Radiography and Computed Tomography Detection of Intimal and Medial Calcifications in Leg Arteries in Comparison to Histology
Abstract
:1. Introduction
2. Materials and Methods
3. Results
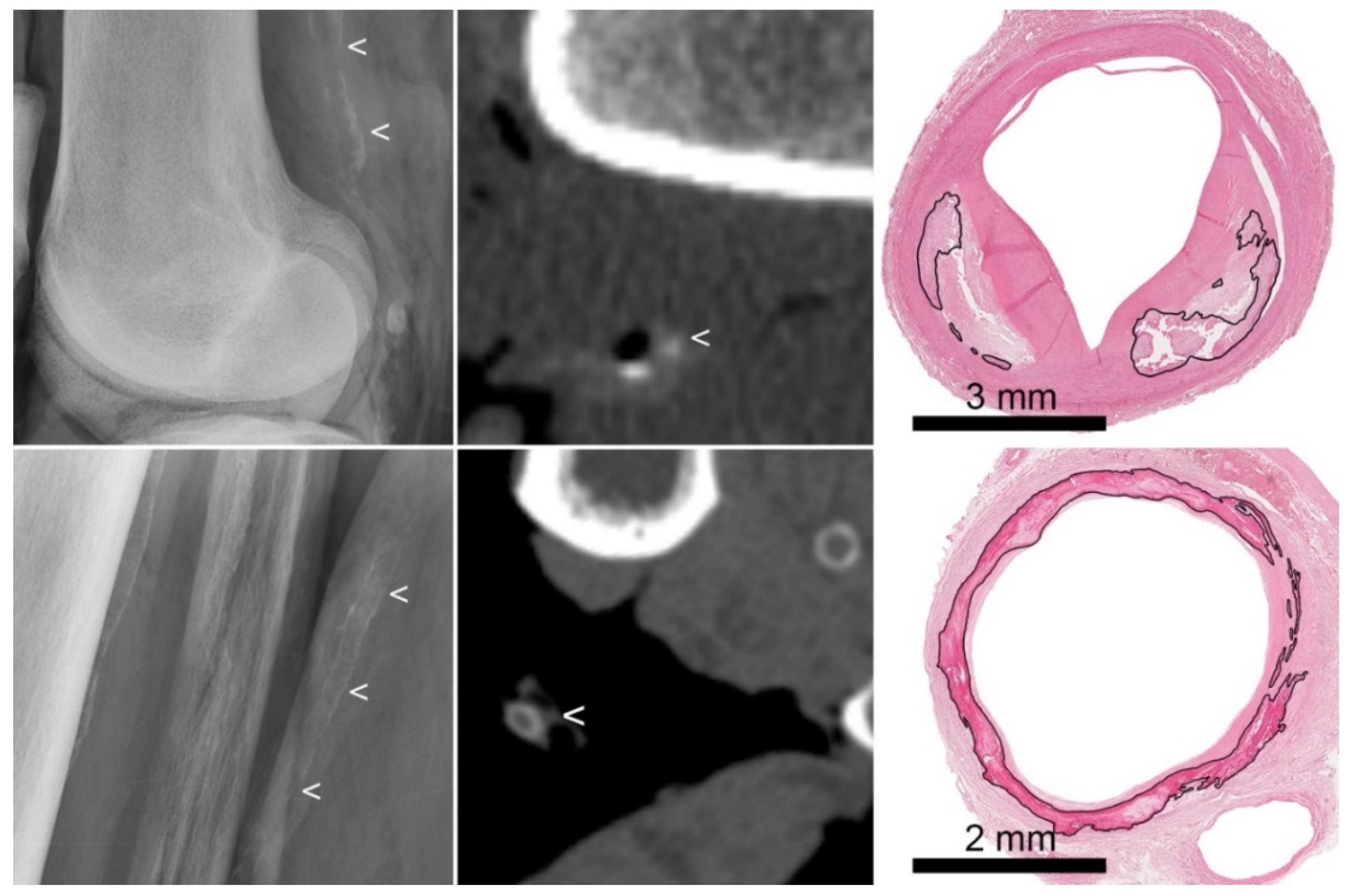
3.1. Histology
3.2. Radiography
3.3. Computed Tomography
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha-Singh, K.J.; Zeller, T.; Jaff, M.R. Peripheral arterial calcification: Prevalence, mechanism, detection, and clinical implications. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2014, 83, E212–E220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, W.C.; Han, K.H.; Schneider, T.M.; Hennigar, R.A. Prevalence of nonatheromatous lesions in peripheral arterial disease. Arter. Thromb. Vasc. Biol. 2015, 35, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Narula, N.; Dannenberg, A.J.; Olin, J.W.; Bhatt, D.L.; Johnson, K.W.; Nadkarni, G.; Min, J.; Torii, S.; Poojary, P.; Anand, S.S.; et al. Pathology of Peripheral Artery Disease in Patients With Critical Limb Ischemia. J. Am. Coll. Cardiol. 2018, 72, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Mustapha, J.A.; Narula, J.; Mori, H.; Saab, F.; Jinnouchi, H.; Yahagi, K.; Sakamoto, A.; Romero, M.E.; Narula, N.; et al. Histopathologic Characterization of Peripheral Arteries in Subjects With Abundant Risk Factors: Correlating Imaging With Pathology. JACC Cardiovasc. Imaging 2019, 12, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Soor, G.S.; Vukin, I.; Leong, S.W.; Oreopoulos, G.; Butany, J. Peripheral vascular disease: Who gets it and why? A histomorphological analysis of 261 arterial segments from 58 cases. Pathology 2008, 40, 385–391. [Google Scholar] [CrossRef]
- Ho, C.Y.; Shanahan, C.M. Medial Arterial Calcification: An Overlooked Player in Peripheral Arterial Disease. Arter. Thromb. Vasc. Biol. 2016, 36, 1475–1482. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, E.J.; de Jong, P.A.; Beulens, J.W.; Mali, W.P.; van der Schouw, Y.T.; Beijerinck, D. Medial Arterial Calcification: Active Reversible Disease in Human Breast Arteries. JACC Cardiovasc. Imaging 2015, 8, 984–985. [Google Scholar] [CrossRef] [Green Version]
- Edouard, T.; Chabot, G.; Miro, J.; Buhas, D.C.; Nitschke, Y.; Lapierre, C.; Rutsch, F.; Alos, N. Efficacy and safety of 2-year etidronate treatment in a child with generalized arterial calcification of infancy. Eur. J. Pediat. 2011, 170, 1585–1590. [Google Scholar] [CrossRef]
- Kranenburg, G.; de Jong, P.A.; Bartstra, J.W.; Lagerweij, S.J.; Lam, M.G.; Ossewaarde-van Norel, J.; Risseeuw, S.; van Leeuwen, R.; Imhof, S.M.; Verhaar, H.J.; et al. Etidronate for Prevention of Ectopic Mineralization in Patients With Pseudoxanthoma Elasticum. J. Am. Coll. Cardiol. 2018, 71, 1117–1126. [Google Scholar] [CrossRef]
- Orr, D.P.; Myerowitz, R.L.; Herbert, D.L.; Friday, P. Correlation of radiographic and histologic findings in arterial calcification. Investig. Radiol. 1978, 13, 110–114. [Google Scholar] [CrossRef]
- Kockelkoren, R.; Vos, A.; Van Hecke, W.; Vink, A.; Bleys, R.L.; Verdoorn, D.; Mali, W.P.; Hendrikse, J.; Koek, H.L.; de Jong, P.A.; et al. Computed Tomographic Distinction of Intimal and Medial Calcification in the Intracranial Internal Carotid Artery. PLoS ONE 2017, 12, e0168360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, A.; Van Hecke, W.; Spliet, W.G.; Goldschmeding, R.; Isgum, I.; Kockelkoren, R.; Bleys, R.L.; Mali, W.P.; de Jong, P.A.; Vink, A. Predominance of Nonatherosclerotic Internal Elastic Lamina Calcification in the Intracranial Internal Carotid Artery. Stroke 2016, 47, 221–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meershoek, A.; van Dijk, R.A.; Verhage, S.; Hamming, J.F.; van den Bogaerdt, A.J.; Bogers, A.J.; Schaapherder, A.F.; Lindeman, J.H. Histological evaluation disqualifies IMT and calcification scores as surrogates for grading coronary and aortic atherosclerosis. Int. J. Cardiol. 2016, 224, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arter. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fishbein, M.C.; Fishbein, G.A. Arteriosclerosis: Facts and fancy. Cardiovasc. Pathol. 2015, 24, 335–342. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Schantl, A.E.; Ivarsson, M.E.; Leroux, J. Investigational Pharmacological Treatments for Vascular Calcification. Adv. Therap. 2019, 2, 1800094. [Google Scholar] [CrossRef] [Green Version]
- Janssen, T.; Bannas, P.; Herrmann, J.; Veldhoen, S.; Busch, J.D.; Treszl, A.; Munster, S.; Mester, J.; Derlin, T. Association of linear (1)(8)F-sodium fluoride accumulation in femoral arteries as a measure of diffuse calcification with cardiovascular risk factors: A PET/CT study. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2013, 20, 569–577. [Google Scholar] [CrossRef]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.; Hiatt, W.R.; Jonsson, B.; Lacroix, P.; et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Tang, G.; Xu, X.; Yan, L.; Liang, M.; Zhang, W.; Liu, X.; Luo, B. Different ultrasound scoring methods for assessing medial arterial calcification: Association with diabetic complications. Ultrasound Med. Biol. 2020, 46, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
| Histology | ||||
|---|---|---|---|---|
| Absent | Intima | Media | ||
| Radiography | Absent | 18 | 1 | 5 |
| Intima | 0 | 12 | 0 | |
| Media | 0 | 5 | 7 | |
| CT | Absent | 34 | 3 | 9 |
| Intima | 3 | 18 | 3 | |
| Media | 0 | 6 | 15 | |
| Indistinguishable | 1 | 2 | 2 | |
| Resident | ||||
|---|---|---|---|---|
| Absent | Intima | Media | ||
| Experienced observer | Absent | 9 | 2 | 13 |
| Intima | 0 | 2 | 10 | |
| Media | 0 | 3 | 9 | |
| Resident | |||||
|---|---|---|---|---|---|
| Absent | Intima | Media | Indistinguishable | ||
| Experienced observer | Absent | 31 | 10 | 3 | 2 |
| Intima | 0 | 9 | 10 | 5 | |
| Media | 0 | 5 | 15 | 1 | |
| Indistinguishable | 0 | 5 | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vos, A.; Vink, A.; Kockelkoren, R.; Takx, R.A.P.; Celeng, C.; Mali, W.P.T.M.; Isgum, I.; Bleys, R.L.A.W.; de Jong, P.A. Radiography and Computed Tomography Detection of Intimal and Medial Calcifications in Leg Arteries in Comparison to Histology. J. Pers. Med. 2022, 12, 711. https://doi.org/10.3390/jpm12050711
Vos A, Vink A, Kockelkoren R, Takx RAP, Celeng C, Mali WPTM, Isgum I, Bleys RLAW, de Jong PA. Radiography and Computed Tomography Detection of Intimal and Medial Calcifications in Leg Arteries in Comparison to Histology. Journal of Personalized Medicine. 2022; 12(5):711. https://doi.org/10.3390/jpm12050711
Chicago/Turabian StyleVos, Annelotte, Aryan Vink, Remko Kockelkoren, Richard A. P. Takx, Csilla Celeng, Willem P. T. M. Mali, Ivana Isgum, Ronald L. A. W. Bleys, and Pim A. de Jong. 2022. "Radiography and Computed Tomography Detection of Intimal and Medial Calcifications in Leg Arteries in Comparison to Histology" Journal of Personalized Medicine 12, no. 5: 711. https://doi.org/10.3390/jpm12050711
APA StyleVos, A., Vink, A., Kockelkoren, R., Takx, R. A. P., Celeng, C., Mali, W. P. T. M., Isgum, I., Bleys, R. L. A. W., & de Jong, P. A. (2022). Radiography and Computed Tomography Detection of Intimal and Medial Calcifications in Leg Arteries in Comparison to Histology. Journal of Personalized Medicine, 12(5), 711. https://doi.org/10.3390/jpm12050711







