An Individualized Approach of Multidisciplinary Heart Team for Myocardial Revascularization and Valvular Heart Disease—State of Art
Abstract
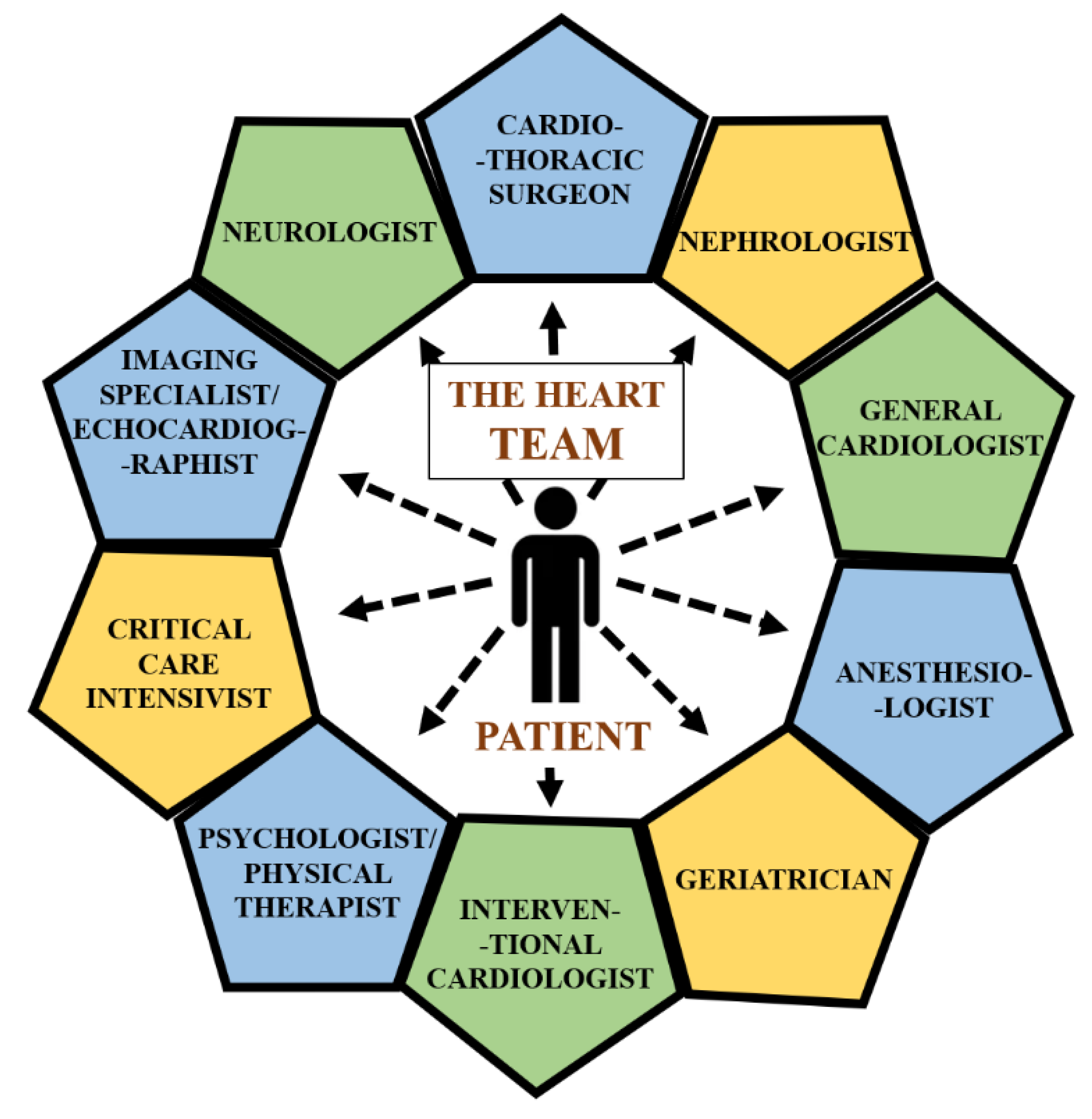
:1. Introduction
2. Heart Team for Myocardial Revascularization
3. Heart Team for Aortic Valve Stenosis
4. Heart Team for Mitral Regurgitation
5. Limitations
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L.; et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012, 126, 3097–3137. [Google Scholar] [CrossRef] [Green Version]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef] [Green Version]
- Beyersdorf, F.; Vahanian, A.; Milojevic, M.; Praz, F.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardio-Thorac. Surg. 2021, 60, 727–800. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef]
- Murphy, M.L.; Hultgren, H.N.; Detre, K.; Thomsen, J.; Takaro, T. Treatment of chronic stable angina. A preliminary report of survival data of the randomized Veterans Administration cooperative study. N. Engl. J. Med. 1977, 297, 621–627. [Google Scholar] [CrossRef]
- Prospective randomised study of coronary artery bypass surgery in stable angina pectoris. Second interim report by the European Coronary Surgery Study Group. Lancet 1980, 2, 491–495. [PubMed]
- Serruys, P.W.; Morice, M.-C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; Ståhle, E.; Feldman, T.E.; Brand, M.V.D.; Bass, E.J.; et al. Percutaneous Coronary Intervention versus Coronary-Artery Bypass Grafting for Severe Coronary Artery Disease. N. Engl. J. Med. 2009, 360, 961–972. [Google Scholar] [CrossRef]
- Head, S.J.; Davierwala, P.M.; Serruys, P.W.; Redwood, S.R.; Colombo, A.; Mack, M.J.; Morice, M.-C.; Holmes, D.R.; Feldman, T.E.; Ståhle, E.; et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: Final five-year follow-up of the SYNTAX trial. Eur. Heart J. 2014, 35, 2821–2830. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Onuma, Y.; Andreini, D.; Sonck, J.; Pompilio, G.; Mushtaq, S.; La Meir, M.; Miyazaki, Y.; De Mey, J.; Gaemperli, O.; et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur. Heart J. 2018, 39, 3689–3698. [Google Scholar] [CrossRef] [PubMed]
- Andreini, D.; Modolo, R.; Katagiri, Y.; Mushtaq, S.; Sonck, J.; Collet, C.; De Martini, S.; Roberto, M.; Tanaka, K.; Miyazaki, Y.; et al. Impact of Fractional Flow Reserve Derived From Coronary Computed Tomography Angiography on Heart Team Treatment Decision-Making in Patients With Multivessel Coronary Artery Disease. Circ. Cardiovasc. Interv. 2019, 12, e007607. [Google Scholar] [CrossRef]
- Banning, A.P.; Serruys, P.; De Maria, G.L.; Ryan, N.; Walsh, S.; Gonzalo, N.; van Geuns, R.J.; Onuma, Y.; Sabate, M.; Davies, J.; et al. Impact of Fractional Flow Reserve Derived From Coronary Computed Tomography Angiography on Heart Team Treatment Decision-Making in Patients With Multivessel Coronary Artery Disease. Insights From the SYNTAX III REVOLUTION Trial. Eur. Heart J. 2022, 43, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Bonzel, T.; Schächinger, V.; Dörge, H. Description of a Heart Team approach to coronary revascularization and its beneficial long-term effect on clinical events after PCI. Clin. Res. Cardiol. 2016, 105, 388–400. [Google Scholar] [CrossRef]
- Abdulrahman, M.; Alsabbagh, A.; Kuntze, T.; Lauer, B.; Ohlow, M.A. Impact of Hierarchy on Multidisciplinary Heart-Team Recommendations in Patients with Isolated Multivessel Coronary Artery Disease. J. Clin. Med. 2019, 8, 1490. [Google Scholar] [CrossRef] [Green Version]
- Patterson, T.; McConkey, H.Z.; Ahmed-Jushuf, F.; Moschonas, K.; Nguyen, H.; Karamasis, G.V.; Perera, D.; Clapp, B.R.; Roxburgh, J.; Blauth, C.; et al. Long-Term Outcomes Following Heart Team Revascularization Recommendations in Complex Coronary Artery Disease. J. Am. Heart Assoc. 2019, 8, 011279. [Google Scholar] [CrossRef]
- Domingues, C.T.; Milojevic, M.; Thuijs, D.J.F.M.; Van Mieghem, N.M.; Daemen, J.; Van Domburg, R.T.; Kappetein, A.P.; Head, S.J. Heart Team decision making and long-term outcomes for 1000 consecutive cases of coronary artery disease. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 206–213. [Google Scholar] [CrossRef]
- Young, M.N.; Kolte, D.; Cadigan, M.E.; Laikhter, E.; Sinclair, K.; Pomerantsev, E.; Fifer, M.A.; Sundt, T.M.; Yeh, R.W.; Jaffer, F.A. Multidisciplinary Heart Team Approach for Complex Coronary Artery Disease: Single Center Clinical Presentation. J. Am. Heart Assoc. 2020, 9, e014738. [Google Scholar] [CrossRef]
- Kezerle, L.; Yohanan, E.; Cohen, A.; Merkin, M.; Ishay, Y.; Weinstein, J.M.; Cafri, C. The impact of Heart Team discussion on decision making for coronary revascularization in patients with complex coronary artery disease. J. Card. Surg. 2020, 35, 2719–2724. [Google Scholar] [CrossRef]
- Tsang, M.B.; Schwalm, J.D.; Gandhi, S.; Sibbald, M.G.; Gafni, A.; Mercuri, M.; Salehian, O.; Lamy, A.; Pericak, D.; Jolly, S.; et al. Comparison of Heart Team vs Interventional Cardiologist Recommendations for the Treatment of Patients With Multivessel Coronary Artery Disease. JAMA Netw. Open 2020, 3, e2012749. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, J.C.; Mercado, N. Treatment Recommendations for Patients With Multivessel Coronary Artery Disease—There Is No “I” in Heart Team, But Is the Heart Team Better Than the I? JAMA Netw. Open 2020, 3, e2013098. [Google Scholar] [CrossRef] [PubMed]
- Jonik, S.; Marchel, M.; Pędzich-Placha, E.; Pietrasik, A.; Rdzanek, A.; Huczek, Z.; Kochman, J.; Budnik, M.; Piątkowski, R.; Scisło, P.; et al. Optimal management of patients with severe coronary artery disease following multidisciplinary Heart Team discussion—Insights from tertiary cardiovascular care center. Int. J. Environ. Res. Public Health 2022, 19, 3933. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef] [Green Version]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef] [Green Version]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Dubois, C.; Coosemans, M.; Rega, F.; Poortmans, G.; Belmans, A.; Adriaenssens, T.; Herregods, M.-C.; Goetschalckx, K.; Desmet, W.; Janssens, S.; et al. Prospective evaluation of clinical outcomes in all-comer high-risk patients with aortic valve stenosis undergoing medical treatment, transcatheter or surgical aortic valve implantation following heart team assessment. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 492–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thyregod, H.G.H.; Holmberg, F.; Gerds, T.A.; Ihlemann, N.; Søndergaard, L.; Steinbrüchel, D.A.; Olsen, P.S. Heart Team therapeutic decision-making and treatment in severe aortic valve stenosis. Scand. Cardiovasc. J. 2016, 50, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Bakelants, E.; Belmans, A.; Verbrugghe, P.; Adriaenssens, T.; Jacobs, S.; Bennett, J.; Meuris, B.; Desmet, W.; Rega, F.; Herijgers, P.; et al. Clinical outcomes of heart-team-guided treatment decisions in high-risk patients with aortic valve stenosis in a health-economic context with limited resources for transcatheter valve therapies. Acta Cardiol. 2018, 74, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Rea, C.W.; Wang, T.K.M.; Ruygrok, P.N.; Sidhu, K.; Ramanathan, T.; Nand, P.; Stewart, J.T.; Webster, M.W. Characteristics and Outcomes of Patients With Severe Aortic Stenosis Discussed by the Multidisciplinary “Heart Team” According to Treatment Allocation. Heart Lung Circ. 2020, 29, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Conte, G.; Espejo-Paeres, C.; Nombela-Franco, L.; Jimenez-Quevedo, P.; Cobiella, J.; Vivas, D.; De Agustín, J.A.; McInerney, A.; Pozo, E.; Salinas, P.; et al. Performance of the heart team approach in daily clinical practice in high-risk patients with aortic stenosis. J. Card. Surg. 2021, 36, 31–39. [Google Scholar] [CrossRef]
- Jonik, S.; Marchel, M.; Pędzich-Placha, E.; Huczek, Z.; Kochman, J.; Ścisło, P.; Czub, P.; Wilimski, R.; Hendzel, P.; Opolski, G.; et al. Heart Team for Optimal Management of Patients with Severe Aortic Stenosis—Long-Term Outcomes and Quality of Life from Tertiary Cardiovascular Care Center. J. Clin. Med. 2021, 10, 5408. [Google Scholar] [CrossRef]
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef]
- Feldman, T.; Kar, S.; Elmariah, S.; Smart, S.C.; Trento, A.; Siegel, R.J.; Apruzzese, P.; Fail, P.; Rinaldi, M.J.; Smalling, R.W.; et al. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. J. Am. Coll. Cardiol. 2015, 66, 2844–2854. [Google Scholar] [CrossRef] [Green Version]
- Heuts, S.; Olsthoorn, J.R.; Hermans, S.M.M.; Streukens, S.A.F.; Vainer, J.; Cheriex, E.C.; Segers, P.; Maessen, J.G.; Nia, P.S. Multidisciplinary decision-making in mitral valve disease: The mitral valve heart team. Neth. Heart J. 2019, 27, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Külling, M.; Corti, R.; Noll, G.; Küest, S.; Hürlimann, D.; Wyss, C.; Reho, I.; Tanner, F.C.; Külling, J.; Meinshausen, N.; et al. Heart team approach in treatment of mitral regurgitation: Patient selection and outcome. Open Heart 2020, 7, e001280. [Google Scholar] [CrossRef] [PubMed]
- Nia, P.S.; Olsthoorn, J.R.; Heuts, S.; van Kuijk, S.M.J.; Vainer, J.; Streukens, S.; Schalla, S.; Segers, P.; Barenbrug, P.; Crijns, H.J.G.M.; et al. Effect of a dedicated mitral heart team compared to a general heart team on survival: A retrospective, comparative, non-randomized interventional cohort study based on prospectively registered data. Eur. J. Cardio-Thorac. Surg. 2021, 60, 263–273. [Google Scholar] [CrossRef]
- Jonik, S.; Marchel, M.; Pędzich-Placha, E.; Pietrasik, A.; Rdzanek, A.; Huczek, Z.; Kochman, J.; Budnik, M.; Piątkowski, R.; Ścisło, P.; et al. Long-term outcomes and quality of life following implementation of dedicated mitral valve Heart Team decisions for patients with severe mitral valve regurgitation in tertiary cardiovascular care center. Cardiol. J. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]

| Study Type | Clinical Characteristics | Results | Ref. No. |
|---|---|---|---|
| prospective, randomized | 1800 patients with 3-VD or/and LMS: CABG—897, PCI—903 Follow-up: 12 months |
| [10], Serruys PW, et al. |
| retrospective analysis of prospective, randomized trial | 1095 patients with 3-VD: CABG—549, PCI—546 Follow-up: 5 years |
| [11], Head SJ, et al. |
| prospective, randomized | 223 patients with de novo 3-VD or LM disease Separate HTs randomized to assess the CAD with either coronary CTA or CA. |
| [12], Collet C, et al. |
| prospective, randomized | 223 patients with 3-VD or LM disease 2 HTs to decide between CABG and PCI FFR analysis in 196 patients FFRCT available for 1030 lesions |
| [13], Andreini D, et al. |
| prospective, nonrandomized | 454 patients with de novo 3-VD without LMS compared with 315 patients from the pre-defined SYNTAX PCI group and 334 patients from the pre-defined SYNTAX CABG cohort. Follow-up: 5 years |
| [14], Banning AP, et al. |
| retrospective | 3408 catheterizations with a first diagnosis of CAD 1527 patients had first PCI Follow-up: 15 years |
| [15], Bonzel T, et al. |
| retrospective | 209 patients with isolated MVD: CABG—141, PCI—59, OMT—9 Impact of hierarchy on multidisciplinary HT recommendations. |
| [16], Abdulrahman M, et al. |
| prospective | 366 patients with LMS, 2-VD, 3-VD or clinical equipoise: CABG—102, PCI—127, OMT—137 Follow-up: 3 years |
| [17], Patterson T, et al. |
| retrospective | 960 patients with CAD—69.4%—simple CAD, 30.6%—complex CAD Median (IQR) follow-up: 4.6 (4.2–5.0) years |
| [18], Dominigues CT, et al. |
| prospective | 166 high-risk patients with complex CAD: CABG—49, PCI—79, OMT—34, hybrid therapy—1 Follow-up: 3 years |
| [19], Young MN, et al. |
| prospective | 186 patients with MVD: 93—HT approach, 93—control group |
| [20], Kezerle L, et al. |
| retrospective | 234 patients with MVD originally treated as recommended by interventional cardiologists (2012–2014) compared with blinded HT treatment recommendations (2017–2018) |
| [21], Tsang MB, et al. Comment: [22], Blankenship JC, et al. |
| retrospective | 1286 patients with 3-VD or/and LMS: CABG—356, PCI—679, OMT—251 Mean (SD) follow-up: 37 (14) months |
| [23], Jonik S, et al. |
| Study Type | Clinical Characteristics | Results | Ref. No. |
|---|---|---|---|
| prospective | 163 high-risk patients with symptomatic AS: SAVR—35, TAVI—73, OMT/PTAV—55 Median (IQR) follow-up: 38 (12–42) months for SAVR, 25 (12–40) months for TAVI, 32 (18–41) months for OMT/PTAV |
| [31], Dubois C, et al. |
| retrospective | 487 patients with severe AS: SAVR—392, TAVI—60, OMT—35 Median (IQR) follow-up: 3.5 (1.87–3.53) years |
| [32], Thyregod HGH, et al. |
| prospective | 405 high-risk patients with AS: SAVR—98, TAVI—188, OMT/PTAV—116 Median follow-up: 12 months |
| [33], Bakelants E, et al. |
| retrospective | 243 patients with severe AS: SAVR—26, TAVI—200, OMT—17 Mean (SD) follow-up: 2.0 (1.4) years |
| [34], Rea CW, et al. |
| prospective | 286 patients with AS: SAVR—53, TAVR—210, OMT—23 Median (IQR) follow-up: 18 (11–26) months |
| [35], Tirado-Conte G, et al. |
| retrospective | 482 patients with severe AS: SAVR—85, TAVR—318, OMT—79 Median follow-up: 866 days |
| [36], Jonik S, et al. |
| Study Type | Clinical Characteristics | Results | Ref. No. |
|---|---|---|---|
| prospective | 158 patients with MV pathology with or without concomitant cardiac disesase: Surgery—67 (MVR or MVP; isolated or concomitant), percutaneous—20 (MC or MVA), OMT—71 30-days mortality and MACCE An estimated (Kaplan-Meier) overall survival with median follow-up: 450 days |
| [40], Heuts S, et al. |
| retrospective | 400 patients with MR: MVR—36, MVP—185, MC—179 Mean (SD) follow-up: 32.2 (17.6) months |
| [41], Külling M, et al. |
| retrospective | 1145 patients with MV disesase: 641—discussed by dedicated mitral HT (surgery—289, transcatheter—101, OMT—251); 504—discussed by general HT (surgery—285, MC—7, OMT—212) Median (IQR) follow-up: 41.1 (22.8–60.0) months |
| [42], Sardari Nia P, et al. |
| retrospective | 157 patients with severe MR: MVR—46, MC—58, OMT—53 Mean (SD) follow-up: 29 (15) months |
| [43], Jonik S, et al. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonik, S.; Marchel, M.; Huczek, Z.; Kochman, J.; Wilimski, R.; Kuśmierczyk, M.; Grabowski, M.; Opolski, G.; Mazurek, T. An Individualized Approach of Multidisciplinary Heart Team for Myocardial Revascularization and Valvular Heart Disease—State of Art. J. Pers. Med. 2022, 12, 705. https://doi.org/10.3390/jpm12050705
Jonik S, Marchel M, Huczek Z, Kochman J, Wilimski R, Kuśmierczyk M, Grabowski M, Opolski G, Mazurek T. An Individualized Approach of Multidisciplinary Heart Team for Myocardial Revascularization and Valvular Heart Disease—State of Art. Journal of Personalized Medicine. 2022; 12(5):705. https://doi.org/10.3390/jpm12050705
Chicago/Turabian StyleJonik, Szymon, Michał Marchel, Zenon Huczek, Janusz Kochman, Radosław Wilimski, Mariusz Kuśmierczyk, Marcin Grabowski, Grzegorz Opolski, and Tomasz Mazurek. 2022. "An Individualized Approach of Multidisciplinary Heart Team for Myocardial Revascularization and Valvular Heart Disease—State of Art" Journal of Personalized Medicine 12, no. 5: 705. https://doi.org/10.3390/jpm12050705
APA StyleJonik, S., Marchel, M., Huczek, Z., Kochman, J., Wilimski, R., Kuśmierczyk, M., Grabowski, M., Opolski, G., & Mazurek, T. (2022). An Individualized Approach of Multidisciplinary Heart Team for Myocardial Revascularization and Valvular Heart Disease—State of Art. Journal of Personalized Medicine, 12(5), 705. https://doi.org/10.3390/jpm12050705








