Abstract
Huntington’s Disease (HD) is a rare, neurodegenerative disorder characterized by chorea, cognitive decline, and behavioral changes. Despite wide clinical use since the mid-1980s, tiapride was recently withdrawn from the Dutch market without rationale. Although alternatives are available, many patients experienced dysregulation after this unwanted change. We provide insight into the impact of sudden tiapride withdrawal by reviewing medical records of HD patients who were using tiapride at the time of withdrawal. In addition, we performed a systematic search in five databases on tiapride efficacy and its safety profile in HD. Original research and expert opinions were included. In our patient group on tiapride, 50% required tiapride import from abroad. Regarding the review, 12 articles on original datasets and three expert opinions were included. The majority of studies showed an improvement in chorea while patients were on tiapride. Due to limited sample sizes, not all studies performed statistical tests on their results. Fifty percent of clinical experts prefer tiapride as initial chorea monotherapy, especially when comorbid behavioral symptoms are present. Side effects are often rare and mild. No safety concerns were reported. In conclusion, tiapride is almost irreplaceable for some patients and is an effective and safe chorea treatment in HD.
1. Introduction
Huntington’s Disease (HD) is an autosomal, dominantly inherited neurodegenerative disorder caused by an expansion of the CAG repeat in the huntingtin (HTT) gene [1,2]. The disease generally manifests during mid-life and is clinically characterized by involuntary movements (chorea), psychiatric and behavioral symptoms, and cognitive decline [3,4,5]. To this date, no cure or disease-modifying therapy exists [6,7]. However, a wide variety of pharmacological and non-pharmacological therapies are used to improve quality of life [7,8,9,10,11,12].
Traditionally, dopamine-blocking agents have been used to treat chorea [13,14]. Haloperidol, developed in the mid-20th century, was one of the first treatments [15,16,17]. In the mid-1980s, tiapride became available [18,19]. Tiapride was originally developed to counteract abnormal involuntary movements in various disorders [20,21,22]. Tiapride is marketed under various brand names, such as tiapridal, tiaprida, hipokin, sereprile, and tiaprid, and is highly affordable at approximately €0.55/day for 200 mg [23,24]. As an antipsychotic and benzamide derivate, tiapride acts by selectively blocking dopamine D2- and D3-receptors [25,26]. Blocking these dopamine receptors improves the regulation of behavioral, sleep, and motor function disturbances [26,27]. Therefore, tiapride is appealing to prescribe in clinically heterogeneous movement disorders such as HD and has been widely prescribed by European HD experts over the last 40 years [28,29,30].
In March 2020, Sanofi S.A. withdraw tiapride from the Dutch market abruptly [31]. This was the only company that supplied this medicine, which was covered by health insurance, in the Netherlands [31,32]. No major safety concerns were reported, whereas many HD patients reported benefits from tiapride [28,33]. The unexpected and sudden shortage of tiapride caused much distress among patients and their caregivers, as illustrated by three representative cases below [34]. Pharmacological alternatives, which had to be introduced quickly, were not always suitable and led to disease aggravation or (severe) side effects in several patients. Therefore, we reviewed the records of all our HD patients who were using tiapride at the time of withdrawal and made a total overview of the subsequent adjustments and the success rate of substitute medicines given. In addition, a systematic literature search was performed to review the effectiveness and safety profile of tiapride in HD. Side effects and potential adverse drug effects are summarized.
2. Case Descriptions
A 60-year-old man was clinically and genetically diagnosed with HD in 2013. His chorea was well controlled with tiapride 100 mg three times a day without any side effects. After market withdrawal, tiapride was tapered down to 50 mg per week and tetrabenazine was started. After three days of tetrabenazine 12.5 mg, the patient contacted the hospital because of severe nausea and dizziness, requiring domperidone. Tetrabenazine was stopped and tiapride reintroduced in increasing doses up to 300 mg per day again and imported at the patient’s own expense. No other explanation for the nausea, such as food poisoning or infection, was found. The nausea disappeared within a few days and after two weeks the patient felt like his normal self again.
A 66-year-old female HD patient with coexisting sleep problems and urge incontinence had been taking tiapride since 2016. Her chorea was well controlled with 150 mg daily without side effects. After market withdrawal, tiapride was tapered down and her chorea increased enormously. The patient’s UHDRS-TMS (Unified Huntington’s Disease Rating Scale—Total Motor Score, with a range from zero to 124 [35]) increased in one year from 37 to 82. This was mostly due to chorea and dystonia, with a 24- and 12-point increase, respectively. Initiation of quetiapine 25 mg daily did not affect chorea but led to nausea and frequent vomiting as side effects. As a second alternative tetrabenazine was started up to three times a day 25 mg. Her chorea improved, but the nausea persisted, and her urge incontinence worsened. Switching to another urinary spasmolytic decreased the urinary frequency. Nausea was partly reduced by dietary measures. A year after tetrabenazine initiation, the patient’s UHDRS-TMS had stabilized at 45 points. In consultation with the patient and her family, it was decided to continue tetrabenazine 25 mg three times daily, although side effects (mostly nausea) persisted.
A 60-year-old man with clinically and genetically diagnosed HD had an extensive cardiovascular medical history and diabetes mellitus type 2 with renal failure that left him dependent on hemodialysis. For HD symptoms, he used mirtazapine 45 mg and tiapride 100 mg twice daily. Since tiapride was no longer available, tetrabenazine was started at 25 mg three times a day. Although no side effects occurred, tetrabenazine was not effective enough and his increased choreatic movements hindered hemodialysis substantially. Hemodialysis was aborted on several occasions and additional haloperidol had to be administered intravenously. The patient then switched back to low doses of tiapride, with good antichoreatic control and no further need for haloperidol.
3. Materials and Methods
Leiden University Medical Center is a large HD expertise center in the Netherlands with more than 500 patient visits per year. Medical records of all Huntington’s Disease patients who were using tiapride at the time of withdrawal, in March 2020, were reviewed. To include all patients, all medical records were searched for tiapride use between 1 January and 31 December 2020. If no hospital tiapride prescription was present during the period of interest, the national healthcare network (Dutch: Landelijk Schakelpunt) for medication prescriptions was consulted. We analyzed what happened after tiapride market withdrawal for each patient. Possible options included tapering down of tiapride, starting a pharmacological alternative within 3 months, and/or tiapride import from abroad. The latter required patients to pay in advance (±€200/year), without certainty of insurance reimbursement.
The databases PubMed, Web of Science, PsychInfo, Embase, and the Cochrane Library were all searched from their respective inception up to 17 November 2021 using a systematic search. This search focused on tiapride and Huntington’s Disease. Synonyms tiapridal and tiaprid were included. Included languages were English, Dutch, German, and French. Original studies, in which effects (either positive or negative) of tiapride in genetically proven (CAG-repeat length ≥36) HD patients were described, and articles (including expert opinions) that described pharmacological alternatives to tiapride in HD were included. Case reports, book chapters, research protocols, and Huntington’s Disease-like syndromes were excluded. The complete search strategy can be found in the Supplementary Materials. All records were screened independently by two authors, Stephanie Feleus and Malu van Schaijk, for English and Dutch articles, or Stephanie Feleus and Raymund A.C. Roos for German and French articles. In case of disagreement on whether to include a record in the next screening phase or not, records were given the benefit of the doubt and stayed in screening. Selected results were screened in full-text and references of included articles were cross-checked for additional records. If a relevant new record was identified based on title, it would enter the phase of title-abstract screening in the selection process.
The tiapride package leaflet and the World Health Organization (WHO) VigiAccess database were consulted for definite and potential side effects of tiapride on 17 November 2021, respectively [33,36]. VigiAccess is a web-based platform that searches VigiBase, the WHO global database of individual case safety reports. VigiAccess data come from a variety of sources, including but not limited to patients, medical practitioners, pharmaceutical companies, and worldwide national regulatory authorities [37]. Drug relatability is variably assessed by data-providing countries, which means that causality cannot be inferred. Since only records that are documented are included and the total number of patients on tiapride is unknown, incidence cannot be assessed, either. To provide an overview of rarely reported potential side effects, adverse drug reactions that were reported 25 times or more (cut-off arbitrarily chosen) in VigiAccess and are not otherwise listed in the patient leaflet are described in this review.
4. Results
4.1. Evaluation of Medical Records
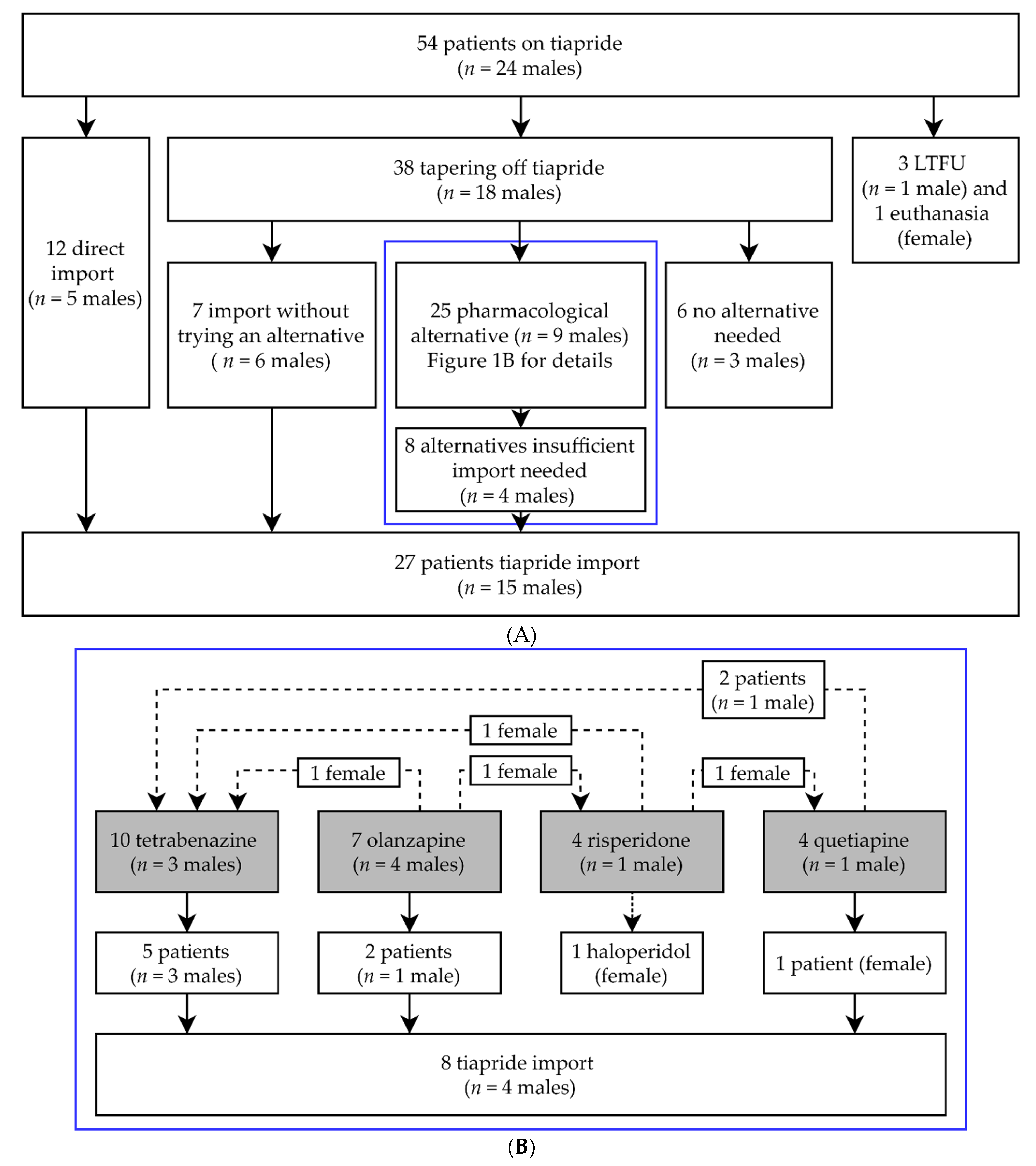
Of all our HD patients, 54 patients (mean age 58.0 years (range 23–86 years), n = 24 males) used tiapride at the time of withdrawal in March 2020. Without first tapering off and trying an alternative, 12 patients (mean age 54.6 years) directly chose tiapride import from abroad at their own expense. For the majority of patients, tiapride was first tapered off (n = 38, mean age 59.6 years). Four patients were either lost to follow-up or passed away (mean age 59.8 years). Details are shown in Figure 1A. No pharmacological alternative was needed for 6 patients (mean age 59.7 years). Most of them were using a low dose (≤100 mg daily), which could explain why no alternative was needed. During tapering off or directly after, seven patients (mean age 56.0 years) required immediate tiapride import (Figure 1A). Twenty-five patients (mean age 59.6 years) were prescribed alternative medications. The alternatives that were used most often were tetrabenazine and olanzapine. Less regularly chosen were quetiapine and risperidone. Several patients switched between alternatives. From those prescribed an alternative, a minority of patients (40%) continued with their first alternative (data not shown). Ultimately, 17 of the 25 patients (68%) stayed on their final pharmacological alternative. A graphical overview is shown in Figure 1B. Finally, for 27 patients (50% of the total group) tiapride needed to be imported. The average age of these 27 patients (n = 15 males) was 55.6 years.
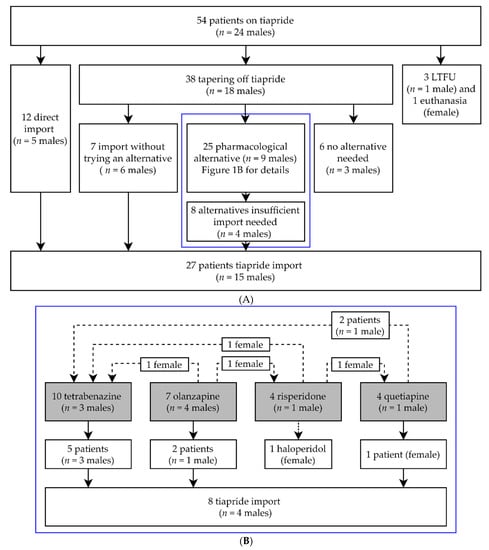
Figure 1.
(A) General overview of clinical follow-up and tiapride import. In the blue box are those patients that were prescribed a pharmacological alternative. (B) describes these pharmacological alternatives in more detail; n = number of; LTFU = Lost to follow-up. (B) Enlargement of blue box (A). Details of pharmacological alternatives used. Gray boxes are the original pharmacological alternatives prescribed. Dotted lines are patients who switched between pharmacological alternatives; n = number of.
4.2. Systematic Literature Review on Tiapride in Huntington’s Disease
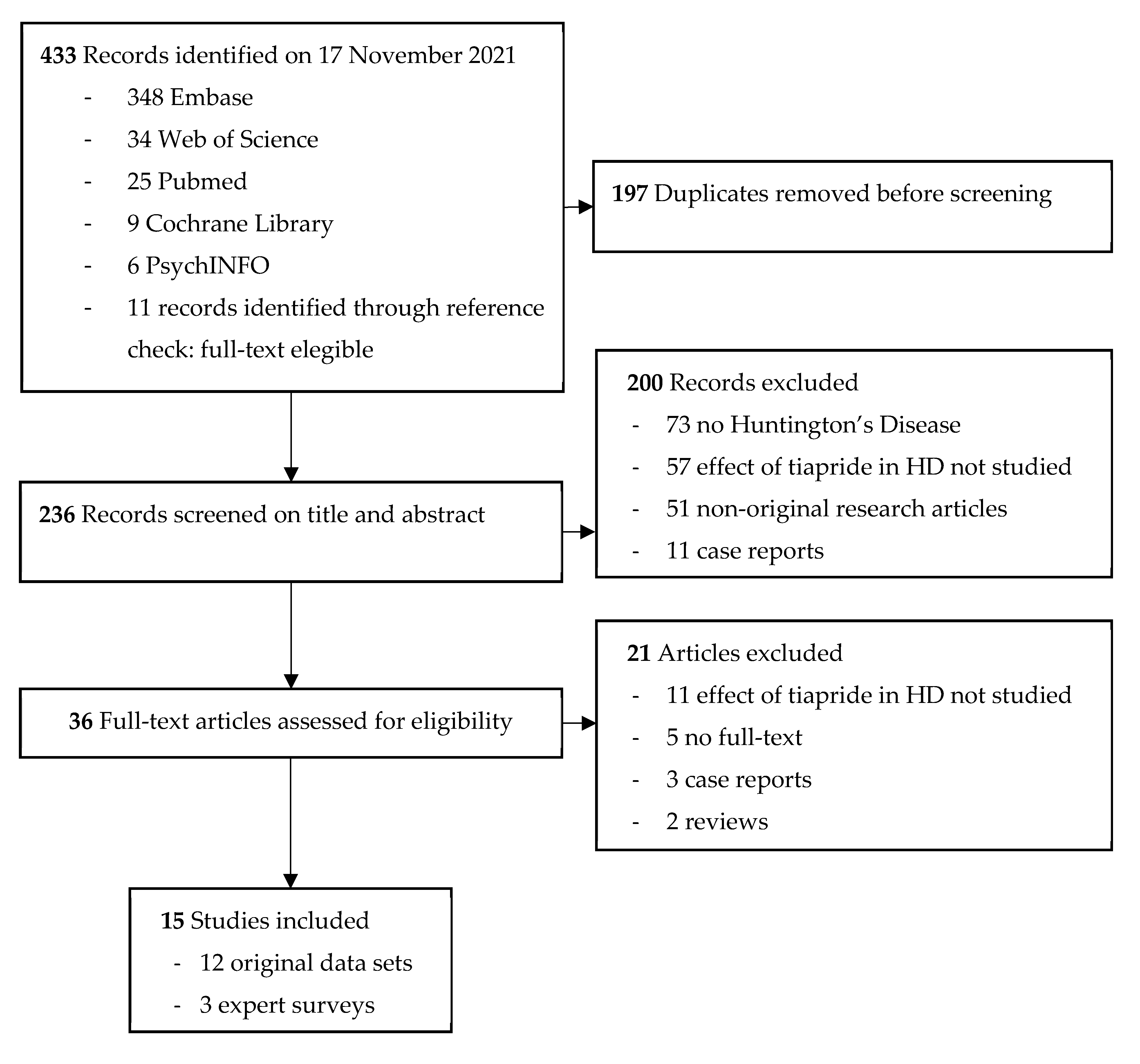
The literature search performed yielded 433 results. After removing duplicates, 236 studies were screened for title and abstract, and 36 studies remained for full-text screening. We were unable to retrieve the full text of five articles [38,39,40,41,42]. When screening the full texts, another 16 articles did not meet the inclusion criteria. Cohen’s kappa (κ) for inter-rater variability between SF, MS, and RACR combined is 0.72, which indicates a substantial interrater agreement. Ultimately, 15 studies were included, of which 12 were original articles and three were expert surveys on prescription preferences. An overview of the selection process with reasons for exclusion is shown in Figure 2.
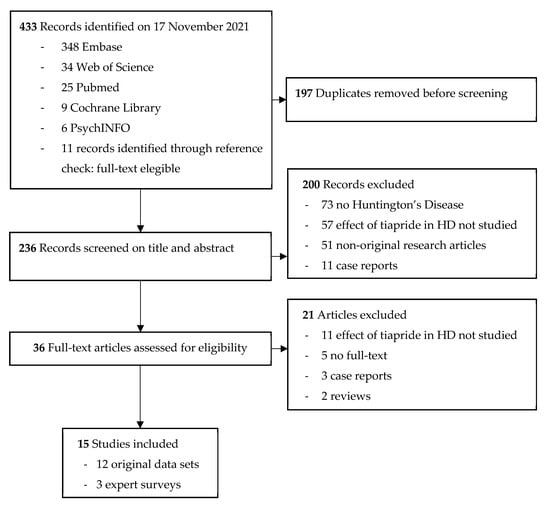
Figure 2.
Flow diagram of selected studies.
Only a small number of studies have explored the treatment potential of tiapride in HD. A detailed overview of these studies is given in Table 1. The majority, nine out of 12, were experimental studies with a time frame ranging from several weeks to one year [18,19,43,44,45,46,47,48,49]. Dosing of tiapride varied greatly between studies, ranging from 300 to 3000 mg/day [18,19,43,44,45,47,48,49]. Many studies used an effect-guided titration scheme for tiapride with an equilibrium between 300 and 900 mg/day [18,43,44,45,47,48]. The largest, although still small with n = 23, experimental, placebo-controlled trial was performed by Deroover et al. [19]. In patients with early to moderate HD, they found a significant chorea improvement (p < 0.05) with tiapride 3000 mg/day for 6 weeks. These results are strengthened by several other studies, which already showed a positive effect on chorea control with lower dosages ranging from 300 to 1200 mg/day [18,43,45,46,47,48]. The second-largest experimental study by Roos et al. was also a placebo-controlled, double-blind, cross-over trial with 22 HD patients on tiapride 300 mg/day for two weeks [49]. With a relatively short follow-up period, merely a trend towards significance (p = 0.10) was found. On the contrary, Girotti et al., who prescribed tiapride 600–800 mg/day in 12 patients for 4 weeks, found no significant chorea improvement relative to pimozide and haloperidol, which gave more side effects [44]. One of the more recent observational studies by Cichecki et al. performed an uncorrected analysis on 20 HD patients with varying although stable tiapride dosages [50]. No significant difference in UHDRS-TMS between baseline and one-year follow-up was found. Only a minority of studies reported the number of males/females at baseline [19,44,47,49,51]. Two studies analyzed potential sex differences in tiapride response. Petit et al. was the only study that described that females might have a reduced tiapride response [47]. Roos et al. could not reproduce these results [49]. In summary, most studies, seven out of 11 that focused on abnormal movements, concluded that tiapride has a positive effect on chorea. None of the studies reported a significant worsening of chorea with tiapride.

Table 1.
Studies on tiapride in Huntington’s Disease.
Although most research has been focused on chorea control, two observational studies described the effect of tiapride on body weight [51,52]. Konvalinkova et al. found no significant weight change, neither weight gain nor weight loss, in 49 patients on tiapride compared to 260 controls [52]. Desamericq et al. reported less weight gain (p < 0.05) with tiapride compared to known weight increasers olanzapine and risperidone in a similar-sized population [51]. Based on these two studies, tiapride appears not to affect body weight [51,52]. As a comparative study, Desamericq et al. commented on functionality changes in different drug groups [51]. Functionality, measured by the Unified Huntington’s Disease Rating Scale—Total Functional Capacity (UHDRS-TFC), describes one’s capacity to live independently and perform activities of daily living [35]. Functional deterioration was more pronounced (p < 0.05) in patients on tiapride than in those on dibenzodiazepines (mostly olanzapine). However, tiapride was given to more diseased HD patients at baseline, so the unadjusted significant decline in UHDRS-TFC tends to be confounded by this. Only one included study was designed to test the efficacy of tiapride on depressive symptoms, anxiety, and irritability in HD [19]. However, at baseline most patients did not experience these psychiatric symptoms. Therefore, the power to deduct any conclusion hereof is too low.
4.3. Safety and Side-Effect Profile of Tiapride
Treatment with tiapride is generally well tolerated, particularly in HD patients (Table 1). Side effects are often rare and mild. After four decades of tiapride use in clinical practice, the most reported (in 1–10% of patients) side effects are somnolence, apathy, agitation, and during the beginning of treatment, rigidity, hypokinesia, and hypersalivation [19,24,44,46,47,49]. Less commonly (0.1–1%) reported side effects are akathisia and symptoms that are a consequence of elevated prolactin levels, such as gynecomastia, galactorrhea, and weight increase [24,46,47]. For detailed descriptions of rare side effects, we refer to the package leaflet [36]. In VigiAccess, 1897 reports of suspected potential side effects were present since tiapride became available. This is a relatively small number of reports considering that tiapride has been available for over four decades and is also used in psychiatric patients. Only those symptoms that were reported 25 times or more (arbitrarily chosen) and that are not otherwise mentioned in the patient leaflet are listed here: confusional state (n = 94), hyponatremia (n = 36), urinary retention (n = 34), loss of consciousness (n = 33), bradycardia (n = 27), thrombocytopenia (n = 26), increased serum creatine phosphokinase (n = 26), aspiration pneumonia (n = 26), and rash (n = 25). Simultaneous use of tiapride with antidepressants, benzodiazepines, analgesics, opiates, or alcohol might increase its sedative side effects [24]. Simultaneous use of tiapride with substances that might prolong QT-interval, such as class IA and III antiarrhythmic agents, domperidone, haloperidol, and pimozide, should be avoided [36].
4.4. Expert Opinions
Three expert surveys were included in this review. Two studies focused on pharmacological chorea control [28,53]. One article summarized preferred treatment options for irritability in HD [29]. A summary of the included expert opinions can be found in Table 2. The survey with the most respondents (n = 200) was conducted among USA neurologists and describes pharmacological treatment options for HD [53]. At the time of publication, solely tetrabenazine was FDA-approved for HD chorea control. Tiapride was and is still not available in North America. However, 54% of respondents perceived tetrabenazine as having minimal or no effectiveness in suppressing HD chorea. Antipsychotics (26%) and amantadine (9.3%) were reported as off-label alternatives. The most commonly prescribed antipsychotics were risperidone (6.9%), quetiapine (6.7%), and haloperidol (5.8%). Burgunder et al. conducted a multinational survey among North American, European and Australian physicians [28]. Regional differences were visible since respondents from North America and Australia equally favored off-label antipsychotics (58%) or tetrabenazine (56%), while European respondents evidently preferred antipsychotics (87%) and specifically tiapride (50%) as initial monotherapy. Tetrabenazine was considered an alternative monotherapy by 67% of European respondents. All experts preferred antipsychotic monotherapy when comorbid behavioral symptoms were present. A combination of tetrabenazine and an antipsychotic was prescribed when symptoms were inadequately controlled by monotherapy. Efficacy and side-effect profiles were considered similar for antipsychotics and tetrabenazine, except that depression is more prevalent in the latter.

Table 2.
Summary of included expert surveys.
One expert opinion focused on the pharmacological treatment of irritability [29]. Worldwide respondents preferred antipsychotics (77%) for severe aggression. In case of milder irritability symptoms, SSRIs (57%), antipsychotics (31%), and mirtazapine (28%) were endorsed. Olanzapine, risperidone, and, to a lesser extent, tiapride are specific drugs from the antipsychotic class that were favored. None of the studies reported sex differences in tiapride treatment response [28,29,53].
5. Discussion
To our knowledge, this is the first review on the efficacy and safety profile of tiapride in HD. A general beneficial trend is seen for tiapride as chorea treatment in HD. Although we were unable to retrieve the full text of five articles, we do not expect our conclusion to change if those articles had been available. One study described potential decreased tiapride efficacy in females [47]. However, treatment duration differed between sexes. Females who did not respond to tiapride treatment were all treated for three weeks or less. In comparison, the treatment period for males was often several months or more. None of the other included studies reported any sex differences in tiapride response. Therefore, it seems unlikely that tiapride affects men and women differently. This is supported by our own patients’ data. The small number of studies included in our review were inconclusive about the effect of tiapride on the weight of HD patients, but research in other patient populations has demonstrated that tiapride sporadically may lead to weight gain [21]. Weight increase is limited to some kilograms during the beginning of treatment. Major weight gain is rarely reported [54] and is likely a consequence of increased prolactin levels and increased appetite [55]. No recommendations for specific disease stages can be made since not all studies described disease severity in their study population (data not shown) and all subgroups were small. Two studies have explored this. Deroover et al. described an increased efficacy in patients in disease stage II, compared to those in stage I of the Shoulson and Fahn HD disease stage classification [56], while Roos et al. were not able to distinguish patients in whom chorea improved based on severity or duration of illness [49]. Larger studies with patient and disease characteristics including disease severity are needed to investigate who benefits most from tiapride and at what dose.
This review showed that side effects of tiapride are often rare and mild. Somnolence is one of them [36]. Prescribing tiapride in the evening could not only treat restlessness during the night but may also benefit pre-existing sleeping problems that are common in HD [57,58]. For tetrabenazine, the most often used pharmacological alternative, caution is advised in those patients who have experienced or currently experience depressive symptoms [59,60]. In addition, nausea can be a limiting side effect of tetrabenazine [59,61]. Tiapride has a more favorable profile considering these points [36]. Antipsychotics, especially those belonging to the first generation, could induce tardive dyskinesia with prolonged use [62]. This iatrogenic syndrome is characterized by involuntary movements most pronounced in the face and oromandibular region. To this date, only a very small number (n = 22) of potential cases of tardive dyskinesia related to tiapride have been described in VigiAccess [33]. These cases did not meet our threshold of being reported 25 times or more. In none of the cases causality was confirmed. The risk of developing tardive dyskinesia is related to a drug’s potency to block the dopaminergic D2-receptors [63]. Even at a higher dosage of 600 mg/day, tiapride does not exceed an 80% D2-receptor occupancy [64]. This could very well be a protective factor against developing tardive dyskinesia. Moreover, in some countries tiapride is registered as a substitute antipsychotic when tardive dyskinesia is present.
Limitations of our review are mostly based on the nature of included studies. Most studies were conducted in the 1970s and 1980s, according to the standards of that time [18,19,43,44,45,46,47,48,49]. Relatively small sample sizes (n < 25) were common [18,19,43,44,45,46,47,48,49,50]. Nearly half of the included articles with original data did not perform statistical tests but reported clinically observed changes instead [18,45,46,47,48]. Different outcome measures were used (Table 1). A formal meta-analysis was therefore not possible. Only two out of 12 therapeutical studies used the UHDRS-TMS as an outcome measure [50,51]. Developed in 1996, this scale is the gold standard nowadays for systematically assessing chorea and other physical characteristics of HD [35]. The majority (nine out of 12) of the included therapeutic studies are dated before 1996 and used various outcome measures instead. These include clinical observation by the researcher, patient and/or caregiver, subjective measures on Likert scales, and qualitative and quantitative scores, such as the Abnormal Involuntary Movement Scale (AIMS) [65]. Studies included in our review also varied substantially in treatment periods and dosages of tiapride. Treatment periods ranged from one week up to eight years (Table 1). Dosages of tiapride ranged from 300 mg/day to 900 mg/day with an outlier of 3000 mg/day [18,19,43,44,45,47,48,49]. Unfortunately, several studies did not even report the administered dosage or treatment period [46,48,50,51,52]. Deducting the optimum dosage is therefore difficult. The dosages most often reported were between 300 and 900 mg/day depending on chorea severity and treatment response. The great diversity in study design further complicates the comparability of studies.
Tiapride is not available in the USA for reasons unknown and unavailable to us. As a consequence, our results show a strong regional preference determined by availability in Europe. Prescribing preferences in countries outside Europe, USA, and Australia are concealed to us. Since European clinicians have more experience with tiapride, it inclines them to prescribe tiapride sooner in the next patient. To include as many records as possible, we enriched our search by including French and German language articles.
We do not know the underlying reasons to abruptly withdraw tiapride from the Dutch market. No safety issues have been reported. Business decisions for a cheap, infrequently used drug could have played a role for Sanofi S.A., a large, international pharmaceutical company. Its decision to withdraw tiapride from one national market might jeopardize other countries as well. The sudden lack of tiapride required health care professionals to swiftly provide pharmaceutical alternatives in a complex patient population while being in the middle of the COVID-19 pandemic lockdown. Alternatives were not suitable for more than half of our patients, and a trial and error of medication was often needed before finding an effective alternative. Reasons based on certain patient characteristics to choose tiapride in the first place could have enlarged this percentage. Several reviews have been conducted in the last years, summarizing possible treatment options for chorea in HD [8,30,66]. Alongside (deu)tetrabenazine, these include quetiapine, olanzapine, risperidone, sulpiride, clozapine, aripiprazole, haloperidol, amantadine, lorazepam, and clonazepam. Aside from varying degrees of expected benefit, each drug has its advantages and disadvantages. Based on the information currently available on tiapride in HD, a large RCT to investigate the true effect of tiapride would be beneficial. A French comparative study, called NEUROHD, is ongoing and will publish its results in the near future [67]. In this multi-center, randomized clinical trial with 180 HD patients, the effects of olanzapine, tetrabenazine, and tiapride on patients’ functionality are compared. Secondary endpoints include motor function, psychiatric and cognitive performance, metabolic parameters, drug tolerance, and cost aspects. However, this is not an approach that takes into account personalized medicine, where patient characteristics such as disease characteristics, pharmacogenetic profile, and comorbidities, could in fact dictate the most effective medicine on an individual basis. Further studies to disentangle these specific characteristics are needed.
6. Conclusions
The available studies on tiapride included in our study do not always meet current research standards. However, a general beneficial trend is seen for tiapride as an anti-choreatic treatment in HD. Interestingly, while tetrabenazine is the only approved anti-choreatic treatment in many countries, many experts in the HD field recommend using antipsychotics as initial monotherapy. Based on regional availability, specific drugs are recommended. In Europe, tiapride is favored by many patients and by half of the clinicians. Furthermore, tiapride is cheap and considered to be well tolerated. Over the past decades, no major safety concerns have arisen. Common side effects are mild and often temporary. In line with the International Therapeutic Guidelines [30], we recommend that clinicians consider tiapride treatment for HD chorea, in particular when the patients have associated personality and/or behavioral or psychotic disorders.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jpm12040589/s1, S1: Search strategy.
Author Contributions
Conceptualization, S.F., R.A.C.R. and S.T.d.B.; methodology, S.F.; formal analysis, S.F.; investigation, S.F., M.v.S. and R.A.C.R.; resources, S.F.; data curation, S.F.; writing—original draft preparation, S.F.; writing—review and editing, S.F., M.v.S., R.A.C.R. and S.T.d.B.; visualization, S.F.; supervision, S.T.d.B.; project administration, S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study. The Medical Research Involving Human Subjects Act is not applicable.
Informed Consent Statement
Patient consent was waived. The Medical Research Involving Human Subjects Act is not applicable.
Data Availability Statement
Due to privacy restrictions, patients’ medical records are not available.
Acknowledgments
Information obtained from VigiAccess is based on VigiBase, the WHO global database of reported potential side effects of medicinal products, developed and maintained by Uppsala Monitoring Centre. We are thankful to Uppsala Monitoring Centre for publicly providing their data. Conclusions drawn are those of the authors and not necessarily those of the Uppsala Monitoring Centre, National Centers, or WHO.
Conflicts of Interest
Authors S. Feleus and S.T. de Bot: Leiden University Medical Center receives grants from the European Huntington’s Disease Network (EHDN) and Cure HD Initiative (CHDI), participates in an EU Horizon 2020 project: Innovative Medicines Initiative (IMI) 2 (IDEA_FAST), and participates in clinical trials sponsored by Hoffmann-LaRoche, and by PRILENIA. The aforementioned sponsors had no role in the design, execution, interpretation, or writing of this current study. Authors M. van Schaijk and R.A.C. Roos declare no conflicts of interest.
Abbreviations
AIMS = Abnormal Involuntary Movement Scale; BL = Baseline; CAG-repeat = Cytosine Adenine Guanine trinucleotide repeat; EU = Europe; EMG = Electromyography; FDA = United States (of America) Food and Drug Administration; FU = Follow-up; HD = Huntington’s Disease; HTT = Huntingtin; LTFU = Lost to follow-up; mg = milligram; n = number of; NA = Not applicable; p = p-value; RCT = Randomized Controlled Trial; UHDRS- TFC = Unified Huntington’s Disease Rating Scale—Total Functional Capacity; UHDRS-TMS = Unified Huntington’s Disease Rating Scale—Total Motor Score; uk = unknown; USA = United States of America; WHO = World Health Organization.
References
- MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.A.; James, M.; Groot, N.; et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef]
- Caron, N.S.; Wright, G.E.B.; Hayden, M.R. Huntington Disease. In GeneReviews(®); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Mirzaa, G.M., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Folstein, S.E.; Folstein, M.F. Psychiatric features of Huntington’s disease: Recent approaches and findings. Psychiatr. Dev. 1983, 1, 193–205. [Google Scholar] [PubMed]
- Roos, R.A.C. Clinical neurology. In Huntington’s Disease, 4th ed.; Bates, G.P., Tabrizi, S.J., Jones, L., Eds.; Oxford University Press: New York, NY, USA, 2014; pp. 25–35. [Google Scholar]
- Baake, V.; Reijntjes, R.; Dumas, E.M.; Thompson, J.C.; Roos, R.A.C. Cognitive decline in Huntington’s disease expansion gene carriers. Cortex 2017, 95, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Rook, M.E.; Southwell, A.L. Antisense Oligonucleotide Therapy: From Design to the Huntington Disease Clinic. BioDrugs 2022. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Rodrigues, F.B.; Duarte, G.S.; Mestre, T.A.; Bachoud-Levi, A.C.; Bentivoglio, A.R.; Burgunder, J.M.; Cardoso, F.; Claassen, D.O.; Landwehrmeyer, G.B.; et al. An MDS Evidence-Based Review on Treatments for Huntington’s Disease. Mov. Disord. 2022, 37, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Coppen, E.M.; Roos, R.A. Current Pharmacological Approaches to Reduce Chorea in Huntington’s Disease. Drugs 2017, 77, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Bilney, B.; Morris, M.E.; Perry, A. Effectiveness of physiotherapy, occupational therapy, and speech pathology for people with Huntington’s disease: A systematic review. Neurorehabil. Neural Repair 2003, 17, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Nance, M.A. Comprehensive Care. In Huntington’s Disease, 4th ed.; Bates, G.P., Tabrizi, S.J., Jones, L., Eds.; Oxford University Press: New York, NY, USA, 2014; pp. 393–409. [Google Scholar]
- Quinn, L.; Kegelmeyer, D.; Kloos, A.; Rao, A.K.; Busse, M.; Fritz, N.E. Clinical recommendations to guide physical therapy practice for Huntington disease. Neurology 2020, 94, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Zarotti, N.; Dale, M.; Eccles, F.; Simpson, J. Psychological Interventions for People with Huntington’s Disease: A Call to Arms. J. Huntington’s Dis. 2020, 9, 231–243. [Google Scholar] [CrossRef]
- Cepeda, C.; Murphy, K.P.; Parent, M.; Levine, M.S. The role of dopamine in Huntington’s disease. Prog. Brain Res. 2014, 211, 235–254. [Google Scholar] [CrossRef] [Green Version]
- Bashir, H.; Jankovic, J. Treatment options for chorea. Expert Rev. Neurother. 2018, 18, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Buis, C.; Flohil, J.M. CLINICAL EXPERIENCES WITH HALOPERIDOL (SERENASE). Ned. Tijdschr. Geneeskd. 1964, 108, 796–800. [Google Scholar] [PubMed]
- Koller, W.C.; Trimble, J. The gait abnormality of Huntington’s disease. Neurology 1985, 35, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.J.; Mones, R.J. Modification of choreiform activity by Haloperidol. JAMA 1971, 216, 675–676. [Google Scholar] [CrossRef]
- Chouza, C.; Romero, S.; Lorenzo, J. Clinical trial of tiapride in patients with dyskinesia. Sem. Hop. 1982, 58, 725–733. [Google Scholar]
- Deroover, J.; Baro, F.; Bourguignon, R.P.; Smets, P. Tiapride versus placebo: A double-blind comparative study in the management of Huntington’s chorea. Curr. Med. Res. Opin. 1984, 9, 329–338. [Google Scholar] [CrossRef]
- Roos, R.A.C.; de Haas, E.J.M.; Buruma, O.J.S.; de Wolff, F.A. Pharmacokinetics of tiapride in patients with tardive dyskinesia and Huntington’s disease. Eur. J. Clin. Pharmacol. 1986, 31, 191–194. [Google Scholar] [CrossRef]
- Eggers, C.; Rothenberger, A.; Berghaus, U. Clinical and neurobiological findings in children suffering from tic disease following treatment with tiapride. Eur. Arch. Psychiatry Neurol. Sci. 1988, 237, 223–229. [Google Scholar] [CrossRef]
- Lnĕnicka, J.; Stará, V. The therapeutic effect of tiapride in the treatment of dyskinetic forms of cerebral palsy in children. Cesk. Pediatr. 1992, 47, 670–672. [Google Scholar]
- Tiapride. Available online: https://www.drugs.com/international/tiapride.html (accessed on 11 February 2022).
- Farmacotherapeutisch Kompas. Tiapride. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/t/tiapride (accessed on 20 July 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5467, Tiapride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tiapride (accessed on 4 September 2020).
- Dose, M.; Lange, H.W. The benzamide tiapride: Treatment of extrapyramidal motor and other clinical syndromes. Pharmacopsychiatry 2000, 33, 19–27. [Google Scholar] [CrossRef]
- Steele, J.W.; Faulds, D.; Sorkin, E.M. Tiapride. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in geriatric agitation. Drugs Aging 1993, 3, 460–478. [Google Scholar] [CrossRef] [PubMed]
- Burgunder, J.M.; Guttman, M.; Perlman, S.; Goodman, N.; van Kammen, D.P.; Goodman, L. An International Survey-based Algorithm for the Pharmacologic Treatment of Chorea in Huntington’s Disease. PLoS Curr. 2011, 3, RRN1260. [Google Scholar] [CrossRef] [PubMed]
- Groves, M.; van Duijn, E.; Anderson, K.; Craufurd, D.; Edmondson, M.C.; Goodman, N.; van Kammen, D.P.; Goodman, L. An International survey-based algorithm for the pharmacologic treatment of irritability in Huntington’s disease. PLoS Curr. 2011, 3, RRN1259. [Google Scholar] [CrossRef] [PubMed]
- Bachoud-Lévi, A.C.; Ferreira, J.; Massart, R.; Youssov, K.; Rosser, A.; Busse, M.; Craufurd, D.; Reilmann, R.; de Michele, G.; Rae, D.; et al. International Guidelines for the Treatment of Huntington’s Disease. Front. Neurol. 2019, 10, 710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ark, T. Regulation of the Minister for Medical Care of January 22, 2021, Reference 217289-1815434-Z, Amending Annexes 1 and 2 of the Health Insurance Scheme in Connection with the Change in the Entitlement to Registered Medicines; Staatscourant: The Hague, The Netherlands, 2021; p. 17. [Google Scholar]
- Zorginstituut Nederland. Reimbursement per DDD 2016-2020 for ATC-subgroup N05AL03: Tiapride. Available online: https://www.gipdatabank.nl/databank?infotype=g&label=00-totaal&tabel_g_00-totaal=B_01-basis&tabel_h_00-totaal=B_01-basis&geg=vg&spec=vg_ddd&item=N05AL03 (accessed on 16 March 2022).
- Lindquist, M. VigiBase, the WHO Global ICSR Database System: Basic facts. Drug Inf. J. 2008, 42, 409–419. [Google Scholar] [CrossRef]
- [Patient Organisation Huntington’s Disease in the Netherlands] Vereniging van Huntington. [CALL TO ACTION! Tiapride has been taken off-market]. Available online: https://www.huntington.nl/nieuws/682-oproep-tot-actie-tiapridal-is-van-de-markt-gehaald.html (accessed on 16 March 2022).
- Huntington Study Group. Unified Huntington’s Disease Rating Scale: Reliability and consistency. Mov. Disord. 1996, 11, 136–142. [Google Scholar] [CrossRef]
- Sanofi Winthrop Industrie. Tiapridal (Tiapride) [Package Leaflet: Information for the User]; Sanofi Winthrop Industrie: Quetigny, France, 2013. [Google Scholar]
- Uppsala Monitoring Centre. Caveat Document; Statement of Reservations, Limitations and Conditions Relating to Data Released from VigiBase, the WHO Global Database of Reported Potential Side Effects of Medicinal Products; Uppsala Monitoring Centre: Uppsala, Sweden, 2021; p. 1. [Google Scholar]
- Brion, S.; Guerin, R. Action d’une molécule neurotrope originale dans certains syndromes neurologiques (mouvements anormaux et al.gies diverses). Sem. Hop. 1977, 53, 40–44. [Google Scholar]
- Costall, B.; Naylor, R.J. Démonstration neupharmacologique de l’effet antidyskinétique du Tiapride. Sem. Hop. 1977, 53, 72–76. [Google Scholar]
- Emile, J.; Bastard, J.; Truelle, J.L. Utilisation du tiapride en neurologie; Resultats préliminaires. Sem. Hop. 1977, 53, 16–20. [Google Scholar]
- Lhermitte, F.; Signoret, J.L.; Agid, Y. Etude des effets d’une molécule originale, le Tiapride, dans le traitement des mouvements anormaux d’origine extrapyramidale. Sem. Hop. 1977, 53, 9–15. [Google Scholar]
- Trillet, M.; Joyeux, O.; Masson, R. Tiapride et mouvements anormaux. Sem. Hop. 1977, 53, 21–27. [Google Scholar]
- Csanda, E.; Tarczy, M.; Jelencsik, I. Tiapride treatment of abnormal movements and painful conditions. Sem. Hop. 1984, 60, 3006–3008. [Google Scholar]
- Girotti, F.; Carella, F.; Scigliano, G.; Grassi, M.P.; Soliveri, P.; Giovannini, P.; Parati, E.; Caraceni, T. Effect of neuroleptic treatment on involuntary movements and motor performances in Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 1984, 47, 848–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grass, H.; Gottschaldt, M. Pharmakologische Therapiemöglichkeit extrapyramidaler Hyperkinesen mit Tiaprid. Psychiatr. Neurol. Med. Psychol. 1983, 35, 222–229. [Google Scholar]
- Mathe, J.F.; Cler, J.M.; Venisse, J.L. Therapeutic use of tiapride in movement disorders. Sem. Hop. 1978, 54, 517–520. [Google Scholar]
- Petit, H. Les indications du Tiapride en pathologie extrapyramidale. Lille Med. 1979, 24, 339–344. [Google Scholar] [PubMed]
- Quinn, N.; Marsden, C.D. Tiapride in 12 Huntington’s disease patients. J. Neurol. Neurosurg. Psychiatry 1985, 48, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, R.A.C.; Buruma, O.J.S.; Bruyn, G.W. Tiapride in the treatment of Huntington’s chorea. Acta Neurol. Scand. 1982, 65, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Cichecki, F.; Witkowski, G.; Zielonka, D.; Sienkiewicz-Jarosz, H.; Stepniak, I.; Ziora-Jakutowicz, K. Treatment of chorea and its efficacy in huntington’s disease (HD) patients. J. Neurol. Neurosurg. Psychiatry 2016, 87, A72. [Google Scholar] [CrossRef]
- Desamericq, G.; Dolbeau, G.; Verny, C.; Charles, P.; Durr, A.; Youssov, K.; Simonin, C.; Azulay, J.P.; Tranchant, C.; Goizet, C.; et al. Effectiveness of anti-psychotics and related drugs in the Huntington french-speaking group cohort. PLoS ONE 2014, 9, e85430. [Google Scholar] [CrossRef]
- Konvalinkova, R.; Dusek, P.; Doleckova, K.; Uhrova, T.; Roth, J.; Klempir, J. Does the risperidone influence weight in Huntington’s disease? J. Neurol. Neurosurg. Psychiatry 2018, 89, A86. [Google Scholar]
- Sung, V.W.; Iyer, R.G.; Gandhi, S.K.; Shah-Manek, B.; DiBonaventura, M.; Abler, V.; Claassen, D.O. Physician perceptions of pharmacologic treatment options for chorea associated with Huntington disease in the United States. Curr. Med. Res. Opin. 2018, 34, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Meisel, A.; Winter, C.; Zschenderlein, R.; Arnold, G. Tourette syndrome: Efficient treatment with ziprasidone and normalization of body weight in a patient with excessive weight gain under tiapride. Mov. Disord. 2004, 19, 991–992. [Google Scholar] [CrossRef] [PubMed]
- Ben-Jonathan, N.; Hugo, E.R.; Brandebourg, T.D.; LaPensee, C.R. Focus on prolactin as a metabolic hormone. Trends Endocrinol. Metab. 2006, 17, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Shoulson, I.; Fahn, S. Huntington disease: Clinical care and evaluation. Neurology 1979, 29, 1–3. [Google Scholar] [CrossRef]
- Anderson, K.E.; van Duijn, E.; Craufurd, D.; Drazinic, C.; Edmondson, M.; Goodman, N.; van Kammen, D.; Loy, C.; Priller, J.; Goodman, L.V. Clinical Management of Neuropsychiatric Symptoms of Huntington Disease: Expert-Based Consensus Guidelines on Agitation, Anxiety, Apathy, Psychosis and Sleep Disorders. J. Huntingtons Dis. 2018, 7, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Ogilvie, A.C.; Nopoulos, P.C.; Schultz, J.L. Sleep disturbances by disease type and stage in Huntington’s disease. Parkinsonism Relat. Disord. 2021, 91, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Sun Pharmaceutical Industries Europe B.V. Package Leaflet: Information for the User. Tetrabenazine 25 mg Tablets; Ranbaxy (UK) Limited a Sun Pharmaceutical Company: Uxbridge, UK, 2021; p. 6. [Google Scholar]
- Huntington Study Group. Tetrabenazine as antichorea therapy in Huntington disease: A randomized controlled trial. Neurology 2006, 66, 366–372. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, P.; Jamwal, S.; Deshmukh, R.; Gauttam, V. Tetrabenazine: Spotlight on Drug Review. Ann. Neurosci. 2016, 23, 176–185. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, TX, USA, 2013. [Google Scholar]
- Klawans, H.L., Jr. The pharmacology of tardive dyskinesias. Am. J. Psychiatry 1973, 130, 82–86. [Google Scholar] [CrossRef]
- Leenders, K.L.; Blauth-Eckmeyer, E. PET study with the benzamide tiapride. Eur. Psychiatry 1996, 11, 416s. [Google Scholar] [CrossRef]
- Guy, W. Abnormal Involuntary Movement Scale (AIMS); ECDEU Assessment Manual for Psychopharmacology; U.S. Department of Health Education and Welfare: Washington, DC, USA, 1976; pp. 534–537. [Google Scholar]
- Armstrong, M.J.; Miyasaki, J.M. Evidence-based guideline: Pharmacologic treatment of chorea in Huntington disease: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2012, 79, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuroleptic and Huntington Disease Comparison of: Olanzapine, la Tetrabenazine and Tiapride (NEUROHD) NCT00632645. Available online: https://ClinicalTrials.gov/show/NCT00632645 (accessed on 11 February 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).