Estimating the Effect of Motivational Interventions in Patients with Eating Disorders: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search and Selection
2.3. Data Extraction
2.4. Quality and Bias Assessment
2.5. Data Analysis
3. Results
3.1. Study Selection
3.2. Bias Assessment
3.3. The Effect of Motivational Interventions
3.3.1. Study Characteristics
3.3.2. Outcomes
Motivation to Change
Eating Disorder Psychopathology
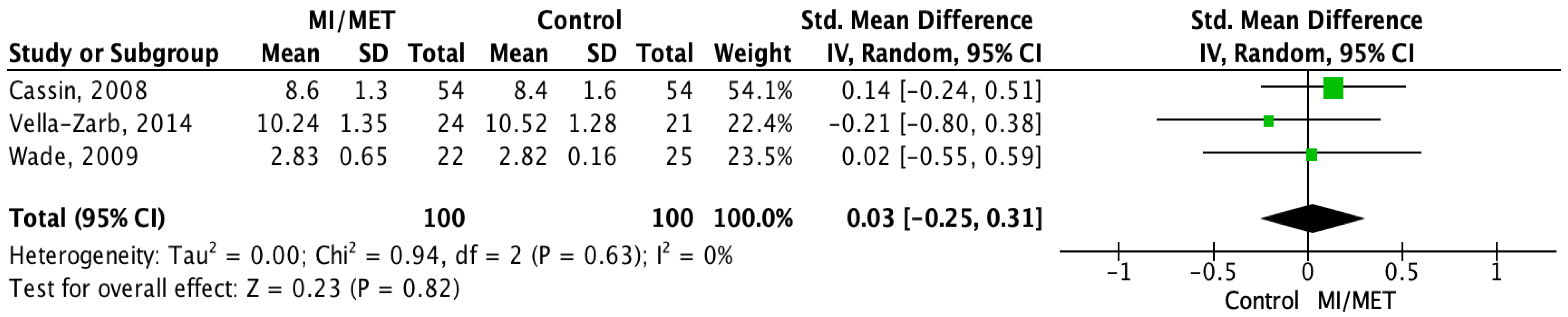
Body Mass Index
3.4. Data Analysis
3.4.1. Meta-Analysis
3.4.2. Meta-Regression
4. Discussion
4.1. The Effect of MET/MI on Motivation to Change
4.2. The Effect of MET/MI on Eating Disorder Psychopathology
4.3. The Effect of MET/MI on Body Mass Index
4.4. Strengths and Limitations
4.5. Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACMTQ | The Autonomous and Controlled Motivations for Treatment Questionnaire |
| AMI | Adapted Motivational Interviewing |
| AN | Anorexia Nervosa |
| AN-B/P | Anorexia Nervosa Bingeing/Purging |
| ANSOCQ | Anorexia Nervosa Stages of Change Questionnaire |
| BE | Binge Eating |
| BED | Binge Eating Disorder |
| BMI | Body Mass Index |
| BN | Bulimia Nervosa |
| CBT | Cognitive Behavioral Therapy |
| DH | Day Hospital |
| ED | Eating Disorder |
| EDDS | Eating Disorder Diagnostic Scale |
| EDE | Eating Disorder Examination |
| EDE-Q | Eating Disorder Examination Questionnaire |
| EDI-2 | Eating Disorders Inventory-2 |
| EDNOS | Eating Disorder Not Otherwise Specified |
| EDP | Eating Disorder Psychopathology |
| MANNA | Motivation-Enhancing Psychotherapy for Inpatients with Anorexia Nervosa |
| MANTRA | Maudsley Model of Anorexia Nervosa Treatment for Adults |
| MD | Mean Difference |
| MET | Motivational Enhancement Therapy |
| MI | Motivational Interviewing |
| Min | Minute |
| MO | Motivation |
| MTC | Motivation to Change Scale |
| OSFED | Other Specified Feeding and Eating Disorder |
| PCED | Pros and Cons of Eating Disorders Scale |
| PE | Psychoeducation |
| PSR | Psychiatric Status Rating |
| RC | Readiness to Change |
| RCT | Randomized Controlled Trial |
| RecoveryMANTRA | Recovery Maudsley Model of Anorexia Nervosa Treatment for Adults |
| RMI | Readiness and Motivation Interview |
| RMT | Readiness and Motivation Therapy |
| SEED | Short Evaluation of Eating Disorders |
| SD | Standard Deviation |
| SH | Self-Help |
| SMD | Standardized Mean Difference |
| SSCM | Specialist Supportive Clinical Management |
| TAU | Treatment-as-Usual |
| TTM | The Transtheoretical Model |
| UFED | Unspecified Feeding and Eating Disorder |
| URICA | The University of Rhode Island Change Assessment Scale |
| WEL | Weight Efficacy Lifestyle Questionnaire |
| WL | Waitlist |
Appendix A
| Study | Participant Characteristics: No. Patients/Drop-Out (No. Females/No. Men) Mean Age in Years +/− SD ED Diagnosis (ED Duration: Mean Years +/− SD) | Recruitment and Concurrent Treatment: Inpatient/Outpatient Status Type of ED Treatment | Study Design 1: Type of Intervention Received in Each Group | Intervention: No. Sessions (Length: min/Session) | Therapist: Background, Training and/or Supervision | Outcome Assessment: Assessment Time Points Instruments Used to Assess: MC and/or RC, EDP and/or BMI | Objectives | Results: Effect on MO/RC, EDP and/or BMI |
|---|---|---|---|---|---|---|---|---|
| Cardi et al. [40] | 187/NR (181/6) All: 27.81 +/− 9.30 AN (All: 7.76 +/− 8.91) | Participants were recruited from outpatient services. | RecoveryMANTRA + TAU vs. TAU | Online SH intervention with access to videoclips, a workbook, and six text-based chat sessions with a mentor (60 min/session). | Background: Postgraduate psychology students, carers, or people recovered from an ED. Training: Two 3-day training sessions in MI and RecoveryMANTRA. 2-day booster training sessions offered twice a year. Supervision: Session transcripts used in weekly supervisions performed by two clinical supervisors by mail or over the phone. | Assessments: Baseline, 6 weeks, 6 months, and 12 months. Tools: MO/RC: (1) ACMTQ (baseline and after 6 weeks) (2) 2 visual analogue scales EDP: EDE-Q BMI: Self-reported and clinical teams. | Primary outcome: Differences in BMI between groups. Secondary outcomes: 1) BMI at 6 and 12 months 2) ED symptoms (EDE-Q) 6 weeks, 6 months, and 12 months 3) Motivation to change | MO/RC: Intervention group had significantly higher levels of confidence in own ability to change compared to control group. EDP: No significant differences. BMI: No significant differences. |
| Cassin et al. [41] | 108/14 (108/0) All: 42.5 +/− 12.7 BED (15.1 +/− 11.6) | Recruited from the community (a large Canadian city) | AMI + SH handbook vs. SH handbook | 1× AMI (81.8 min) | Background: Two clinical psychology doctoral students. Training: Read about MI, observed training videotapes and engaged in role-play exercises. Supervision: Audiotaped sessions were rated by trained undergraduate research assistants. | Assessments: Baseline, post-intervention, 4 weeks, 8 weeks, and 16 weeks. Tools: MO/RC: Change ratings (not measured at baseline) EDP: (1) SCID-I (2) WEL (3) Timeline Follow-Back Interview BMI: Self-report at baseline. | BE frequency and size | MO/RC: AMI + SH significantly more confident in ability to change. EDP: AMI + SH significantly improved BE, and more participants no longer met DSM-IV frequency criteria for BED. BMI: Only baseline. |
| Dunn et al. [42] | 90/31 2 (79/11) All: 19 +/− 2.64 BN, BED (Full and subthreshold) (Duration: NR) | College students recruited through a screening. | MET + SH vs. SH | Intervention with MET (Total: 90 min, MET: 45 min). | Background: Undergraduate research assistants (psychology) and master’s-level graduate students (clinical psychology). Training: 2 × 4 h MI-workshops (lectures, demonstrations, videotapes, role playing, and trainee practice with feedback). The 1st author educated the students and assistants on ED and their treatment (3 h session). Supervision: 1 interview was performed with another therapist (received feedback) and then 1 with the 1st author. All sessions were audiotaped and rated by 1st author. | Assessments: Baseline, 2 months and 4 months. Tools: MO/RC: URICA (baseline + immediately post treatment) EDP: (1) EDE-Q (2) EDDS BMI: Not specified. | 1) Improvement in motivation (readiness to change) 2) Reduction in ED symptoms | MO/RC: MET significantly better at enhancing readiness to change in BE patients. EDP: Significantly more were abstinent from bingeing in MET. BMI: Only baseline. |
| Geller et al. [43] | 181/68 (NR) RMT: 28.12 +/− 6.42 Control: 28.68 +/− 8.6 AN-B/P, AN-R, BN, EDNOS (RMT: 9.86 +/− 6.75, control: 11.63 +/− 9.63) | Participants were recruited from a tertiary care Canadian eating disorder treatment program (outpatient). | RMT vs. WL | 1× RMT weekly for 5 weeks (60 min/session). | Background: Clinical psychologists, counseling psychologists and nurse clinicians. Training: 1st and 3rd authors trained the therapists. Study manual and audiotaped pilot sessions were available. Supervision: 5 RMT sessions with pilot participants under supervision. These sessions were recorded and reviewed. RMT sessions audiotaped and randomly reviewed in weekly meetings with study therapists. | Assessments: Baseline, 6 weeks, and 3 months. Tools: MO/RC: RMI (with EDE) EDP: EDI-2 BMI: Self-report at baseline. | Primary outcome: Readiness to change Secondary outcome: EDP | MO/RC: Significiantly fewer patients were ambivalent about change in RMT group. EDP: No significant differences. BMI: Only baseline. |
| Katzman et al. [46] | 225/124 3 (NR) All: 29.3 +/− 7.5 BN, EDNOS (Duration: NR) | Patients were recruited from primary or secondary care settings (outpatient). | (1) MET-I vs. (2) MET-G vs. (3) CBT-G | (1) 4× individual MET + 8× individual CBT (MET-I) vs. (2) 4× individual MET + 8×group CBT (MET-G) vs. (3) 4× individual CBT + 8×group CBT (CBT-G) (Individual sessions: 50 min, group sessions: 90 min) | Background: Psychologists, psychiatrists, nurses, or occupational therapists experienced in the delivering MET and CBT. Training: A 2-day MET-workshop, a training day on the use of the group program and a 2-day manual-guided CBT workshop Supervision: Weekly mandatory supervision. | Assessments: Baseline, 4 weeks, 12 weeks, 1-year, and 2.5-year. Tools: MO/RC: URICA (only baseline) EDP: (1) Standardized semistructured interview for suitability (2) SEED BMI: Not specified. | Primary outcome: Reduction in ED symptoms: BE, self-induced vomiting, laxative, and/or diuretic use. Additional outcome: Motivation to change | MO/RC: Only baseline. EDP: No significant differences.BMI: No significant differences. |
| MacDonald et al. [29] | 44/11 (44/0) All: 27.3 +/− 8.4 BN, PD (All: 9.9 +/− 7.6) | Participants were recruited from a waitlist before admittance at DH. | MI + TAU vs. CBT-RR + TAU vs. TAU | 4× weekly (60 min/session) | Background: Therapists (1st and 4th authors) were clinical psychology doctoral-level students. Training: Experienced in treating ED using both CBT and MI. Supervision: Therapists were trained and supervised by 2nd author (registered psychologist). | Assessments: Baseline and after DH, but self-reported items about ED behavior/symptoms were answered and collected weekly. Tools: MO/RC: Not assessed. EDP: (1) Total binge/purge frequency during first 4 weeks (2) Normalized eating during first 4 weeks (adherence to meal plan) (3) EDE (abbreviated version, diagnostic items only) (4) EDE-Q Weight and Shape Concerns (5) Binge/vomit/laxative frequency (6) Binge/vomit/laxative abstinence (7) Participants also self-reported eating disorder symptoms/behavior. BMI: Clinical charts. | (1) RR (2) BE/vomit/laxative frequency (3) Overvaluation of weight and shape | MO/RC: Not measured. EDP: CBT-group patients had significantly fewer bingeing and purging episodes and improved on EDE-Q Weight and Shape compared to MI-group. BMI: Only baseline. |
| Schmidt et al., 2012 [38] | 72/NR (67/5) All: 26.6 +/− 7.9 AN-R, AN-BP, EDNOS-R, EDNOS-BP (6.72 +/− 5.98) | Eating disorders outpatient service of the South London and Maudsley NHS Foundation Trust. | MANTRA vs. SSCM | 1× weekly for 20 weeks + 1× monthly for 4 months. Participants were offered 2 more sessions (24–26 sessions). Low-weight (BMI ≤ 15 kg/m2) patients could receive 1 session weekly for 30 weeks and 4 follow-ups, participants were offered 2 more sessions (34–36 sessions). (Length: NR) | Background: Experienced ED therapists performed 1 of 2 treatments (MANTRA or SSCM). Training: Workshops. Supervision: Weekly supervision. Interventions were audiotaped. | Assessments: Baseline, 6 months, and 12 months. Tools: MO/RC: Not assessed. EDP: EDE BMI: Height (measured at initial assessment) and weight (measured at each assessment). | Primary outcomes (at 6 and 12 months): (1) BMI (2) Weight (3) Global score in EDE | MO/RC: Not assessed. EDP: No significant differences. BMI: No significant differences. |
| Schmidt et al., 2015 [37] | 142/31 (139/3) All: 26.7 +/− 7.7 AN-R, AN-BP, EDNOS (8.3 +/− 7.3) | Recruitment from 4 ED services in the United Kingdom (outpatient) | MANTRA vs. SSCM | 1× weekly for 20 weeks + 1× monthly for 4 months. Participants were offered 2 more sessions (24–26 sessions, 50 min/session, but SSCM sessions could be reduced to 30 min). Patients with BMI ≤ 15 kg/m2 received additional 10 sessions (34–36 sessions). | Background: Experienced therapists in ED performed both types of interventions. Training: 2 days of training in both interventions. Supervision: Weekly supervision. Interventions were audiotaped. | Assessments: Baseline, 6 months, and 12 months. Tools: MO/RC: Not assessed. EDP: (1) EDE (2) EDE-Q BMI: Not specified. | Primary outcome: BMI at 12 months Secondary outcome EDP | MO/RC: Not assessed. EDP: No significant differences. BMI: No significant differences. |
| Treasure et al. [16] | 125/38 (125/0) All: 28.5 +/− 7.2 BN (MET: 10.8 +/− 8.4, CBT: 11.4 +/− 6.4) | Inpatient and outpatient treatment at the ED Unit of the Bethlem and Maudsley Hospital. | (1) MET + group CBT vs. (2) MET + individual CBT vs. (3) Individual CBT + group CBT | 12 weeks in total in all intervention groups: (1) 4× MET for 4 weeks + 8× group CBT for 8 weeks (2) 4× MET for 4 weeks + 8× individual CBT for 8 weeks (3) 4× individual CBT for 4 weeks + 8× group CBT for 8 weeks (Length: NR) | Background: Therapists trained in conducting both MET and CBT therapy. Training: MET training by an expert. Supervision: By experienced clinicians. | Assessments: Baseline and 4 weeks. Tools: MO/RC: URICA (at week 1 and week 4) EDP: Unnamed scale used by European-wide COST Action B6 Project (Frequency of BE, vomiting, and laxative abuse) BMI: Not specified. | (1) Change in symptoms (2) Effect of stage of change on improvement (3) Change in stages | MO/RC: No significant differences. EDP: No significant differences. BMI: Only baseline. |
| Vella-Zarb et al. [47] | 47/12 (45/2 4) All: 24.88 +/− 6.91 BED (full and subthreshold), BN (non-Purging Subtype) (Duration: NR) | Recruitment from York University and the community. | MI + SH manual vs. PE + SH manual | 1 session (60 min) | Background: Only the 1st author (senior-level PhD clinical psychology student) performed the intervention. Training: Trained in MI and attended MI workshops. Supervision: Audiotaped sessions. Random sessions were monitored by two psychologists. | Assessments: Baseline, 1 month, and 4 months. Tools: MO/RC: URICA (Baseline and immediately after intervention) EDP: (1) EDE-Q (2) WEL (Baseline and immediately after intervention) BMI: Not specified. | Primary Outcomes: (1) ED behaviors and attitudes (2) BE abstinence Secondary Outcomes: (1) Readiness to change (2) Eating self-efficacy | MO/RC: MI significantly more effective at increasing RC and eating self-efficacy in individuals who binge eat than PE. EDP: No significant differences. BMI: Only baseline. |
| Wade et al. [28] | 47/8 (45/2) All: 21.85 +/− 5.37 AN (full and subthreshold) (MI: 6.93 +/− 7.22, TAU: 3.16 +/− 3.41) | Inpatients at a weight disorder unit in Adelaide. | MI + TAU vs. TAU | Up to 4 sessions over 2 weeks (60 min/session). | Background: Novice therapists. Training: NR. Supervision: Sessions were recorded. Evaluated by a clinical psychologist. | Assessments: Baseline, 2 week, and 6 week. Tools: MO/RC: (1) ANSOCQ (2) 6 self-report questions EDP: EDE BMI: From “case notes”. | (1) Changes in eating disorder psychopathology (2) Changes in motivation | MO/RC: No significant differences. EDP: No significant differences. BMI: No significant differences. |
| Weiss et al. [44] | 32/7 (30/2) All: 28 +/− 8.8 AN, BN, EDNOS (0.7 years +/− 8.9) | WL patients at inpatient and DH (outpatient) units at Toronto General Hospital. | MI + TAU +WL for intensive care vs. TAU + WL for intensive care | 4× MI weekly for 4 weeks (50 min/session). | Background: Therapy performed by 1st author. Training: 2-day workshop on MI. Supervision: Experienced MI and ED therapists using live observations and videotape reviews. 20% of MI sessions were assessed by independent raters. | Assessments: Baseline and 4 weeks. Tools: MO/RC: MTC EDP: PCED BMI: Not specified. | (1) Changes in PCED (2) Changes in MTC | MO/RC: No significant differences. EDP: No significant differences. BMI: Only baseline. |
| Ziser et al. [45] | 22/9 5 (22/0) All: 31.9 +/− 12.6 AN (Intervention: 10.9 +/− 8.6, control: 7.2 +/− 5.9) | Inpatient treatment at two university hospitals. | MANNA vs. TAU | 1× MANNA weekly for 10 weeks (Length of session: NR) | Background: NR. Training: Two experts trained the therapists who performed the MI sessions. Supervision: NR. | Assessments: 1 week, 5 weeks, and 10 weeks. Tools: MO/RC: URICA-S EDP: (1) EDE-Q (2) PSR BMI: Height and weight registered in the patient’s file during treatment at hospital. | (1) Motivation to change (2) Weight gain (3) EDP | MO/RC: No significant differences. EDP: No significant differences. BMI: No significant differences. |
| Article | BMIControl | BMIIntervention | EDPControl | EDPIntervention |
|---|---|---|---|---|
| Sample size (MacDonald et al.) | 23 | 21 | 23 | 21 |
| Pearson’s r (MacDonald et al.) | 0.997 | 0.988 | 0.28 (EDE-Q) | 0.24 (EDE-Q) |
| Fisher’s Z (MacDonald et al.) | 3.250 | 2.555 | 0.288 | 0.245 |
| Sample size (Wade et al.) | 25 | 22 | 25 | 22 |
| Pearson’s r (Wade et al.) | 0.75 | 0.42 | 0.77 (EDE) | 0.72 (EDE) |
| Fisher’s Z (Wade et al.) | 3.250 | 2.555 | 0.288 | 0.245 |
| Average Fisher’s Z | 2.064 | 1.477 | 0.669 | 0.583 |
| Average Pearson’s r | 0.968 | 0.901 | 0.584 | 0.526 |
References
- Volpe, U.; Tortorella, A.; Manchia, M.; Monteleone, A.M.; Albert, U.; Monteleone, P. Eating disorders: What age at onset? Psychiatry Res. 2016, 238, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Keski-Rahkonen, A.; Mustelin, L. Epidemiology of eating disorders in Europe: Prevalence, incidence, comorbidity, course, consequences, and risk factors. Curr. Opin. Psychiatry 2016, 29, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Steinhausen, H.C. The outcome of anorexia nervosa in the 20th century. Am. J. Psychiatry 2002, 159, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Steinhausen, H.C.; Weber, S. The outcome of bulimia nervosa: Findings from one-quarter century of research. Am. J. Psychiatry 2009, 166, 1331–1341. [Google Scholar] [CrossRef]
- Arcelus, J.; Mitchell, A.J.; Wales, J.; Nielsen, S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch. Gen. Psychiatry 2011, 68, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Iwajomo, T.; Bondy, S.J.; de Oliveira, C.; Colton, P.; Trottier, K.; Kurdyak, P. Excess mortality associated with eating disorders: Population-based cohort study. Br. J. Psychiatry 2020, 219, 487–493. [Google Scholar] [CrossRef]
- Harris, E.C.; Barraclough, B. Excess mortality of mental disorder. Br. J. Psychiatry 1998, 173, 11–53. [Google Scholar] [CrossRef]
- Weissman, R.S.; Rosselli, F. Reducing the burden of suffering from eating disorders: Unmet treatment needs, cost of illness, and the quest for cost-effectiveness. Behav. Res. Ther. 2017, 88, 49–64. [Google Scholar] [CrossRef]
- Price-Evans, K.; Treasure, J. The Use of Motivational Interviewing in Anorexia Nervosa. Child Adolesc. Ment. Health 2011, 16, 65–70. [Google Scholar] [CrossRef]
- Vitousek, K.; Watson, S.; Wilson, G.T. Enhancing motivation for change in treatment-resistant eating disorders. Clin. Psychol. Rev. 1998, 18, 391–420. [Google Scholar] [CrossRef]
- Sjogren, J.M. On motivation as a Target for Intervention in Anorexia Nervosa. Iris Publ. 2019, 5, 1–4. [Google Scholar] [CrossRef]
- Reid, M.; Burr, J.; Williams, S.; Hammersley, R. Eating disorders patients’ views on their disorders and on an outpatient service: A qualitative study. J. Health Psychol. 2008, 13, 956–960. [Google Scholar] [CrossRef] [Green Version]
- DiClemente, C.C.; Prochaska, J.O.; Fairhurst, S.K.; Velicer, W.F.; Velasquez, M.M.; Rossi, J.S. The process of smoking cessation: An analysis of precontemplation, contemplation, and preparation stages of change. J. Consult. Clin. Psychol. 1991, 59, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Zaitsoff, S.L.; Taylor, A. Factors related to motivation for change in adolescents with eating disorders. Eur. Eat. Disord. Rev. 2009, 17, 227–233. [Google Scholar] [CrossRef]
- Clausen, L.; Lubeck, M.; Jones, A. Motivation to change in the eating disorders: A systematic review. Int. J. Eat. Disord. 2013, 46, 755–763. [Google Scholar] [CrossRef]
- Treasure, J.L.; Katzman, M.; Schmidt, U.; Troop, N.; Todd, G.; de Silva, P. Engagement and outcome in the treatment of bulimia nervosa: First phase of a sequential design comparing motivation enhancement therapy and cognitive behavioural therapy. Behav. Res. Ther. 1999, 37, 405–418. [Google Scholar] [CrossRef]
- Sansfacon, J.; Booij, L.; Gauvin, L.; Fletcher, E.; Islam, F.; Israel, M.; Steiger, H. Pretreatment motivation and therapy outcomes in eating disorders: A systematic review and meta-analysis. Int. J. Eat. Disord. 2020, 53, 1879–1900. [Google Scholar] [CrossRef]
- Prochaska, J.O.; Velicer, W.F. The transtheoretical model of health behavior change. Am. J. Health Promot. 1997, 12, 38–48. [Google Scholar] [CrossRef]
- Miller, W.R.; Rollnick, S. Ten things that motivational interviewing is not. Behav. Cogn. Psychother. 2009, 37, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Lundahl, B.; Burke, B.L. The effectiveness and applicability of motivational interviewing: A practice-friendly review of four meta-analyses. J. Clin. Psychol. 2009, 65, 1232–1245. [Google Scholar] [CrossRef]
- Sjogren, J.M. Anorexia Nervosa and Motivation for Behavioral Change—Can it be Enhanced? J. Psychol. Clin. Psychiatry 2017, 8, 00489. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research; World Health Organization: Genève, Switzerland, 1993.
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2021; Chapter 8. [Google Scholar]
- Higgins, J.P.; Li, T.; Deeks, J.J. Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2021; Chapter 6. [Google Scholar]
- Wade, T.D.; Frayne, A.; Edwards, S.A.; Robertson, T.; Gilchrist, P. Motivational change in an inpatient anorexia nervosa population and implications for treatment. Aust. N. Z. J. Psychiatry 2009, 43, 235–243. [Google Scholar] [CrossRef]
- MacDonald, D.E.; McFarlane, T.L.; Dionne, M.M.; David, L.; Olmsted, M.P. Rapid response to intensive treatment for bulimia nervosa and purging disorder: A randomized controlled trial of a CBT intervention to facilitate early behavior change. J. Consult. Clin. Psychol. 2017, 85, 896–908. [Google Scholar] [CrossRef]
- Sedgwick, P.; Marston, L. Meta-analyses: Standardised mean differences. BMJ 2013, 347, f7257. [Google Scholar] [CrossRef] [Green Version]
- Andrade, C. Mean Difference, Standardized Mean Difference (SMD), and Their Use in Meta-Analysis: As Simple as It Gets. J. Clin. Psychiatry 2020, 81, 20f13681. [Google Scholar] [CrossRef]
- Del Re, A.C. A Practical Tutorial on Conducting Meta-Analysis in R. Quant. Methods Psychol. 2015, 11, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-on Guide; Chapmann & Hall/CRC Press: Boca Raton, FL, USA; London, UK, 2021. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U.; Magill, N.; Renwick, B.; Keyes, A.; Kenyon, M.; Dejong, H.; Lose, A.; Broadbent, H.; Loomes, R.; Yasin, H.; et al. The Maudsley Outpatient Study of Treatments for Anorexia Nervosa and Related Conditions (MOSAIC): Comparison of the Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA) with specialist supportive clinical management (SSCM) in outpatients with broadly defined anorexia nervosa: A randomized controlled trial. J. Consult. Clin. Psychol. 2015, 83, 796–807. [Google Scholar] [CrossRef]
- Schmidt, U.; Oldershaw, A.; Jichi, F.; Sternheim, L.; Startup, H.; McIntosh, V.; Jordan, J.; Tchanturia, K.; Wolff, G.; Rooney, M.; et al. Out-patient psychological therapies for adults with anorexia nervosa: Randomised controlled trial. Br. J. Psychiatry 2012, 201, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, U.; Renwick, B.; Lose, A.; Kenyon, M.; Dejong, H.; Broadbent, H.; Loomes, R.; Watson, C.; Ghelani, S.; Serpell, L.; et al. The MOSAIC study—Comparison of the Maudsley Model of Treatment for Adults with Anorexia Nervosa (MANTRA) with Specialist Supportive Clinical Management (SSCM) in outpatients with anorexia nervosa or eating disorder not otherwise specified, anorexia nervosa type: Study protocol for a randomized controlled trial. Trials 2013, 14, 160. [Google Scholar] [CrossRef] [Green Version]
- Cardi, V.; Albano, G.; Ambwani, S.; Cao, L.; Crosby, R.D.; Macdonald, P.; Schmidt, U.; Treasure, J. A randomised clinical trial to evaluate the acceptability and efficacy of an early phase, online, guided augmentation of outpatient care for adults with anorexia nervosa. Psychol. Med. 2019, 50, 2610–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassin, S.E.; von Ranson, K.M.; Heng, K.; Brar, J.; Wojtowicz, A.E. Adapted motivational interviewing for women with binge eating disorder: A randomized controlled trial. Psychol. Addict. Behav. 2008, 22, 417–425. [Google Scholar] [CrossRef]
- Dunn, E.C.; Neighbors, C.; Larimer, M.E. Motivational enhancement therapy and self-help treatment for binge eaters. Psychol. Addict. Behav. 2006, 20, 44–52. [Google Scholar] [CrossRef]
- Geller, J.; Brown, K.E.; Srikameswaran, S. The efficacy of a brief motivational intervention for individuals with eating disorders: A randomized control trial. Int. J. Eat. Disord. 2010, 44, 497–505. [Google Scholar] [CrossRef]
- Weiss, C.V.; Mills, J.S.; Westra, H.A.; Carter, J.C. A preliminary study of motivational interviewing as a prelude to intensive treatment for an eating disorder. J. Eat. Disord. 2013, 1, 34. [Google Scholar] [CrossRef] [Green Version]
- Ziser, K.; Rheindorf, N.; Keifenheim, K.; Becker, S.; Resmark, G.; Giel, K.E.; Skoda, E.M.; Teufel, M.; Zipfel, S.; Junne, F. Motivation-Enhancing Psychotherapy for Inpatients With Anorexia Nervosa (MANNA): A Randomized Controlled Pilot Study. Front. Psychiatry 2021, 12, 632–660. [Google Scholar] [CrossRef]
- Katzman, M.A.; Bara-Carril, N.; Rabe-Hesketh, S.; Schmidt, U.; Troop, N.; Treasure, J. A randomized controlled two-stage trial in the treatment of bulimia nervosa, comparing CBT versus motivational enhancement in Phase 1 followed by group versus individual CBT in Phase 2. Psychosom. Med. 2010, 72, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Vella-Zarb, R.A.; Mills, J.S.; Westra, H.A.; Carter, J.C.; Keating, L. A Randomized controlled trial of motivational interviewing + self-help versus psychoeducation + self-help for binge eating. Int. J. Eat. Disord. 2015, 48, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Knowles, L.; Anokhina, A.; Serpell, L. Motivational interventions in the eating disorders: What is the evidence? Int. J. Eat. Disord. 2013, 46, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, P.; Hibbs, R.; Corfield, F.; Treasure, J. The use of motivational interviewing in eating disorders: A systematic review. Psychiatry Res. 2012, 200, 1–11. [Google Scholar] [CrossRef] [PubMed]





| Article | BMIControl | BMIIntervention | EDPControl | EDPIntervention |
|---|---|---|---|---|
| Pearson’s r (MacDonald et al.) | 0.997 | 0.988 | 0.28 (EDE-Q) | 0.24 (EDE-Q) |
| Pearson’s r (Wade et al.) | 0.75 | 0.42 | 0.77 (EDE) | 0.72 (EDE) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fetahi, E.; Søgaard, A.S.; Sjögren, M. Estimating the Effect of Motivational Interventions in Patients with Eating Disorders: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 577. https://doi.org/10.3390/jpm12040577
Fetahi E, Søgaard AS, Sjögren M. Estimating the Effect of Motivational Interventions in Patients with Eating Disorders: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2022; 12(4):577. https://doi.org/10.3390/jpm12040577
Chicago/Turabian StyleFetahi, Egzona, Anders Stjerne Søgaard, and Magnus Sjögren. 2022. "Estimating the Effect of Motivational Interventions in Patients with Eating Disorders: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 12, no. 4: 577. https://doi.org/10.3390/jpm12040577
APA StyleFetahi, E., Søgaard, A. S., & Sjögren, M. (2022). Estimating the Effect of Motivational Interventions in Patients with Eating Disorders: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 12(4), 577. https://doi.org/10.3390/jpm12040577







