Body Representation in Patients with Severe Spinal Cord Injury: A Pilot Study on the Promising Role of Powered Exoskeleton for Gait Training
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Conventional Physical Therapy (CPT)
2.3. Ekso Training
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
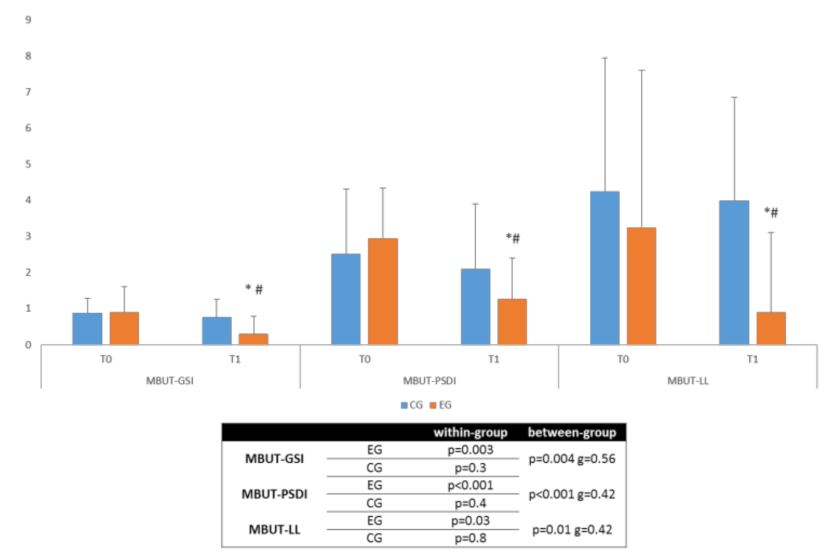
3.1. Primary Outcome
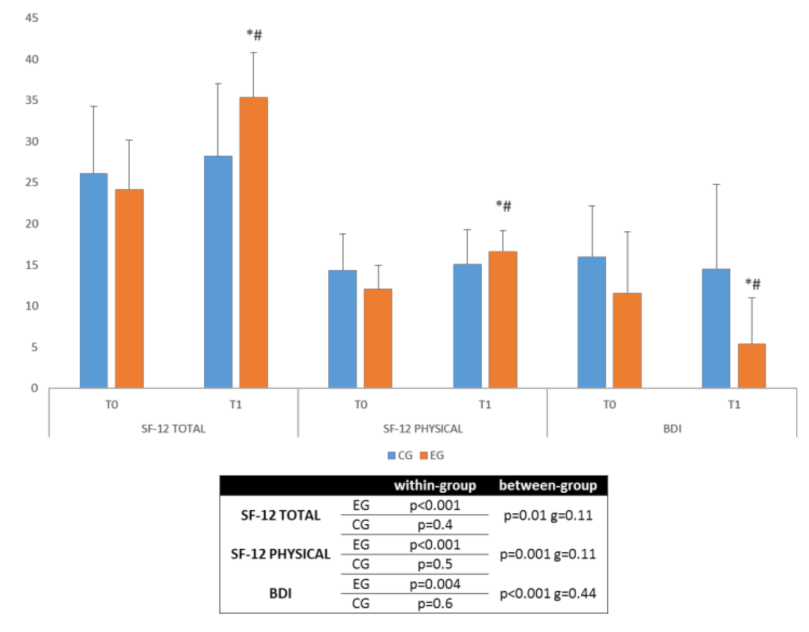
3.2. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, W.-Y.; He, D.-W. Current trends in spinal cord injury repair. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3340–3344. [Google Scholar]
- De Vivo, M.J. Epidemiology of traumatic spinal cord injury: Trends and future implications. Spinal Cord 2012, 50, 365–372. [Google Scholar] [CrossRef]
- Pagliacci, M.C.; Celani, M.G.; Spizzichino, L.; Zampolini, M.; Aito, S.; Citterio, A.; Finali, G.; Loria, D.; Ricci, S.; Taricco, M.; et al. Spinal cord lesion management in Italy: A 2-year survey. Spinal Cord 2003, 41, 620–628. [Google Scholar] [CrossRef] [Green Version]
- Geyh, S.; Ballert, C.; Sinnott, A.; Charlifue, S.; Catz, A.; Greve, J.; Post, M.W.M. Quality of life after spinal cord injury: A comparison across six countries. Spinal Cord 2012, 51, 322–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boakye, M.; Leigh, B.C.; Skelly, A.C. Quality of life in persons with spinal cord injury: Comparisons with other populations. J. Neurosurg. Spine 2012, 17, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.; Dorstyn, D.; Burke, A.L.J. Psychosocial aspects of spinal cord injury pain: A meta-analysis. Spinal Cord 2016, 54, 640–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutenbrunner, C.; Blumenthal, M.; Geng, V.; Egen, C. Rehabilitation Services Provision and Payment. Am. J. Phys. Med. Rehabil. 2017, 96, S35–S40. [Google Scholar] [CrossRef]
- Selvarajah, S.; Hammond, E.R.; Haider, A.H.; Abularrage, C.J.; Becker, D.; Dhiman, N.; Hyder, O.; Gupta, D.; Black, J.H.; Schneider, E.B. The Burden of Acute Traumatic Spinal Cord Injury among Adults in the United States: An Update. J. Neurotrauma 2014, 31, 228–238. [Google Scholar] [CrossRef]
- Munce, S.; Wodchis, W.P.; Guilcher, S.; Couris, C.M.; Verrier, M.; Fung, K.; Craven, B.; Jaglal, S.B. Direct costs of adult traumatic spinal cord injury in Ontario. Spinal Cord 2013, 51, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Gamblin, A.S.; Garry, J.G.; Wilde, H.W.; Reese, J.C.; Sherrod, B.; Karsy, M.; Guan, J.; Mortenson, J.; Flis, A.; Rosenbluth, J.P.; et al. Cost Analysis of Inpatient Rehabilitation after Spinal Injury: A Retrospective Cohort Analysis. Cureus 2019, 11, e5747. [Google Scholar] [CrossRef] [Green Version]
- Marini, M.; Reale, L. Bisogni e Costi Delle Persone Con Lesione Midollare e Dei Nuclei Familiari di Riferimento; Istituto per gli Affari Sociali: Milan, Italy, 2010; p. 105. [Google Scholar]
- Baricich, A.; Amico, A.P.; Zampolini, M.; Gimigliano, F.; Cisari, C.; Fiore, P. People with Spinal Cord Injury in Italy. Am. J. Phys. Med. Rehabil. 2017, 96, S80–S82. [Google Scholar] [CrossRef] [PubMed]
- Paillard, J. Body schema and body image—A double dissociation. In Motor Control, Today and Tomorrow; Academic Publishing House: Sofia, Bulgaria, 1999; pp. 197–214. [Google Scholar]
- Gallagher, S. Body image and body schema: A conceptual clarification. J. Mind Behav. 1986, 7, 541–554. [Google Scholar]
- De Vignemont, F. Body schema and body image—Pros and cons. Neuropsychologia 2010, 48, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Schwoebel, J.; Coslett, H.B. Evidence for multiple, distinct representations of the human body. J. Cogn. Neurosci. 2005, 17, 543–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, M.R.; Azañón, E.; Haggard, P. More than skin deep: Body representation beyond primary somatosensory cortex. Neuropsychologia 2010, 48, 655–668. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, N.; Izumi, S.-I.; Ota, J.; Ueda, J. Neural Plasticity on Body Representations: Advancing Translational Rehabilitation. Neural Plast. 2016, 2016, 9737569. [Google Scholar] [CrossRef] [PubMed]
- Cash, T.F. The psychosocial consequences of androgenetic alopecia: A review of the research literature. Br. J. Dermatol. 1999, 141, 398–405. [Google Scholar] [CrossRef]
- Bassett, R.L.; The SHAPE-SCI Research Group; Ginis, K.M. More than looking good: Impact on quality of life moderates the relationship between functional body image and physical activity in men with SCI. Spinal Cord 2008, 47, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Dewis, M.E. Spinal cord injured adolescents and young adults: The meaning of body changes. J. Adv. Nurs. 1989, 14, 389–396. [Google Scholar] [CrossRef]
- Maresca, G.; Maggio, M.G.; Caliri, S.; De Cola, M.C.; Scarcella, I.; Andaloro, A.; Latella, D.; Accorinti, M.; De Luca, R.; Calabrò, R.S. The role of body image changes in neurorehabilitation outcomes: A preliminary study. Psychol. Health Med. 2019, 25, 10–16. [Google Scholar] [CrossRef]
- Bassett, R.L.; The SHAPE-SCI Research Group; Ginis, K.M.; Buchholz, A.C. A pilot study examining correlates of body image among women living with SCI. Spinal Cord 2009, 47, 496–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chau, L.; Hegedus, L.; Praamsma, M.; Smith, K.; Tsukada, M.; Yoshida, K.; Renwick, R. Women Living with a Spinal Cord Injury: Perceptions About Their Changed Bodies. Qual. Health Res. 2008, 18, 209–221. [Google Scholar] [CrossRef]
- Kennedy, P.; Gorsuch, N.; Marsh, N. Childhood onset of spinal cord injury: Self-esteem and self-perception. Br. J. Clin. Psychol. 1995, 34, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, C.-A.; Khan, G. Sexual Self-esteem and Body Image of South African Spinal Cord Injured Adolescents. Sex. Disabil. 2005, 23, 1–20. [Google Scholar] [CrossRef]
- Sheldon, A.P.; Renwick, R.; Yoshida, K.K. Exploring Body Image and Self-Concept of Men with Acquired Spinal Cord Injuries. Am. J. Men’s Health 2011, 5, 306–317. [Google Scholar] [CrossRef] [Green Version]
- Stensman, R. Body image among 22 persons with acquired and congenital severe mobility impairment. Spinal Cord 1989, 27, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Taleporos, G.; McCabe, M.P. Body image and physical disability—Personal perspectives. Soc. Sci. Med. 2002, 54, 971–980. [Google Scholar] [CrossRef]
- Conomy, J.P. Disorders of body image after spinal cord injury. Neurology 1973, 23, 842. [Google Scholar] [CrossRef]
- Evans, J.H. On disturbance of the body image in paraplegia. Brain 1962, 85, 687–700. [Google Scholar] [CrossRef]
- Bors, E. Phantom limbs of patients with spinal cord injury. Arch. Neurol. Psychiatry 1951, 66, 610–631. [Google Scholar] [CrossRef]
- Hicks, A.L.; Martin, K.A.; Ditor, D.S.; Latimer, A.; Craven, B.; Bugaresti, J.; McCartney, N. Long-term exercise training in persons with spinal cord injury: Effects on strength, arm ergometry performance and psychological well-being. Spinal Cord 2003, 41, 34–43. [Google Scholar] [CrossRef]
- Leemhuis, E.; Giuffrida, V.; De Martino, M.L.; Forte, G.; Pecchinenda, A.; De Gennaro, L.; Giannini, A.M.; Pazzaglia, M. Rethinking the Body in the Brain after Spinal Cord Injury. J. Clin. Med. 2022, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, E.; De Gennaro, L.; Pazzaglia, A.M. Disconnected Body Representation: Neuroplasticity Following Spinal Cord Injury. J. Clin. Med. 2019, 8, 2144. [Google Scholar] [CrossRef] [Green Version]
- Nardone, R.; Höller, Y.; Brigo, F.; Seidl, M.; Christova, M.; Bergmann, J.; Golaszewski, S.; Trinka, E. Functional brain reorganization after spinal cord injury: Systematic review of animal and human studies. Brain Res. 2013, 1504, 58–73. [Google Scholar] [CrossRef]
- Van Diemen, T.; van Leeuwen, C.; van Nes, I.; Geertzen, J.; Post, M. Body Image in Patients with Spinal Cord Injury during Inpatient Rehabilitation. Arch. Phys. Med. Rehabil. 2017, 98, 1126–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del-Ama, A.J.; Gil-Agudo, A.; Pons, J.L.; Moreno, J.C. Hybrid gait training with an overground robot for people with incom-plete spinal cord injury: A pilot study. Front Hum. Neurosci. 2014, 8, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routhier, F.; Vincent, C.; Desrosiers, J.; Nadeau, S. Mobility of wheelchair users: A proposed performance assessment framework. Disabil. Rehabil. 2003, 25, 19–34. [Google Scholar] [CrossRef]
- Sorbera, C.; Portaro, S.; Cimino, V.; Leo, A.; Accorinti, M.; Silvestri, G.; Bramanti, P.; Naro, A.; Calabrò, R.S. ERIGO: A possible strategy to treat orthostatic hypotension in progressive supranuclear palsy? A feasibility study. Funct. Neurol. 2019, 34, 93–97. [Google Scholar] [PubMed]
- Miller, L.; Zimmermann, A.; Herbert, W. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: Systematic review with meta-analysis. Med. Devices Évid. Res. 2016, 9, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, J.C.; Patsakos, E.M.; Maltais, D.B.; Wolfe, D.L.; Gagnon, D.H.; Craven, B.C. Rehabilitation Interventions to modify endocrine-metabolic disease risk in In-dividuals with chronic Spinal cord injury living in the Community (RIISC): A systematic review and scoping perspective. J. Spinal Cord Med. 2017, 40, 733–747. [Google Scholar] [CrossRef]
- Nordström, B.; Nyberg, L.; Ekenberg, L.; Näslund, A. The psychosocial impact on standing devices. Disabil. Rehabil. Assist. Technol. 2014, 9, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kressler, J.; Thomas, C.K.; Field-Fote, E.; Sanchez, J.; Widerstrom-Noga, E.; Cilien, D.C.; Gant, K.; Ginnety, K.; González, H.; Martinez, A.; et al. Understanding Therapeutic Benefits of Overground Bionic Ambulation: Exploratory Case Series in Persons with Chronic, Complete Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2014, 95, 1878–1887.e4. [Google Scholar] [CrossRef] [PubMed]
- Kolakowsky-Hayner, S.A.; Crew, J.; Moran, S.; Shah, A. Safety and Feasibility of using the Ekso™ Bionic Exoskeleton to Aid Ambulation after Spinal Cord Injury. J. Spine 2013, S4, 003. [Google Scholar]
- Esquenazi, A.; Talaty, M.; Packel, A.; Saulino, M. The ReWalk powered exoskeleton to restore ambulatory function to indi-viduals with thoracic-level motor-complete spinal cord injury. Am. J. Phys. Med. Rehabil. 2012, 91, 911–921. [Google Scholar] [CrossRef] [Green Version]
- Palermo, A.E.; Maher, J.L.; Baunsgaard, C.B.; Nash, M.S. Clinician-Focused Overview of Bionic Exoskeleton Use after Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2017, 23, 234–244. [Google Scholar] [CrossRef]
- Kandilakis, C.; Sasso-Lance, E. Exoskeletons for Personal Use after Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2021, 102, 331–337. [Google Scholar] [CrossRef]
- Chiș, L.C.; Copotoiu, M.; Moldovan, L. Different Types of Exoskeletons can Improve the Life of Spinal Cord Injury’s Patients—A Meta-Analysis. Procedia Manuf. 2020, 46, 844–849. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, A.; Lobo-Prat, J.; Font-Llagunes, J.M. Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments. J. Neuroeng. Rehabil. 2021, 18, 22. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sørensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [Green Version]
- Maggio, M.G.; Naro, A.; Manuli, A.; Maresca, G.; Balletta, T.; Latella, D.; De Luca, R.; Calabrò, R.S. Effects of Robotic Neu-rorehabilitation on Body Representation in Individuals with Stroke: A Preliminary Study Focusing on an EEG-Based Approach. Brain Topogr. 2021, 34, 348–362. [Google Scholar] [CrossRef]
- Birch, N.; Graham, J.; Priestley, T.; Heywood, C.; Sakel, M.; Gall, A.; Nunn, A.; Signal, N. Results of the first intermediate analysis of the RAPPER II study in patients with spinal cord injury: Walking and functional exercise programs in the walking aid system fed with REX. J. Neuroeng. Rehabil. 2017, 14, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuzzolaro, M.; Vetrone, G.; Marano, G.F.; Battacchi, M.W. BUT: Una nuova scala per la valutazione del disagio relativo all’immagine del corpo. Psichiatr. Dell’infanzia E Dell’adolescenza 1999, 66, 417–428. [Google Scholar]
- Apolone, G.; Mosconi, P.; Quattrociocchi, L.; Gianicolo, E.; Groth, N.; Ware, J. Questionario sullo stato di salute SF-12. In Versione Italiana; Guerini e Associati: Florence, Italy, 2001. [Google Scholar]
- Sica, C.; Ghisi, M. The Italian Versions of the Beck Anxiety Inventory and the Beck Depression Inventory-II: Psychometric Properties and Discriminant Power; Leading-Edge Psychological Tests and Testing Research; Lange, M.A., Ed.; Nova Science Publishers: New York, NY, USA, 2007. [Google Scholar]
- Akiyama, Y.; Fukui, Y.; Okamoto, S.; Yamada, Y. Effects of exoskeletal gait assistance on the recovery motion following tripping. PLoS ONE 2020, 15, e0229150. [Google Scholar] [CrossRef] [PubMed]
- Portaro, S.; Naro, A.; Leo, A.; Cimino, V.; Balletta, T.; Buda, A.; Accorinti, M.; Calabrò, R. Overground exoskeletons may boost neuroplasticity in myotonic dystrophy type1 rehabilitation: A case report. Medicine 2019, 98, e17582. [Google Scholar] [CrossRef] [PubMed]
- Swank, C.; Sikka, S.; Driver, S.; Bennett, M.; Callender, L. Feasibility of integrating robotic exoskeleton gait training in inpatient rehabilitation. Disabil. Rehabil. Assist. Technol. 2019, 15, 409–417. [Google Scholar] [CrossRef]
- Rojek, A.; Mika, A.; Łukasz, O.; Stolarczyk, A.; Kielnar, R. Effects of Exoskeleton Gait Training on Balance, Load Distribution, and Functional Status in Stroke: A Randomized Controlled Trial. Front. Neurol. 2020, 10, 1344. [Google Scholar] [CrossRef] [Green Version]
- Baunsgaard, C.B.; Nissen, U.V.; Brust, A.K.; Frotzler, A.; Ribeill, C.; Kalke, Y.-B.; León, N.; Gómez, B.; Samuelsson, K.; Antepohl, W.; et al. Gait training after spinal cord injury: Safety, feasibility and gait function following 8 weeks of training with the exoskeletons from Ekso Bionics. Spinal Cord 2018, 56, 106–116. [Google Scholar] [CrossRef] [Green Version]
- AlAmro, R.A.; Chisholm, A.E.; Williams, A.M.M.; Carpenter, M.G.; Lam, T. Overground walking with a robotic exoskeleton elicits trunk muscle activity in people with high-thoracic motor-complete spinal cord injury. J. Neuroeng. Rehabil. 2018, 15, 109. [Google Scholar] [CrossRef]
- Baunsgaard, C.; Nissen, U.; Brust, A.; Frotzler, A.; Ribeill, C.; Kalke, Y.; León, N.; Gómez, B.; Samuelsson, K.; Antepohl, W.; et al. Exoskeleton gait training after spinal cord injury: An exploratory study on secondary health conditions. J. Rehabil. Med. 2018, 50, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Gorgey, A.S.; Wade, R.; Sumrell, R.; Villadelgado, L.; Khalil, R.E.; Lavis, T. Exoskeleton Training May Improve Level of Physical Activity after Spinal Cord Injury: A Case Series. Top. Spinal Cord Inj. Rehabil. 2017, 23, 245–255. [Google Scholar] [CrossRef]
- Scandola, M.; Aglioti, S.M.; Lazzeri, G.; Avesani, R.; Ionta, R.; Moro, V. Visuo-motor and interoceptive influences on peri-personal space representation following spinal cord injury. Sci. Rep. 2020, 10, 5162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scandola, M.; Aglioti, S.M.; Avesani, R.; Bertagnoni, G.; Marangoni, A.; Moro, V. Corporeal illusions in chronic spinal cord injuries. Conscious. Cogn. 2017, 49, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, M.; Zantedeschi, M. Plasticity and Awareness of Bodily Distortion. Neural Plast. 2016, 2016, 9834340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takakusaki, K. Forebrain control of locomotor behaviors. Brain Res. Rev. 2008, 57, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Cook, A.W.; Druckemiller, W.H. Phantom limb in paraplegic patients; report of two cases and an analysis of its mechanism. J. Neurosurg. 1952, 9, 508–516. [Google Scholar] [CrossRef]
- Enander, J.M.D.; Jones, A.M.; Kirkland, M.; Hurless, J.C.; Jorntell, H.; Loeb, G.E. A Model for Self-Organization of Sensorimotor Function: The Spinal Monosynaptic Loop. J. Neurophysiol. 2022. [Google Scholar] [CrossRef]
- Donoghue, J.P. Plasticity of adult sensorimotor representations. Curr. Opin. Neurobiol. 1995, 5, 749–754. [Google Scholar] [CrossRef]
- Øyvind, F.S. Re-embodiment: Incorporation through embodied learning of wheelchair skills. Med. Health Care Philos. 2011, 14, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Pazzaglia, M.; Galli, G.; Scivoletto, G.; Molinari, M. A Functionally Relevant Tool for the Body following Spinal Cord Injury. PLoS ONE 2013, 8, e58312. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, C.T.; Pazzaglia, M.; Longo, M.R.; Scivoletto, G.; Haggard, P. Body image distortions following spinal cord injury. J. Neurol. Neurosurg. Psychiatry 2013, 84, 201–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, K.A.; Gammage, K.L.; van Ingen, C.; Ditor, D.S. It’s all about acceptance: A qualitative study exploring a model of positive body image for people with spinal cord injury. Body Image 2015, 15, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Arazpour, M.; Chitsazan, A.; Hutchins, S.W.; Mousavi, M.E.; Ebrahimi-Takamjani, I.; Ghomsheh, F.T.; Aminian, G.; Rahgozar, M.; Bani, M.A. Evaluation of a novel powered gait orthosis for walking by a spinal cord injury patient. Prosthet. Orthot. Int. 2012, 36, 239–246. [Google Scholar] [CrossRef]
- Hartigan, C.; Kandilakis, C.; Dalley, S.; Clausen, M.; Wilson, E.; Morrison, S.; Etheridge, S.; Farris, R.J. Mobility Outcomes Following Five Training Sessions with a Powered Exoskeleton. Top. Spinal Cord Inj. Rehabil. 2015, 21, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juszczak, M.; Gallo, E.; Bushnik, T. Examining the Effects of a Powered Exoskeleton on Quality of Life and Secondary Im-pairments in People Living with Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2018, 24, 336–342. [Google Scholar] [CrossRef] [PubMed]


| Experimental Group | Control Group | All | p-Value | |
|---|---|---|---|---|
| Patients | 21 | 21 | 42 | |
| Age (years) | 58.6 ± 15.0 | 52.6 ± 9.0 | 55.6 ± 12.6 | 0.12 |
| Gender | ||||
| Female | 10 (40.0%) | 7 (33.3%) | 17 (40.5%) | 0.35 |
| Male | 11 (60.0%) | 14 (66.7%) | 25 (59.5%) | |
| Education | ||||
| Elementary school | - | - | - | 0.23 |
| Middle school | 5 (23.8%) | 3 (14.3%) | 8 (19.0%) | |
| High school | 14 (66.7%) | 12 (57.1%) | 26 (62.0%) | |
| University | 2 (9.5%) | 6 (28.6%) | 8 (19.0%) | |
| Spinal Injury Disability (AIS) | ||||
| AIS—A patients | 10 (47.6%) | 10 (47.6%) | 20 (47.6%) | 0.99 |
| AIS—B patients | 11 (52.3%) | 11 (52.3%) | 22 (52.3%) | |
| Time Post-Injury | ||||
| AIS—A patients | 7 ± 1 | 6 ± 2 | 7 ± 2 | 0.93 |
| AIS—B patients | 6 ± 2 | 7 ± 2 | 7 ± 2 |
| Clinical Scales | Group Analysis | Median (IQR) | p-Value | |
|---|---|---|---|---|
| BUT-A (GSI) | T0–T0 between groups | CG A T0/EG A T0 | 0.74 (0.4)–1.1 (0.9) | 0.60 |
| CG B T0/EG B T0 | 1.04 (0.4)–0.7 (0.5) | 0.14 | ||
| CG A T0/CG B T0 | 0.74 (0.4)–1.04 (0.4) | 0.12 | ||
| EG A T0/EG B T0 | 1.1 (0.9)–0.7 (0.5) | 0.31 | ||
| T1–T1 between groups | CG A T1/EG A T1 | 0.62 (0.5)–0.45 (0.7) | 0.49 | |
| CG B T1/EG B T1 | 0.9 (0.5)–0.14 (0.3) | <0.001 * | ||
| CG A T1/CG B T1 | 0.62 (0.5)–0.9 (0.5) | 0.18 | ||
| EG A T1/EG B T1 | 0.45 (0.7)–0.14 (0.3) | 0.18 | ||
| T0–T1 within group | CG A T0/CG A T1 | 0.74 (0.4)–0.62 (0.5) | 0.09 | |
| CG B T0/CG B T1 | 1.04 (0.4)–0.9 (0.5) | 0.09 | ||
| EG A T0/EG A T1 | 1.1 (0.9)–0.45 (0.7) | <0.001 * | ||
| EGB T0/EG B T1 | 0.7 (0.5)–0.14 (0.3) | <0.001 * | ||
| BUT-B (PSDI) | T0–T0 between groups | CG A T0/EG A T0 | 2.01 (0.5)–2.9 (1.7) | 0.10 |
| CG B T0/EG B T0 | 3.03 (3.1)–3 (1.6) | 0.95 | ||
| CG A T0/CG B T0 | 2.01 (0.5)–3.03 (3.1) | 0.32 | ||
| EG A T0/EG B T0 | 2.9 (1.7)–3 (1.6) | 0.91 | ||
| T1–T1 between groups | CG A T1/EG A T1 | 1.5 (0.5)–1.22 (1.3) | 0.54 | |
| CG B T1/EG B T1 | 2.7 (3.1)–1.3 (1.0) | 0.17 | ||
| CG A T1/CG B T1 | 1.5 (0.5)–2.7 (3.1) | 0.25 | ||
| EG A T1/EG B T1 | 1.22 (1.3)–1.3 (1.0) | 0.88 | ||
| T0–T1 within group | CG A T0/CG A T1 | 2.01 (0.5)–1.5 (0.5) | 0.01 | |
| CG B T0/CG B T1 | 3.03 (3.1)–2.7 (3.1) | 0.07 | ||
| EG A T0/EG A T1 | 2.9 (1.7)–1.22 (1.3) | 0.01 | ||
| EGB T0/EG B T1 | 3 (1.6)–1.3 (1.0) | <0.001 * | ||
| BUT-B LL | T0–T0 between groups | CG A T0/EG A T0 | 2.9 (3.7)–3.6 (6.0) | 0.76 |
| CG B T0/EG B T0 | 5.6 (3.7)–2.9 (2.7) | 0.07 | ||
| CG A T0/CG B T0 | 2.9 (3.7)–5.6 (3.7) | 0.11 | ||
| EG A T0/EG B T0 | 3.6 (6.0)–2.9 (2.7) | 0.73 | ||
| T1–T1 between groups | CG A T1/EG A T1 | 3.1 (2.7)–1.3 (3.4) | 0.21 | |
| CG B T1/EG B T1 | 4.9 (3.0)–0.5 (1.0) | <0.001 * | ||
| CG A T1/CG B T1 | 3.1 (2.7)–4.9 (3.0) | 0.16 | ||
| EG A T1/EG B T1 | 1.3 (3.4)–0.5 (1.0) | 0.49 | ||
| T0–T1 within group | CG A T0/CG A T1 | 2.9 (3.7)–3.1 (2.7) | 0.68 | |
| CG B T0/CG B T1 | 5.6 (3.7)–4.9 (3.0) | 0.29 | ||
| EG A T0/EG A T1 | 3.6 (6.0)–1.3 (3.4) | 0.05 | ||
| EGB T0/EG B T1 | 2.9 (2.7)–0.5 (1.0) | 0.001 * | ||
| Clinical Scales | Group Analysis | Median (IQR) | p-Value | |
|---|---|---|---|---|
| SF-12 TOTAL | T0–T0 between groups | CG A T0/EG A T0 | 27.8 (8.1)–21.1 (5.7) | 0.39 |
| CG B T0/EG B T0 | 24.5 (8.2)–26.9 (6.2) | 0.45 | ||
| CG A T0/CG B T0 | 27.8 (8.1)–24.5 (8.2) | 0.37 | ||
| EG A T0/EG B T0 | 21.1 (5.7)–26.9 (6.2) | 0.49 | ||
| T1–T1 between groups | CG A T1/EG A T1 | 30.3 (10.2)–35 (4.5) | 0.19 | |
| CG B T1/EG B T1 | 26.1 (7.5)–35.7 (6.4) | <0.001 * | ||
| CG A T1/CG B T1 | 30.3 (10.2)–26.1 (7.5) | 0.29 | ||
| EG A T1/EG B T1 | 35 (4.5)–35.7 (6.4) | 0.77 | ||
| T0–T1 within group | CG A T0/CG A T1 | 27.8 (8.1)–30.3 (10.2) | 0.19 | |
| CG B T0/CG B T1 | 24.5 (8.2)–26.1 (7.5) | 0.03 | ||
| EG A T0/EG A T1 | 21.1 (5.7)–35 (4.5) | <0.001 * | ||
| EG B T0/EG B T1 | 26.9 (6.2)–35.7 (6.4) | <0.001 * | ||
| SF-12 PHYSICAL | T0–T0 between groups | CG A T0/EG A T0 | 15.4 (5.3)–12.2 (2.4) | 0.01 |
| CG B T0/EG B T0 | 13.3 (3.6)–11.9 (3.5) | 0.38 | ||
| CG A T0/CG B T0 | 15.4 (5.3)–13.3 (3.6) | 0.29 | ||
| EG A T0/EG B T0 | 12.2 (2.4)–11.9 (3.5) | 0.83 | ||
| T1–T1 between groups | CG A T1/EG A T1 | 15.2 (4.7)–16.8 (2.6) | 0.36 | |
| CG B T1/EG B T1 | 15 (3.7)–16.5 (2.4) | 0.29 | ||
| CG A T1/CG B T1 | 15.2 (4.7)–15 (3.7) | 0.91 | ||
| EG A T1/EG B T1 | 16.8 (2.6)–16.5 (2.4) | 0.75 | ||
| T0–T1 within group | CG A T0/CG A T1 | 15.4 (5.3)–15.2 (4.7) | 0.90 | |
| CG B T0/CG B T1 | 13.3 (3.6)–15 (3.7) | 0.07 | ||
| EG A T0/EG A T1 | 12.2 (2.4)–16.8 (2.6) | <0.001 * | ||
| EGB T0/EG B T1 | 11.9 (3.5)–16.5 (2.4) | <0.001 * | ||
| BDI | T0–T0 between groups | CG A T0/EG A T0 | 17.5 (6.3)–12.5 (7.3) | 0.12 |
| CG B T0/EG B T0 | 14.5 (6.1)–107 (7.6) | 0.21 | ||
| CG A T0/CG B T0 | 17.5 (6.3)–14.5 (6.1) | 0.29 | ||
| EG A T0/EG B T0 | 15.5 (7.3)–10.7 (7.6) | 0.59 | ||
| T1–T1 between groups | CG A T1/EG A T1 | 16.6 (4.3)–5.6 (6.3) | <0.001 * | |
| CG B T1/EG B T1 | 12.5 (6.2)–5.2 (4.8) | 0.005 | ||
| CG A T1/CG B T1 | 16.6 (4.3)–12.5 (6.2) | 0.09 | ||
| EG A T1/EG B T1 | 5.6 (6.3)–5.2 (4.8) | 0.87 | ||
| T0–T1 within group | CG A T0/CG A T1 | 17.5 (6.3)–16.6 (14.3) | 0.43 | |
| CG B T0/CG B T1 | 14.5 (6.1)–12.5 (6.2) | <0.001 * | ||
| EG A T0/EG A T1 | 12.5 (7.3)–5.6 (6.3) | <0.001 * | ||
| EG B T0/EG B T1 | 10.7 (7.6)–5.2 (4.8) | 0.14 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggio, M.G.; Naro, A.; De Luca, R.; Latella, D.; Balletta, T.; Caccamo, L.; Pioggia, G.; Bruschetta, D.; Calabrò, R.S. Body Representation in Patients with Severe Spinal Cord Injury: A Pilot Study on the Promising Role of Powered Exoskeleton for Gait Training. J. Pers. Med. 2022, 12, 619. https://doi.org/10.3390/jpm12040619
Maggio MG, Naro A, De Luca R, Latella D, Balletta T, Caccamo L, Pioggia G, Bruschetta D, Calabrò RS. Body Representation in Patients with Severe Spinal Cord Injury: A Pilot Study on the Promising Role of Powered Exoskeleton for Gait Training. Journal of Personalized Medicine. 2022; 12(4):619. https://doi.org/10.3390/jpm12040619
Chicago/Turabian StyleMaggio, Maria Grazia, Antonino Naro, Rosaria De Luca, Desiree Latella, Tina Balletta, Lory Caccamo, Giovanni Pioggia, Daniele Bruschetta, and Rocco Salvatore Calabrò. 2022. "Body Representation in Patients with Severe Spinal Cord Injury: A Pilot Study on the Promising Role of Powered Exoskeleton for Gait Training" Journal of Personalized Medicine 12, no. 4: 619. https://doi.org/10.3390/jpm12040619
APA StyleMaggio, M. G., Naro, A., De Luca, R., Latella, D., Balletta, T., Caccamo, L., Pioggia, G., Bruschetta, D., & Calabrò, R. S. (2022). Body Representation in Patients with Severe Spinal Cord Injury: A Pilot Study on the Promising Role of Powered Exoskeleton for Gait Training. Journal of Personalized Medicine, 12(4), 619. https://doi.org/10.3390/jpm12040619









