities. Despite less invasive treatment this group had poorer/reduced survival. 3. Charlson Comorbidity Index (CCI) and the treatment method were relevant survival factors in patients treated due to cT1 renal cancer tumours.
1. Objectives
To evaluate the results of treatment for cT1 renal tumours with the use of NSS and TA.
2. Introduction
In 2018, according to the GLOBOCAN registry, 403,362 new kidney cancer cases were recorded worldwide [1]. This accounts for 3% of all cancers in adults and is ranked 12th among all neoplasms (9th in men and 14th in women) [2,3]. More than 59% of kidney cancers are diagnosed in developed countries, mostly in Europe, North America and Australia, with fewer tumors diagnosed in Africa, India and China. Kidney cancer is most frequently diagnosed in the Czech Republic, Slovakia and Lithuania, as far as Europe is concerned [4].
Kidney cancer occurs mainly in men (75%) aged of 60 and older [5]. The root cause of this neoplasm is not precisely determined. Cancer risk factors include chemical factors, with smoking being the leading factor. The risk of developing renal cell carcinoma among smokers is greater by 54% among men, and 22% among women [6].
The introduction of ultrasonography (USG), computed tomography (CT) and magnetic resonance imaging (MRI), into abdominal cavity diagnostics, enabled early detection of asymptomatic types of renal cancers. The resection of tumours or kidneys with tumours became the standard renal tumour treatment method.
The research on the optimal method of treatment for small asymptomatic renal tumours is pending [7,8,9,10]. Current guidelines for the tumour’s diameter of up to 7 cm recommend maximum sparing of renal parenchyma (NSS) [11].
Nephron loss influence on cardiovascular diseases is observed [12]. Patients with numerous comorbidities, disabling regular surgery for anaesthesiological or general reasons, are investigated. Experimental methods include thermal ablation, cryoablation, brachytherapy or stereotactic radiotherapy. Stereotactic radiotherapy is a unique method of treating pathological lesions, which consists of administering one or more large doses of radiation to the tumour area, with a minimal exposure of surrounding tissues. Stereotactic radiosurgery is used as part of radical, as well as palliative and analgesic, treatment. In many cases, it is a reasonable alternative to a riskier classical surgical treatment [13,14,15,16]. In this paper, we compare the outcome/results of treating cT1 renal cancer with 4 cm diameter in patients, with the use of NSS techniques and ultrasound-guided TA.
3. Material
Retrospective analysis of 140 patients with T1N0M0 renal carcinoma treated in 2nd Department of Urology of Medical University of Lodz between 2014 and 2017 was performed. The analysis/investigation was approved by the Ethics Committee of Medical University of Lodz. Nephron-sparing surgery was used in 56 cases, while percutaneous high radio frequency thermal ablation (RFA) was used in 84 cases.
4. Methods
Renal tumours were diagnosed with the use of ultrasonography, computed tomography and magnetic resonance imaging. Histopathology examination of removed tumour was performed afterwards. In patients treated with TA, ultrasonography-guided biopsy was performed. Comorbidities were evaluated with Charlson Comorbidity Index. Nephrometry parameters were evaluated with R.E.N.A.L. score. Post-treatment complications were evaluated with the use of Clavien-Dindo classification [17,18,19]. Patients with small T1 renal tumours with peripheral or intermediate location per R.E.N.A.L. score were qualified to NSS, which was open laparotomy (ONSS) without clamping renal vessels. Patients with small T1 renal tumours with peripheral or intermediate location per R.E.N.A.L. score but who had objections related to age or general conditions, had renal contraindications (single kidney, tumours in both kidneys) or who had not consented to surgery were qualified to thermal ablation.
Thermal ablation was performed with monopolar Cool-tip RF ablation system (Covidien, Mansfield, MA, USA) [20,21]. Urea, creatinine and potassium levels were examined before and after operation.
Imaging examinations such as ultrasound, CT, MRI, PET/CT were performed 3, 6 and 12 months after ablation followed by CT and MRI 6-monthly for the next 2 years. Patient follow-up was completed in 2020. Patients’ death data were obtained from the Ministry of Digitalisation after obtaining respective approvals.
Statistical analysis was performed with the use of statistical packet STATISTICA 13.1 licensed by Medical University of Lodz. Nominal variables were displayed as a count of observations and percentage values calculated for investigated and control groups. Chi2 test was used for comparison. For low count of observations to increase conservatism of the test, the Fisher’s exact test was used. Continuous variables due to non-normal distribution pattern (verified with Shapiro-Wilk test) were displayed in a form of median and upper- and lower-quartile values (25–75%). Both groups were compared with the use of Mann-Whitney U test. For survival analysis Kaplan-Meier’s curve and single regression Cox proportional hazards model were applied. To eliminate the bias caused by clinical variables which substantially varied between investigated and control groups, multiple regression Cox proportional hazards model was used considering only those variables which were significant or almost significant (p < 0.1) in single regression model. The results of model application were presented as regression factor, odds ratio with 95% confidence interval (95% CI) and p value.
Clinical data of treated patients are presented in Table 1 and Table 2. Table 1 contains nominal variables and Table 2 continuous variables. In the group of 140 patients with T1a renal tumour there were 86 (61.43%) men and 54 (38%) women. Median age was 67.5 years (59–74.5). Among 140 patients, only 1 patient did not have comorbidities; others had between one and three comorbidities and median value in Charlson comorbidity index was 2 (0–4). Lab values for analysed group were within normal ranges. We found that 76 (54.29%) patients had tumour located in left kidney and 64 (45.71) in right kidney. Median diameter of tumour was 28 mm (23–34.5 mm). Further, 126 (90%) tumours were classified as cT1a and 14 (10%) as cT1b.

Table 1.
Comparison of distribution of nominal variables in the whole group and between NSS-treated and TA-treated group.

Table 2.
Comparison of continuous variables between NSS-treated and TA-treated groups.
In R.E.N.A.L. nephrometry score the location of tumour was exophytic in 128 cases (91.43) or intermediate in 12 cases (8.57%), which proves the right selection of patients to the applied treatment. As such, 56 patients (40%) underwent partial resection of parenchyma containing tumour and in 84 patients (60%) thermal ablation was performed; 133 patients (95%) had no complications, 3 patients (2.14) had minor complications and 2 patients (1.43%) major ones. Two patients (1.43) died during perioperative period.
The average duration of hospitalisation was 8 days. In 123 cases clear cell carcinoma was confirmed in histopathology. Other cases were papillary type I (6 cases (4.29%)), papillary type II (8 cases (5.71%)) and chromophobe renal carcinoma (3 cases (2.14%)) respectively. Fuhrman nuclear grade value (malignancy) was 1 to 2 in 123 cases (87.86). During 3-year follow-up, 25 patients died in both groups. Probability of survival of both groups was 81% (Figure 1). Initial clinical features of both patient groups (NSS and TA) were split into nominal variables and are presented in Table 1, and continuous variables presented in Table 2.
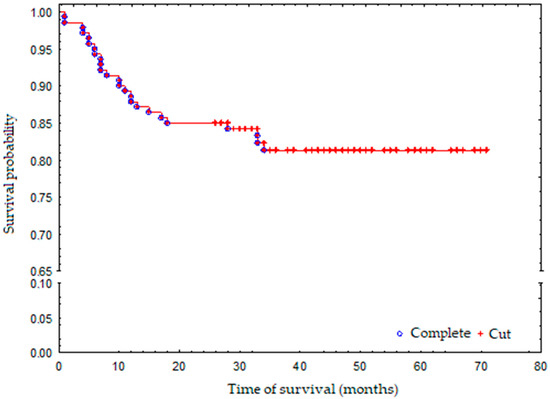
Figure 1.
Survival probability in the whole group.
5. Results Overview and Discussion
Surgical treatment is the standard way of managing renal carcinoma. The type of procedure depends on the size and the location of the tumour, as well as the coexistence of plugs, made of cancer cells, in the renal vein and vena cava inferior. Patients disqualified from surgical treatment can be treated with various forms of tumour ablation and stereotactic radiotherapy [13,14,15]. Ablation of pT1 tumours is safe and associated with low incidence of complications. Efficacy and survival period after NSS remain controversial [22]. Patients treated in our centre formed a homogenous group, as far as TMF grading is concerned (T1N0M0), and the same lab assays and imaging examinations were performed. The median age of the group was 67, which is the age when renal tumours occur most frequently [23]. The group treated with partial resection (NSS) was 10 years younger and the median age of this group was 68. The median age of the group treated with thermal ablation (TA) was 79. In the NSS group, only three patients lived to 75 years of age, while in the TA group, there were 32 such (38.09%) patients. These correspond with the observations of other authors [24,25]. In the treated group, renal cancer occurred more often in men than in women (2:1), and we did not observe relevant differences in frequency between left and right kidney. The presence of comorbidities expressed in the Charlson comorbidity index enabled the assessment of the treatment. The average index value for the whole group was 2 points, while for the NSS group, 3 points, and for the TA group, 5 points, respectively. According to Benegas M. P. et al., patients with Charlson index >1, who were treated with NSS but not nephrectomy, have a greater chance of maintaining better glomerular filtration by 2.5-fold, smaller risk of development of chronic kidney insufficiency, as well as occurrence of cardiovascular events [25]. The American Urological Association (AUA) recommends thermal ablation with the following comorbidities: diabetes, hypertension, chronic renal disease, cerebrovascular and cardiovascular diseases and high surgical and anaesthesiologic risk [26]. In our material, probably due to the elderly age of patients treated with the use of TA, the Charlson index is higher than in other authors’ publications [13]. Lab assays performed prior to the procedure were all within normal ranges (Table 2). Red cells and haemoglobin levels were statistically higher in patients treated with NSS, compared to those treated with TA (Table 2). This indicates a better general condition of patients undergoing open surgery. Platelet levels were comparable in both groups. Nephrometry analysis showed treated tumours were comparable in size and location in the left or right kidney. However, tumour diameters in patients treated with TA were significantly larger than in patients treated with NSS. The R.E.N.A.L. scoring system was implemented to unify the anatomical classification of tumours [27,28,29,30,31,32]. This system is used for the evaluation of NSS and TA treatment. The system describes tumour size and its exophytic or endophytic placement, as well as distance to the renal pelvis and sinus and location within kidney. The use of the system enabled the selection of comparable groups, nephrometry wise (1% of tumours were of exophytic and 8% of intermediate placement). NSS was performed as open laparotomy and patients qualified to this treatment method had to meet ASA classification criteria values = 1 or 2. The tumours in all operated patients had negative resection margin. Ficarra V. et al., in their paper, published in 2018, confirmed that positive resection margin was observed in 6.7% of resected tumours [33]. NSS patients were hospitalised significantly longer compared to TA patients. No post-treatment complications were observed in 95% of cases. Minor post-treatment complications were observed in three patients who had undergone NSS. Severe post-treatment complications were observed in one patient, who had undergone NSS, and in one patient who had undergone TA. Perioperative mortality was comparable in both groups. One NSS patient died due to bleeding and cardiovascular complications and one TA patient died due to thermal intestine injury, followed by peritonitis. No differences were observed in histopathological type and Fuhrman malignancy grading in either group. There were no deaths due to kidney cancer in either group of patients (treated with NSS and thermoablation) for 36 months, despite the differences in the nephrometry assessment of the R.E.N.A.L score in both groups.
During the follow-up period, 25 patients died: 2 NSS patients (3.5%) and 23 TA patients (27.3%), which is highly statistically significant. Three-year survival probability for the whole group was 81% (Figure 1). The death risk in the TA-treated group was 9-fold higher than that observed in the NSS-treated group, which was mostly associated with a greater number of additional illnesses and more advanced age of the patients who have undergone TA (Figure 2). The observed overall survival of patients in both groups did not depend on the neoplastic disease, but on the age of the patients (patients treated with renal-saving surgery were at average 10 years younger than patients treated with thermoablation) and their comorbidity-associated general condition (patients treated with thermoablation had statistically significantly more comorbidities than those treated with renal-sparing surgery, which was reflected in higher Charlson Comorbidity Index).
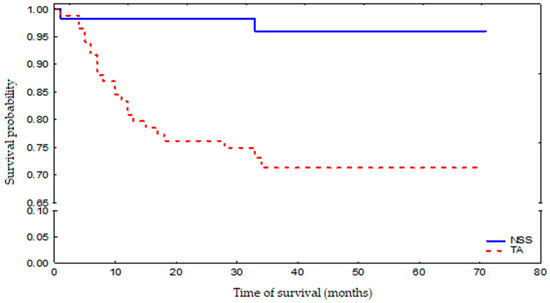
Figure 2.
Survival probability in NSS-treated and TA-treated patient groups.
6. Conclusions
- NSS was associated with a slightly higher side effect rate but resulted in prolonged survival;
- TA was applied to elderly patients with comorbidities. Despite less invasive treatment, this group had poorer survival;
- The aim of presenting two widely different methods for small kidney tumour treatment was to demonstrate their application for various age groups and clinical conditions.
Author Contributions
All Authors participated in: conceptualization, methodology, software, validation, formal analysis, investigation, resources data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition, as follow: M.R. 40%, M.S. 5%, W.R. 5%, B.J. 5%, M.M. 20%, M.L. 5%, J.W. 20%. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Medical University of Łódź, (RNN/232/18/KE 12 June 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. J. Clin. 2018, 68, 394–4241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ziegelmüller, B.K.; Spek, A.; Szabados, B.; Casuscelli, J.; Clevert, D.A.; Staehler, M. Epidemiology and diagnostic assessment of small renal masses. Der. Urologe. Ausg. A 2018, 57, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Zequi, S.; da Costa, W.H.; Korkes, F.; Dos Reis, R.B.; Busato, W.F.S.; Matheus, W.E.; Soares, A. Renal cell cancer treatment: Anex pert panel recommendation from the Latin American cooperative group-genitourinary and the Latin American renal cancer group: Focus on burger. Ther. Adv. Urol. 2019, 11, 1756287219872324. [Google Scholar]
- Protzel, C.; Maruschke, M.; Hakenberg, O.W. Epidemiology, aetiology and pathogenesis of renal cell carcicoma. EAU-Eur. Urol. Suppl. 2012, 11, 52–59. [Google Scholar] [CrossRef]
- European Association of Urology Guidelines 2019 Edition. Renal Cell Carcinoma 7.1.4.3. Ablative Therapies. Available online: https://uroweb.org/guidelines/renal-cell-carcinoma (accessed on 6 December 2021).
- Yan, S.; Yang, W.; Zhu, C.-M.; Yan, P.-M.; Wang, Z.-C. Comparison among cryoablation, radiofrequency ablation, and partial nephrectomy for renal cell carcinomas sized smaller than 2 cm or sized 2–4 cm: A population-based study. Medicine 2019, 98, e15610. [Google Scholar] [CrossRef]
- Uhlig, J.; Kokabi, N.; Xing, M.; Kim, H.S. Ablation versus Resection for Stage 1A Renal Cell Carcinoma: National Variation in Clinical Management and Selected Outcomes. Radiology 2018, 288, 889–897. [Google Scholar] [CrossRef] [Green Version]
- Pantelidou, M.; Challacombe, B.; McGrath, A.; Brown, M.; Ilyas, S.; Katsanos, K.; Adam, A. Percutaneous Radiofrequency Ablation Versus Robotic-Assisted Partial Nephrectomy for the Treatment of Small Renal Cell Carcinoma. Cardiovasc. Interv. Radiol. 2016, 39, 1595–1603. [Google Scholar] [CrossRef] [Green Version]
- Van Poppel, H.; Da Pozzo, L.; Albrecht, W.; Matveev, V.; Bono, A.; Borkowski, A. Eastern Cooperative Oncology Group. A Prospective, randomized EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur. Urol. 2011, 59, 543. [Google Scholar] [CrossRef]
- Kates, M.; Badalato, G.M.; Pitman, M.; McKiernan, J.M. Increased risk of overall and cardiovascular mortality after radical nephrectomy for renal cell carcinoma 2 cm or less. J. Urol. 2011, 186, 1247. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Atwell, T.; Schmit, G.; Lohse, C.M.; Kurup, A.N.; Weisbrod, A.; Leibovich, B.C. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur. Urol. 2015, 67, 252. [Google Scholar] [CrossRef]
- Gunn, A.J.; Gervais, D.A. Percutaneous Ablation of the Small Renal Mass—Techniques and Outcomes. Semin. Interv. Radiol. 2014, 31, 033–041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanathan, R.; Leveillee, R.J. Ablative therapies for renal tumors. Ther. Adv. Urol. 2010, 2, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Wegner, R.E.; Abel, S.; Vemana, G.; Mao, S.; Fuhrer, R. Utilization of stereotactic ablative body radiation therapy for intact renal cell carcinoma: Trends in treatment and predictors of outcome. Adv. Radiat. Oncol. 2019, 5, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E. Charlson Comorbidity Index. Available online: https://www.mdcalc.com/charlson-comorbidity-index-cci (accessed on 6 December 2021).
- Garrido, D.; Dutra, S.; Amante, S.; Chaves, M.; Brum, M. The RENAL Nephrometry Score–What, Why and How. Eur. Congr. Radiol. 2018. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Salagierski, M.; Salagierski, M.; Różański, W. Termoablacja alternatywne rozwiązania i dylematy związane z postępowaniem w przypadkach nowotworów nerek. Urol. Pol. 2008, 61, 4. [Google Scholar]
- Salagierski, M.; Salagierski, M.; Różański, W. Ablacja termiczna guzów nerek monito. rowana w czasie trwania zabiegu. Przegląd Urol. 2010, 2, 60. [Google Scholar]
- Abu-Ghanem, Y.; Fernández-Pello, S.; Bex, A.; Ljungberg, B.; Albiges, L.; Dabestani, S.; Giles, R.H.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; et al. Limitations of Available Studies Prevent Reliable Comparison Between Tumour Ablation and Partial Nephrectomy for Patients with Localised Renal Masses: A Systematic Review from the European Association of Urology Renal Cell Cancer Guideline Panel. Eur. Urol. Oncol. 2020, 3, 433–452. [Google Scholar] [CrossRef]
- Yin, X.; Cui, L.; Li, F.; Qi, S.; Yin, Z.; Gao, J. Radiofrequency Ablation Versus Partial Nephrectomy in Treating Small Renal Tumors: A systematic revive and meta-analysi. Medicine 2015, 94, e2255. [Google Scholar] [CrossRef]
- Stern, J.M.; Svatek, R.; Park, S.; Hermann, M.; Lotan, Y.; Sagalowsky, A.I.; Cadeddu, J.A. Intermediate comparison of partial nephrectomy and radiofrequency ablation for clinical T1a renal tumours. Br. J. Urol. 2007, 100, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Banegas, M.; Harlan, L.; Mann, B.; Yabroff, K.R. Toward greater adoption of minimally-invasive and nephron-sparing surgical techniques for renal cell cancer in the U.S. Urol. Oncol. 2016, 34, 433.e9–433.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.-W.; Cui, X.-M.; Huang, H.; Huang, Y.; Li, L.; Wang, Z.-J.; Qu, F.-J.; Gao, Y.; Cui, X.-G.; Xu, D.-F. Radiofrequency ablation versus partial nephrectomy for treatment of renal masses: A systematic review and meta-analysis. Kaohsiung J. Med. Sci. 2015, 31, 649–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osawa, T.; Hafez, K.S.; Miller, D.C.; Montgomery, J.S.; Morgan, T.M.; Palapattu, G.S.; Weizer, A.Z.; Caoili, E.M.; Ellis, J.H.; Kunju, L.P.; et al. Age, Gender and R.E.N.A.L. Nephrometry Score do not Improve the Accuracy of a Risk Stratification Algorithm Based on Biopsy and Mass Size for Assigning Surveillance versus Treatment of Renal Tumors. J. Urol. 2015, 195, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Kutikov, A.; Uzzo, R.G. The R.E.N.A.L. Nephrometry Score: A Comprehensive Standardized System for Quantitating Renal Tumor Size, Location and Depth. J. Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef]
- Basu, S.; Khan, I.A.; Das, R.K.; Khan, D.; Agarwal, V. RENAL nephrometry score: Predicting perioperative outcomes following open partial nephrectomy. Urol. Ann. 2019, 11, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Huang, W.C.; Skolnik, E.Y.; Gervais, D.A.; Braithwaite, R.S.; Pandharipande, P.V. Tumor anatomy scoring and renal function for nephron-sparing treatment selection in patients with small renal masses: A micro simulation-based decision analysis. AJR Am. J. Roentgenol. 2016, 207, 344–353. [Google Scholar] [CrossRef]
- Konstantinidis, C.; Trilla, E.; Serres-Créixams, X.; Montealegre, C.; Lorente, D.; Castellón, R.; Morote, J. Association among the R.E.N.A.L. nephrometry score and clinical outcomes in patients with small renal masses treated with percutaneous contrast enhanced ultrasound radiofrequency ablation. Cent. Eur. J. Urol. 2019, 72, 92–99. [Google Scholar] [CrossRef]
- Veccia, A.; Antonelli, A.; Uzzo, R.G.; Novara, G.; Kutikov, A.; Ficarra, V.; Simeone, C.; Mirone, V.; Hampton, L.J.; Derweesh, I.; et al. Predictive Value of Nephrometry Scores in Nephron-sparing Surgery: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2019, 6, 490–504. [Google Scholar] [CrossRef]
- Ficarra, V.; Crestani, A.; Inferrera, A.; Novara, G.; Rossanese, M.; Subba, E.; Giannarini, G. Positive Surgical Margins After Partial Nephrectomy: A Systematic Review and Meta-Analysis of Comparative Studies. Kidney Cancer 2018, 2, 133–145. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).