Modulation of Secondary Cancer Risks from Radiation Exposure by Sex, Age and Gonadal Hormone Status: Progress, Opportunities and Challenges
Abstract
:1. Introduction
2. Sex and Age Modulate Cancer Risks from Wartime Exposure to External Radiation
3. Sex and Age Modulate Secondary Cancers Attributable to External Radiation Exposure in Medical Practice
3.1. Radiation Therapy
3.2. Sex and Age Modulate Secondary Cancers Attributable to Radiation from Medical Imaging
4. Effects of Accidental Exposure to Internal Radiation
5. Sex, Hormonal Status and Targeted Radionuclide Therapy: A Challenge and an Opportunity
5.1. Sex and Age Modulate Cancer Risks from Radioactive Iodine Therapy of Thyroid Disease
5.2. TRT for Central Nervous System, Neuroendocrine and Other Tumors: Too Early to Call?
5.3. The Challenge of TRT Risk Prediction and Modulation in Females
6. Summary and Path Forward
- Solid tumor incidence secondary to exposure following the atomic bombing of Hiroshima and Nagasaki, the largest cohort subjected to continued surveillance over more than 60 years, is markedly higher (~2-fold) in women.
- This excess risk varies by organ and age at exposure, with the largest sex differences (6- to 10-fold) found in female thyroid and breasts exposed between birth and menopause (~50 years old) when compared to males in the same age group.
- The risk of secondary breast or thyroid cancer in females decreases more steeply with age at exposure in females compared to males.
- Secondary breast cancers take a long time (10–20 years) to manifest and the increased risk due to radiation is sustained for decades.
- The pattern of vulnerability is remarkably consistent across a variety of exposure mechanisms, radiation doses, mathematical models and risk metrics, such that young females are at a highly elevated risk of secondary solid tumors, especially breast and thyroid, when exposed to wartime, therapeutic or diagnostic radiation.
- Smaller cohort studies in humans and animals also show large changes in cell proliferation rates, radiotracer accumulation and target density in female reproductive and non-reproductive organs, including breast, thyroid and brain, in conjunction with physiological changes in gonadal hormone (especially estradiol) levels such as occur during the menstrual cycle and menopause. NB, while these effects may explain higher propensity of females to radiation induced cancers of the breast and the thyroid, they do not explain the sex difference in lung cancer, the biological origin of whch is still unknown.
Author Contributions
Funding
Conflicts of Interest
References
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamada, N.; Fujimichi, Y. Classification of radiation effects for dose limitation purposes: History, current situation and future prospects. J. Radiat. Res. 2014, 55, 629–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of the Environment, Government of Japan. BOOKLET to Provide Basic Information Regarding Health Effects of Radiation. 2019. Available online: https://www.env.go.jp/en/chemi/rhm/basic-info/1st/10-03-11.html (accessed on 30 January 2020).
- National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII-Phase 2; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Sadetzki, S.; Chetrit, A.; Lubina, A.; Stovall, M.; Novikov, I. Risk of thyroid cancer after childhood exposure to ionizing radiation for tinea capitis. J. Clin. Endocrinol. Metab. 2006, 91, 4798–4804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, S.R.; Kitahara, C.M.; Cha, E.S.; Kim, S.; Bang, Y.J.; Lee, W.J. Cancer Risk After Radioactive Iodine Treatment for Hyperthyroidism: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2125072. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Law, M.W.; Khong, P.L. Whole-body PET/CT Scanning: Estimation of Radiation Dose and Cancer Risk. Radiology 2009, 251, 166–174. [Google Scholar] [CrossRef]
- Rampinelli, C.; De Marco, P.; Origgi, D.; Maisonneuve, P.; Casiraghi, M.; Veronesi, G.; Spaggiari, L.; Bellomi, M. Exposure to Low Dose Computed Tomography for Lung Cancer Screening and Risk of Cancer: Secondary Analysis of Trial Data and Risk-Benefit Analysis. BMJ 2017, 356, j347. [Google Scholar] [CrossRef] [Green Version]
- Musgrove, E.A.; Sutherland, R.L. Cell cycle control by steroid hormones. Semin. Cancer Biol. 1994, 5, 381–389. [Google Scholar]
- Forster, C.; Makela, S.; Warri, A.; Kietz, S.; Becker, D.; Hultenby, K.; Warner, M.; Gustafsson, J.A. Involvement of estrogen receptor beta in terminal differentiation of mammary gland epithelium. Proc. Natl. Acad. Sci. USA 2002, 99, 15578–15583. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wei, F.; Zhang, J.; Hao, L.; Jiang, J.; Dang, L.; Mei, D.; Fan, S.; Yu, Y.; Jiang, L. Bisphenol A and estrogen induce proliferation of human thyroid tumor cells via an estrogen-receptor-dependent pathway. Arch. Biochem. Biophys. 2017, 633, 29–39. [Google Scholar] [CrossRef]
- Committee Examining Radiation Risks of Internal Emitters. Report of the Committee Examining Radiation Risks of Internal Emitters (CERRIE); Committee Examining Radiation Risks of Internal Emitters: Chilton, UK, 2004; ISBN 0-85951-545-1. Available online: www.cerrie.org (accessed on 6 February 2022).
- Jacob, P.; Kenigsberg, Y.; Zvonova, I.; Goulko, G.; Buglova, E.; Heidenreich, W.F.; Golovneva, A.; Bratilova, A.A.; Drozdovitch, V.; Kruk, J.; et al. Childhood exposure due to the Chernobyl accident and thyroid cancer risk in contaminated areas of Belarus and Russia. Br. J. Cancer 1999, 80, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Farahati, J.; Demidchik, E.P.; Biko, J.; Reiners, C. Inverse association between age at the time of radiation exposure and extent of disease in cases of radiation-induced childhood thyroid carcinoma in Belarus. Cancer 2000, 88, 1470–1476. [Google Scholar] [CrossRef]
- Moysich, K.B.; Menezes, R.J.; Michalek, A.M. Chernobyl-related ionising radiation exposure and cancer risk: An epidemiological review. Lancet Oncol. 2002, 3, 269–279. [Google Scholar] [CrossRef]
- Williams, D. Cancer after nuclear fallout: Lessons from the Chernobyl accident. Nat. Rev. Cancer 2002, 2, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Eskin, B.A.; Parker, J.A.; Bassett, J.G.; George, D.L. Human Breast Uptake of Radioactive Iodine. Obstet. Gynecol. 1974, 44, 398–402. [Google Scholar] [PubMed]
- Kitahara, C.M.; de Gonzalez, A.B.; Bouville, A.; Brill, A.B.; Doody, M.M.; Melo, D.R.; Simon, S.L.; Sosa, J.A.; Tulchinsky, M.; Villoing, D.; et al. Association of Radioactive Iodine Treatment With Cancer Mortality in Patients with Hyperthyroidism. JAMA Intern. Med. 2019, 179, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, B.S.; Siegel, J.A.; Hassan, A.; Silberstein, E.B. There is No Association of Radioactive Iodine Treatment with Cancer Mortality in Patients with Hyperthyroidism. J. Nucl. Med. 2019, 60, 1500–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudkov, S.V.; Shilyagina, N.Y.; Vodeneev, V.A.; Zvyagin, A.V. Targeted Radionuclide Therapy of Human Tumors. Int. J. Mol. Sci. 2015, 17, 33. [Google Scholar] [CrossRef]
- Goldsmith, S.J. Targeted Radionuclide Therapy: A Historical and Personal Review. Semin. Nucl. Med. 2020, 50, 87–97. [Google Scholar] [CrossRef]
- Thompson, D.E.; Mabuchi, K.; Ron, E.; Soda, M.; Tokunaga, M.; Ochikubo, S.; Sugimoto, S.; Ikeda, T.; Terasaki, M.; Izumi, S.; et al. Cancer incidence in atomic bomb survivors. Part II: Solid tumors, 1958–1987. Radiat. Res. 1994, 137, S17–S67. [Google Scholar] [CrossRef]
- Preston, D.L.; Kusumi, S.; Tomonaga, M.; Izumi, S.; Ron, E.; Kuramoto, A.; Dohy, N.; Matsuo, T. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950–1987. Radiat. Res. 1994, 137, S68–S97. [Google Scholar] [CrossRef]
- Furukawa, K.; Preston, D.; Funamoto, S.; Yonehara, S.; Ito, M.; Tokuoka, S.; Sugiyama, H.; Soda, M.; Ozasa, K.; Mabuchi, K. Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure. Int. J. Cancer 2013, 132, 1222–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozasa, K.; Shimizu, Y.; Suyama, A.; Kasagi, F.; Soda, M.; Grant, E.J.; Sakata, R.; Sugiyama, H.; Kodama, K. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: An overview of cancer and noncancer diseases. Radiat. Res. 2012, 177, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, K.; Preston, D.L.; Lönn, S.; Funamoto, S.; Yonehara, S.; Matsuo, T.; Egawa, H.; Tokuoka, S.; Ozasa, K.; Kasagi, F.; et al. Radiation and smoking effects on lung cancer incidence among atomic bomb survivors. Radiat. Res. 2010, 174, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, A.V.; Preston, D.L.; Sakata, R.; Sugiyama, H.; de Gonzalez, A.B.; French, B.; Utada, M.; Cahoon, E.K.; Sadakane, A.; Ozasa, K.; et al. Incidence of Breast Cancer in the Life Span Study of Atomic Bomb Survivors: 1958–2009. Radiat. Res. 2018, 190, 433–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirota, K.; Honjo, H.; Shintani, M. Factors affecting menopause. Adv. Obstet. Gynecol. 1995, 47, 389–392. [Google Scholar]
- Schaapveld, M.; Aleman, B.M.P.; van Eggermond, A.M.; Janus, C.P.M.; Krol, A.D.G.; van der Maazen, R.W.M.; Roesink, J.; Raemaekers, J.M.M.; de Boer, J.P.; Zijlstra, J.M.; et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef]
- Hilgendorf, I.; Bergelt, C.; Bokemeyer, C.; Kaatsch, P.; Seifart, U.; Stein, A.; Langer, T. Long-Term Follow-Up of Children, Adolescents, and Young Adult Cancer Survivors. Oncol. Res. Treat. 2021, 44, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Yasui, Y.; Robison, L.L.; Birch, J.M.; Bogue, M.K.; Diller, L.; DeLaat, C.; Fossati-Bellani, F.; Morgan, E.; Oberlin, O.; et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: Report from the Late Effects Study Group. J. Clin. Oncol. 2003, 21, 4386–4394. [Google Scholar] [CrossRef]
- Kenney, L.B.; Yasui, Y.; Inskip, P.D.; Hammond, S.; Neglia, J.P.; Mertens, A.C.; Meadows, A.T.; Friedman, D.; Robison, L.L.; Diller, L. Breast cancer after childhood cancer: A report from the Childhood Cancer Survivor Study. Ann. Intern. Med. 2004, 141, 590–597. [Google Scholar] [CrossRef]
- Travis, L.B.; Hill, D.A.; Dores, G.M.; Gospodarowicz, M.; van Leeuwen, F.E.; Holowaty, E.; Glimelius, B.; Andersson, M.; Wiklund, T.; Lynch, C.F.; et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA 2003, 290, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Travis, L.B.; Hill, D.; Dores, G.M.; Gospodarowicz, M.; van Leeuwen, F.E.; Holowaty, E.; Glimelius, B.; Andersson, M.; Pukkala, E.; Lynch, C.F.; et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J. Natl. Cancer Inst. 2005, 97, 1428–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.J.; Winter, D.L.; Stiller, C.A.; Murphy, M.; Hawkins, M.M. Risk of breast cancer in female survivors of childhood Hodgkin’s disease in Britain: A population-based study. Int. J. Cancer 2007, 120, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.O.; Amsterdam, A.; Bhatia, S.; Hudson, M.M.; Meadows, A.T.; Neglia, J.P.; Diller, L.R.; Constine, L.S.; Smith, R.A.; Mahoney, M.C.; et al. Systematic review: Surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann. Intern. Med. 2010, 152, 444–455. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, M.L.; Sparidans, J.; van’t Veer, M.B.; Noordijk, E.M.; Louwman, M.W.; Zijlstra, J.M.; van den Berg, H.; Russell, N.S.; Broeks, A.; Baaijens, M.H.; et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma: Lower risk after smaller radiation volumes. J. Clin. Oncol. 2009, 10, 4239–4246. [Google Scholar] [CrossRef] [PubMed]
- Krul, I.M.; Opstal-van Winden, A.W.J.; Aleman, B.M.P.; Janus, C.P.M.; van Eggermond, A.M.; De Bruin, M.L.; Hauptmann, M.; Krol, A.D.G.; Schaapveld, M.; Broeks, A.; et al. Breast Cancer Risk After Radiation Therapy for Hodgkin Lymphoma: Influence of Gonadal Hormone Exposure. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, C.S.; Chou, J.F.; Wolden, S.L.; Bernstein, J.L.; Malhotra, J.; Friedman, D.N.; Mubdi, N.Z.; Begg, C.B.; Oeffinger, K.C. Breast cancer after chest radiation therapy for childhood cancer. J. Clin. Oncol. 2014, 32, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Easton, D.F.; Ford, D.; Bishop, D.T. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am. J. Hum. Genet. 1995, 56, 265–271. [Google Scholar] [PubMed]
- Begg, C.B.; Haile, R.W.; Borg, A.; Malone, K.E.; Concannon, P.; Thomas, D.C.; Langholz, B.; Bernstein, L.; Olsen, J.H.; Lynch, C.F.; et al. Variation of breast cancer risk among BRCA1/2 carriers. JAMA 2008, 299, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Mulder, R.L.; Kremer, L.C.M.; Hudson, M.M.; Bhatia, S.; Landier, W.; Levitt, G.; Constine, L.S.; Wallace, W.H.; van Leeuwen, F.E.; Ronckers, C.M.; et al. Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2013, 14, e621–e629. [Google Scholar] [CrossRef] [Green Version]
- Elezaby, M.; Lees, B.; Maturen, K.E.; Barroilhet, L.; Wisinski, K.B.; Schrager, S.; Wilke, L.G.; Sadowski, E. BRCA Mutation Carriers: Breast and Ovarian Cancer Screening Guidelines and Imaging Considerations. Radiology 2019, 291, 554–569. [Google Scholar] [CrossRef]
- Bhatti, P.; Veiga, L.H.; Ronckers, C.M.; Sigurdson, A.J.; Stovall, M.; Smith, S.A.; Weathers, R.; Leisenring, W.; Mertens, A.C.; Hammond, S.; et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: An update from the childhood cancer survivor study. Radiat. Res. 2010, 174, 741–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inskip, P.D.; Sigurdson, A.J.; Veiga, L.; Bhatti, P.; Ronckers, C.; Rajaraman, P.; Boukheris, H.; Stovall, M.; Smith, S.; Hammond, S.; et al. Radiation-Related New Primary Solid Cancers in the Childhood Cancer Survivor Study: Comparative Radiation Dose Response and Modification of Treatment Effects. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 800–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inskip, P.D.; Robison, L.L.; Stovall, M.; Smith, S.A.; Hammond, S.; Mertens, A.C.; Whitton, J.A.; Diller, L.; Kenney, L.; Donaldson, S.S.; et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J. Clin. Oncol. 2009, 27, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Stovall, M.; Smith, S.A.; Langholz, B.M.; Boice, J.D., Jr.; Shore, R.E.; Andersson, M.; Buchholz, T.A.; Capanu, M.; Bernstein, L.; Lynch, C.F.; et al. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1021–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grantzau, T.; Thomsen, M.S.; Væth, M.; Overgaard, J. Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother. Oncol. 2014, 111, 366–373. [Google Scholar] [CrossRef]
- Grantzau, T.; Overgaard, J. Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for primary breast cancer: A systematic review and meta-analysis of population-based studies including 522,739 patients. Radiother. Oncol. 2016, 121, 402–413. [Google Scholar] [CrossRef]
- Bazire, L.; De Rycke, Y.; Asselain, B.; Fourquet, A.; Kirova, Y.M. Risks of second malignancies after breast cancer treatment: Long-term results. Cancer Radiother. 2017, 21, 10–15. [Google Scholar] [CrossRef]
- Long, Q.; Wang, Y.; Che, G. Primary Lung Cancer After Treatment for Breast Cancer. Int. J. Women’s Health 2021, 13, 1217–1225. [Google Scholar] [CrossRef]
- Hou, P.Y.; Hsieh, C.H.; Wu, L.J.; Hsu, C.X.; Kuo, D.Y.; Lu, Y.F.; Tien, H.J.; Hsiao, H.W.; Shueng, P.W.; Hsu, S.M. Modern Rotational Radiation Techniques with Volumetric Modulated Arc Therapy or Helical Tomotherapy for Optimal Sparing of the Lung and Heart in Left-Breast Cancer Radiotherapy Plus Regional Nodal Irradiation: A Comparative Dosimetric Analysis. Cancers 2021, 13, 5043. [Google Scholar] [CrossRef]
- McKeown, S.R.; Hatfield, P.; Prestwich, R.J.; Shaffer, R.E.; Taylor, R.E. Radiotherapy for benign disease; assessing the risk of radiation-induced cancer following exposure to intermediate dose radiation. Br. J. Radiol. 2015, 88, 20150405. [Google Scholar] [CrossRef] [Green Version]
- Boaventura, P.; Pereira, D.; Mendes, A.; Teixeira-Gomes, J.; Sobrinho-Simões, M.; Soares, P. Thyroid and parathyroid tumours in patients submitted to X-ray scalp epilation during the tinea capitis eradication campaign in the North of Portugal (1950–1963). Virchows Arch. 2014, 465, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Shore, R.E.; Woodard, E.; Hildreth, N.; Dvoretsky, P.; Hempelmann, L.; Pasternack, B. Thyroid tumors following thymus irradiation. J. Nat. Cancer Inst. 1985, 74, 177–184. [Google Scholar]
- Modan, B.; Chetrit, A.; Alfandary, E.; Katz, L. Increased risk of breast cancer after low-dose irradiation. Lancet 1989, 1, 629–631. [Google Scholar] [CrossRef]
- Fazel, R.; Krumholz, H.M.; Wang, Y.; Ross, J.S.; Chen, J.; Ting, H.H.; Shah, N.D.; Nasir, K.; Einstein, A.J.; Nallamothu, B.K. Exposure to Low-Dose Ionizing Radiation from Medical Imaging Procedures. N. Engl. J. Med. 2009, 361, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, D.M.; Lonstein, J.E.; Stovall, M.; Hacker, D.G.; Luckyanov, N.; Land, C.E.; US Scoliosis Cohort Study Collaborators. Breast cancer mortality after diagnostic radiography: Findings from the U.S. Scoliosis Cohort Study. Spine 2000, 25, 2052–2063. [Google Scholar] [CrossRef]
- Ronckers, C.M.; Land, C.E.; Miller, J.S.; Stovall, M.; Lonstein, J.E.; Doody, M.M. Cancer mortality among women frequently exposed to radiographic examinations for spinal disorders. Radiat. Res. 2010, 174, 83–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith-Bindman, R.; Lipson, J.; Marcus, R.; Kim, K.P.; Mahesh, M.; Gould, R.; de González, A.B.; Miglioretti, D.L. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 2009, 169, 2078–2086. [Google Scholar] [CrossRef]
- Eisenberg, M.J.; Afilalo, J.; Lawler, P.R.; Abrahamowicz, M.; Richard, H.; Pilote, L. Cancer risk related to low-dose ionizing radiation from cardiac imaging in patients after acute myocardial infarction. CMAJ 2011, 183, 430–436. [Google Scholar] [CrossRef] [Green Version]
- Nekolla, E.A.; Brix, G.; Griebel, J. Lung Cancer Screening with Low-Dose CT: Radiation Risk and Benefit-Risk Assessment for Different Screening Scenarios. Diagnostics 2022, 12, 364. [Google Scholar] [CrossRef]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Craft, A.W.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Miglioretti, D.L.; Johnson, E.; Williams, A.; Greenlee, R.T.; Weinmann, S.; Solberg, L.I.; Feigelson, H.S.; Roblin, D.; Flynn, M.J.; Vanneman, N.; et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013, 167, 700–707. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration, Center for Devices and Radiological Health. White Paper: Initiative to Reduce Unnecessary Radiation Exposure from Medical Imaging. 2019. Available online: https://www.fda.gov/radiation-emitting-products/initiative-reduce-unnecessary-radiation-exposure-medical-imaging/white-paper-initiative-reduce-unnecessary-radiation-exposure-medical-imaging (accessed on 28 January 2022).
- de Gonzalez, A.B.; Daniels, R.D.; Cardis, E.; Cullings, H.M.; Gilbert, E.; Hauptmann, M.; Kendall, G.; Laurier, D.; Linet, M.S.; Little, M.P.; et al. Epidemiological Studies of Low-Dose Ionizing Radiation and Cancer: Rationale and Framework for the Monograph and Overview of Eligible Studies. JNCI Monogr. 2020, 2020, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.K.; Chang, S.C.; Yuan, M.C.; Foo, N.P.; Chan, S.H.; Wang, S.Y.; Lin, C.L.; Hsu, C.Y.; Kao, C.H. Pediatric Nuclear Medicine Examinations and Subsequent Risk of Neoplasm: A Nationwide Population-Based Cohort Study. Front. Med. 2021, 8, 764849. [Google Scholar] [CrossRef] [PubMed]
- Harach, H.R.; Williams, E.D. Childhood thyroid cancer in England and Wales. Br. J. Cancer 1995, 72, 777–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivkind, N.; Stepanenko, V.; Belukha, I.; Guenthoer, J.; Kopecky, K.J.; Kulikov, S.; Kurnosova, I.; Onstad, L.; Porter, P.; Shklovskiy-Kordi, N.; et al. Female breast cancer risk in Bryansk Oblast, Russia, following prolonged low dose rate exposure to radiation from the Chernobyl power station accident. Int. J. Epidemiol. 2020, 49, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, E.K.; Preston, D.; Zhang, R.; Vij, V.; Little, M.P.; Mabuchi, K.; Drozdovitch, V.; Chizhov, K.; Yauseyenka, V.V.; Rozhko, A.V.; et al. Breast cancer risk in residents of Belarus exposed to Chernobyl fallout while pregnant or lactating: Standardized incidence ratio analysis, 1997 to 2016. Int. J. Epidemiol. 2021, dyab226. [Google Scholar] [CrossRef]
- Cahoon, E.K.; Nadyrov, E.A.; Polyanskaya, O.N.; Yauseyenka, V.V.; Veyalkin, I.V.; Yeudachkova, T.I.; Maskvicheva, T.I.; Minenko, V.F.; Liu, W.; Drozdovitch, V.; et al. Risk of Thyroid Nodules in Residents of Belarus Exposed to Chernobyl Fallout as Children and Adolescents. J. Clin. Endocrinol. Metab. 2017, 102, 2207–2217. [Google Scholar] [CrossRef]
- Davis, F.G.; Yu, K.L.; Preston, D.; Epifanova, S.; Degteva, M.; Akleyev, A.V. Solid Cancer Incidence in the Techa River Incidence Cohort: 1956–2007. Radiat. Res. 2015, 184, 56–65. [Google Scholar] [CrossRef]
- Yamashita, S.; Suzuki, S.; Suzuki, S.; Shimura, H.; Saenko, V. Lessons from Fukushima: Latest Findings of Thyroid Cancer after the Fukushima Nuclear Power Plant Accident. Thyroid 2018, 28, 11–22. [Google Scholar] [CrossRef]
- Wei, W.; Rosenkrans, Z.T.; Liu, J.; Huang, G.; Luo, Q.Y.; Cai, W. ImmunoPET: Concept, Design, and Applications. Chem. Rev. 2020, 120, 3787–3851. [Google Scholar] [CrossRef]
- Rondon, A.; Rouanet, J.; Degoul, F. Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials. Cancers 2021, 13, 5570. [Google Scholar] [CrossRef] [PubMed]
- Roncali, E.; Capala, J.; Benedict, S.H.; Akabani, G.; Bednarz, B.; Bhadrasain, V.; Bolch, W.E.; Buchsbaum, J.C.; Coleman, N.C.; Dewaraja, Y.K.; et al. Overview of the First NRG-NCI Workshop on Dosimetry of Systemic Radiopharmaceutical Therapy (RPT). J. Nucl. Med. 2020, 62, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ao, E.; Lambert, B.; Brans, B.; Vandenberghe, S.; Mok, G. Quantitative Imaging for Targeted Radionuclide Therapy Dosimetry—Technical Review. Theranostics 2017, 7, 4551–4565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, D.V.; Sawin, C.T. Radioiodine and thyroid disease: The beginning. Semin. Nucl. Med. 1996, 26, 155–164. [Google Scholar] [CrossRef]
- Sawka, A.M.; Brierley, J.D.; Tsang, R.W.; Thabane, L.; Rotstein, L.; Gafni, A.; Straus, S.; Goldstein, D.P. An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol. Metab. Clin. N. Am. 2008, 37, 457–480. [Google Scholar] [CrossRef]
- Dottorini, M.E.; Lomuscio, G.; Mazzucchelli, L.; Vignati, A.; Colombo, L. Assessment of female fertility and carcinogenesis after iodine-131 therapy for differentiated thyroid carcinoma. J. Nucl. Med. 1995, 36, 21–27. [Google Scholar]
- Al-Jabri, A.; Cooke, J.; Cournane, S.; Healy, M.L. Gender differences in estimating I-131 thyroid uptake from Tc-99m thyroid uptake for benign thyroid disease. Br. J. Radiol. 2021, 94, 20200700. [Google Scholar] [CrossRef]
- Teng, C.J.; Hu, Y.W.; Chen, S.C.; Yeh, C.-M.; Chiang, H.-L.; Chen, T.-J.; Liu, C.-J. Use of radioactive iodine for thyroid cancer and risk of second primary malignancy: A nationwide population-based study. J. Natl. Cancer Inst. 2015, 108, djv314. [Google Scholar] [CrossRef] [Green Version]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Hoefnagel, C.A.; Voute, P.A.; de Kraker, J.; Marcuse, H.R. Radionuclide diagnosis and therapy of neural crest tumors using iodine-131 metaiodobenzylguanidine. J. Nucl. Med. 1987, 28, 308–314. [Google Scholar]
- Yoshimoto, M.; Ogawa, K.; Washiyama, K.; Shikano, N.; Mori, H.; Amano, R.; Kawai, K. Alpha(v)beta(3) Integrin-targeting radionuclide therapy and imaging with monomeric RGD peptide. Int. J. Cancer 2008, 123, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Maecke, H.R.; Reubi, J.C. Somatostatin Receptors as Targets for Nuclear Medicine Imaging and Radionuclide Treatment. J. Nucl. Med. 2011, 52, 841–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthay, K.K.; Weiss, B.; Villablanca, J.G.; Maris, J.M.; Yanik, G.A.; Dubois, S.G.; Stubbs, J.; Groshen, S.; Tsao-Wei, D.; Hawkins, R.; et al. Dose escalation study of no-carrier-added 131I-metaiodobenzylguanidine for relapsed or refractory neuroblastoma: New approaches to neuroblastoma therapy consortium trial. J. Nucl. Med. 2012, 53, 1155–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshinaga, K.; Oriuchi, N.; Wakabayashi, H.; Tomiyama, Y.; Jinguji, M.; Higuchi, T.; Kayano, D.; Fukuoka, M.; Inaki, A.; Toratani, A.; et al. Effects and safety of 131-metaiodobenzylguanidine (MIBG) radiotherapy in malignant neuroendocrine tumors: Results from a multicenter observational registry. Endocr. J. 2014, 61, 1171–1180. [Google Scholar] [CrossRef] [Green Version]
- George, S.L.; Falzone, N.; Chittenden, S.; Kirk, S.J.; Lancaster, D.; Vaidya, S.J.; Mandeville, H.; Saran, F.; Pearson, A.D.; Du, Y.; et al. Individualized 131I-mIBG therapy in the management of refractory and relapsed neuroblastoma. Nucl. Med. Commun. 2016, 37, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Kenny, L. The Use of Novel PET Tracers to Image Breast Cancer Biologic Processes Such as Proliferation, DNA Damage and Repair, and Angiogenesis. J. Nucl. Med. 2016, 57 (Suppl. 1), 89S–95S. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. National Library of Medicine (US). NCT03936426, Peptide Receptor Radionuclide Therapy Administered to Participants with Meningioma with 67Cu-SARTATE™. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03936426 (accessed on 6 February 2022).
- Alain, C.; Pascal, N.; Valérie, G.; Thierry, V. Orexins/Hypocretins and Cancer: A Neuropeptide as Emerging Target. Molecules 2021, 26, 4849. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. National Library of Medicine (US). NCT04790708, Peptide Receptor Radionuclide Therapy (PRRT) in Tumors with High Expression of Somatostatin Receptors (Phase 2) (FENET-2016). 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04790708 (accessed on 6 February 2022).
- ClinicalTrials.gov. National Library of Medicine (US). NCT04711135, Study to Evaluate Safety and Dosimetry of Lutathera in Adolescent Patients with GEP-NETs and PPGLs. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04711135 (accessed on 6 February 2022).
- Laverdière, C.; Liu, Q.; Yasui, Y.; Nathan, P.C.; Gurney, J.G.; Stovall, M.; Diller, L.R.; Cheung, N.K.; Wolden, S.; Robison, L.L.; et al. Long-term outcomes in survivors of neuroblastoma: A report from the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2009, 101, 1131–1140. [Google Scholar] [CrossRef]
- Clement, S.C.; Kraal, K.C.; van Eck-Smit, B.L.; van den Bos, C.; Kremer, L.C.; Tytgat, G.A.; van Santen, H.M. Primary ovarian insufficiency in children after treatment with 131I-metaiodobenzylguanidine for neuroblastoma: Report of the first two cases. J. Clin. Endocrinol. Metab. 2014, 99, E112–E116. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, C.; Adams, F.; Boschmann, M.; Tank, J.; Haertter, S.; Diedrich, A.; Biaggioni, I.; Luft, F.C.; Jordan, J. Phenotypical evidence for a gender difference in cardiac norepinephrine transporter function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R851–R856. [Google Scholar] [CrossRef]
- Moldovanova, I.; Schroeder, C.; Jacob, G.; Hiemke, C.; Diedrich, A.; Luft, F.C.; Jordan, J. Hormonal influences on cardiovascular norepinephrine transporter responses in healthy women. Hypertension 2008, 51, 1203–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garske-Román, U.; Sandström, M.; Fröss Baron, K.; Lundin, L.; Hellman, P.; Welin, S.; Johansson, S.; Khan, T.; Lundqvist, H.; Eriksson, B.; et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): Feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 970–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, V.L.; Richmond, I.; Maguiness, S.; Robinson, J.; Helboe, L.; Adams, I.P.; Drummond, N.S.; Atkin, S. Somatostatin Receptor 2 Expression in the Human Endometrium Through the Menstrual Cycle. Clin. Endocrinol. 2002, 56, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Fasciani, A.; Quilici, P.; Biscaldi, E.; Flamini, M.; Fioravanti, A.; Orlandi, P.; Oliviero, J.; Repetti, F.; Bandelloni, R.; Danesi, R.; et al. Overexpression and Functional Relevance of Somatostatin Receptor-1, -2, and -5 in Endometrium and Endometriotic Lesions. J. Clin. Endocrinol. Metab. 2010, 95, 5315–5319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djordjijevic, D.; Zhang, J.; Priam, M.; Viollet, C.; Gourdji, D.; Kordon, C.; Epelbaum, J. Effect of 17beta-estradiol on somatostatin receptor expression and inhibitory effects on growth hormone and prolactin release in rat pituitary cell cultures. Endocrinology 1998, 139, 2272–2277. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Di Zazzo, E.; Galasso, G.; De Rosa, C.; Abbondanza, C.; Sinisi, A.A.; Altucci, L.; Migliaccio, A.; Castoria, G. Estrogens Modulate Somatostatin Receptors Expression and Synergize With the Somatostatin Analog Pasireotide in Prostate Cells. Front. Pharmacol. 2019, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xiong, Z.; Wu, Y.; Cai, W.; Tseng, J.R.; Gambhir, S.S.; Chen, X. Quantitative PET Imaging of Tumor Integrin αvβ3 Expression with 18F-FRGD2. J. Nucl. Med. 2006, 47, 113–121. [Google Scholar]
- Perrone, M.G.; Malerba, P.; Uddin, J.; Vitale, P.; Panella, A.; Crews, B.C.; Daniel, C.K.; Ghebreselasie, K.; Nickels, M.; Tantawy, M.N.; et al. PET Radiotracer [18F]-P6 Selectively Targeting COX-1 as a Novel Biomarker in Ovarian Cancer: Preliminary investigation. Eur. J. Med. Chem. 2014, 80, 562–568. [Google Scholar] [CrossRef] [Green Version]
- Von Wolff, M.; Strowitzki, T.; Becker, V.; Zepf, C.; Tabibzadeh, S.; Thaler, C.J. Endometrial Osteopontin, a Ligand of beta3-integrin, is Maximally Expressed Around the Time of the “Implantation Window”. Fertil. Steril. 2001, 76, 775–781. [Google Scholar] [CrossRef]
- Von Wolff, M.; Thaler, C.J.; Zepf, C.; Becker, V.; Beier, H.M.; Strowitzki, T. Endometrial Expression and Secretion of Interleukin-6 Throughout the Menstrual Cycle. Gynecol. Endocrinol. 2002, 16, 121–129. [Google Scholar] [CrossRef]
- Milne, S.A.; Perchick, G.B.; Boddy, S.C.; Jabbour, H.N. Expression, Localization, and Signaling of PGE(2) and EP2/EP4 Receptors in Human Nonpregnant Endometrium Across the Menstrual Cycle. J. Clin. Endocrinol. Metab. 2001, 86, 4453–4459. [Google Scholar] [CrossRef] [PubMed]
- Nitkiewicz, A.; Smolinska, N.; Przala, J.; Kaminski, T. Expression of orexin receptors 1 (OX1R) and 2 (OX2R) in the porcine ovary during the oestrous cycle. Regul. Pept. 2010, 165, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Kroll, S.; Zasadny, K.R. Patient-specific whole-body dosimetry: Principles and a simplified method for clinical implementation. J. Nucl. Med. 1998, 39, 14s–20s. [Google Scholar] [PubMed]
- Matthay, K.K.; Panina, C.; Huberty, J.; Price, D.; Glidden, D.V.; Tang, H.R.; Hawkins, R.A.; Veatch, J.; Hasegawa, B. Correlation of tumor and whole-body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with (131)I-MIBG. J. Nucl. Med. 2001, 42, 1713–1721. [Google Scholar] [PubMed]
- ClinicalTrials.gov. National Library of Medicine (US). NCT04917484, Dosimetry Based PRRT versus Standard Dose PRRT with Lu-177-DOTATOC in NEN Patients (DOBATOC). 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04917484 (accessed on 6 February 2022).
- Srivastava, S.C. Paving the way to personalized medicine: Production of some promising theragnostic radionuclides at Brookhaven National Laboratory. Semin. Nucl. Med. 2012, 42, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Vahidfar, N.; Eppard, E.; Farzanehfar, S.; Yordanova, A.; Fallahpoor, M.; Ahmadzadehfar, H. An Impressive Approach in Nuclear Medicine: Theranostics. PET Clin. 2021, 16, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Marini, I.; Sansovini, M.; Bongiovanni, A.; Nicolini, S.; Grassi, I.; Ranallo, N.; Monti, M.; Di Iorio, V.; Germanó, L.; Caroli, P.; et al. Theragnostic in neuroendocrine tumors. Q. J. Nucl. Med. Mol. Imaging 2021, 65, 342–352. [Google Scholar] [CrossRef]
- Koziorowski, J.; Ballinger, J. Theragnostic radionuclides: A clinical perspective. Q. J. Nucl. Med. Mol. Imaging 2021, 65, 306–314. [Google Scholar] [CrossRef]
- Di Stasio, G.D.; Buonomano, P.; Travaini, L.L.; Grana, C.M.; Mansi, L. From the Magic Bullet to Theragnostics: Certitudes and Hypotheses, Trying to Optimize the Somatostatin Model. Cancers 2021, 13, 3474. [Google Scholar] [CrossRef]
- Rizzo, A.; Annunziata, S.; Salvatori, M. Side effects of theragnostic agents currently employed in clinical practice. Q. J. Nucl. Med. Mol. Imaging 2021, 65, 315–326. [Google Scholar] [CrossRef]
- Söderqvist, G.; Isaksson, E.; von Schoultz, B.; Carlström, K.; Tani, E.; Skoog, L. Proliferation of breast epithelial cells in healthy women during the menstrual cycle. Am. J. Obstet. Gynecol. 1997, 176, 123–128. [Google Scholar] [CrossRef]
- Choi, D.; Yoon, S.; Lee, E.; Hwang, S.; Song, S.; Kim, J.; Yoon, B.K.; Lee, J.H. Characterization of Cyclin D2 Expression in Human Endometrium. J. Soc. Gynecol. Investig. 2002, 9, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Ding, H.J.; Liu, C.S.; Chen, Y.K.; Lin, C.C.; Kao, C.H. Correlation Between the Intensity of Breast FDG Uptake and Menstrual Cycle. Acad. Radiol. 2007, 14, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Benign Ovarian and Endometrial Uptake on FDG PET-CT: Patterns and Pitfalls. Ann. Nucl. Med. 2009, 23, 107–112. [Google Scholar] [CrossRef]
- Peppicelli, S.; Andreucci, E.; Ruzzolini, J.; Bianchini, F.; Calorini, L. FDG uptake in cancer: A continuing debate. Theranostics 2020, 10, 2944–2948. [Google Scholar] [CrossRef] [PubMed]
- Lerman, H.; Metser, U.; Grisaru, D.; Fishman, A.; Lievshitz, G.; Even-Sapir, E. Normal and Abnormal 18F-FDG Endometrial and Ovarian Uptake in Pre- and Postmenopausal Patients: Assessment by PET/CT. J. Nucl. Med. 2004, 45, 266–271. [Google Scholar] [PubMed]
- Nishizawa, S.; Inubushi, M.; Okada, H. Physiological 18F-FDG Uptake in the Ovaries and Uterus of Healthy Female Volunteers. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 549–556. [Google Scholar] [CrossRef]
- Park, H.H.; Shin, J.Y.; Lee, J.Y.; Jin, G.H.; Kim, H.S.; Lyu, K.Y.; Lee, T.S. Discussion on the Alteration of 18F-FDG Uptake by the Breast According to the Menstrual Cycle in PET Imaging. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2013, 2013, 2469–2472. [Google Scholar] [CrossRef]
- Biegon, A.; Alexoff, D.L.; Kim, S.W.; Logan, J.; Pareto, D.; Schlyer, D.; Wang, G.J.; Fowler, J.S. Aromatase Imaging with [N-methyl-11C]vorozole PET in Healthy Men and Women. J. Nucl. Med. 2015, 56, 580–585. [Google Scholar] [CrossRef] [Green Version]
- Czoty, P.W.; Riddick, N.V.; Gage, H.D.; Sandridge, M.; Nader, S.H.; Garg, S.; Bounds, M.; Garg, P.K.; Nader, M.A. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology 2009, 34, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Banu, S.K.; Govindarajulu, P.; Aruldhas, M.M. Testosterone and estradiol differentially regulate TSH-induced thyrocyte proliferation in immature and adult rats. Steroids 2002, 67, 573–579. [Google Scholar] [CrossRef]
- Terry, M.B.; Michels, K.B.; Brody, J.G.; Byrne, C.; Chen, S.; Jerry, D.J.; Malecki, K.M.C.; Martin, M.B.; Miller, R.L.; Neuhausen, S.L.; et al. Breast Cancer and the Environment Research Program (BCERP). Environmental exposures during windows of susceptibility for breast cancer: A framework for prevention research. Breast Cancer Res. 2019, 21, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, T.R.; Amurao, M. Medical imaging radiation safety for the female patient: Rationale and implementation. Radiographics 2012, 32, 1829–1837. [Google Scholar] [CrossRef]
- Mankoff, D.A.; Peterson, L.M.; Tewson, T.J.; Link, J.M.; Gralow, J.R.; Graham, M.M.; Krohn, K.A. [18F]fluoroestradiol radiation dosimetry in human PET studies. J. Nucl. Med. 2001, 42, 679–684. [Google Scholar] [PubMed]
- Beauregard, J.M.; Croteau, E.; Ahmed, N.; van Lier, J.E.; Bénard, F. Assessment of human biodistribution and dosimetry of 4-fluoro-11beta-methoxy-16alpha-18F-fluoroestradiol using serial whole-body PET/CT. J. Nucl. Med. 2009, 50, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, R.L. The endocrinology of the menstrual cycle. Methods Mol. Biol. 2014, 1154, 145–169. [Google Scholar] [PubMed]
- Esfahani, S.A.; Salcedo, S.; Heidari, P.; Catalano, O.A.; Pauplis, R.; Hesterman, J.; Kronauge, J.F.; Mahmood, U. A phase one, single-dose, open-label, clinical safety and PET/MR imaging study of (68)Ga-DOTATOC in healthy volunteers. Am. J. Nucl. Med. Mol. Imaging 2017, 7, 53–62. [Google Scholar] [PubMed]
- Hjørnevik, T.; Cipriano, P.W.; Shen, B.; Park, J.H.; Gulaka, P.; Holley, D.; Gandhi, H.; Yoon, D.; Mittra, E.S.; Zaharchuk, G.; et al. Biodistribution and radiation dosimetry of (18)F-FTC-146 in humans. J. Nucl. Med. 2017, 58, 2004–2009. [Google Scholar] [CrossRef] [Green Version]
- O’Donoghue, J.A.; Lewis, J.S.; Pandit-Taskar, N.; Fleming, S.E.; Schoder, H.; Larson, S.M.; Beylergil, V.; Ruan, S.; Lyashchenko, S.; Zanzonico, P.B.; et al. Pharmacokinetics, biodistribution, and radiation dosimetry for (89)Zr-trastuzumab in patients with esophagogastric cancer. J. Nucl. Med. 2017, 56, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Pandit-Taskar, N.; Zanzonico, P.B.; Staton, K.D.; Carrasquillo, J.A.; Reidy-Lagunes, D.; Lyashchenko, S.K.; Burnazi, E.; Zhang, H.; Lewis, J.S.; Blasberg, R.; et al. Biodistribution and dosimetry of (18)F-Meta Fluorobenzyl Guanidine (MFBG): A first-in-human PET-CT imaging study of patients with neuroendocrine malignancies. J. Nucl. Med. 2017, 59, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, C.V.; Acosta, E.P.; Strykowski, J.M. Gender differences in human pharmacokinetics and pharmacodynamics. J. Adolesc. Health 1994, 15, 619–629. [Google Scholar] [CrossRef]
- Huxley, V.H. Sex and the cardiovascular system: The intriguing tale of how women and men regulate cardiovascular function differently. Adv. Physiol. Educ. 2007, 31, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Saganti, P.B. Race and ethnic group dependent space radiation cancer risk predictions. Sci. Rep. 2022, 12, 2028. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry. 1993. Available online: https://www.fda.gov/media/71107/download (accessed on 6 February 2022).
- Holdcroft, A. Gender bias in research: How does it affect evidence based medicine? J. R. Soc. Med. 2007, 100, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Mumford, S.L.; Sjaarda, L.A. Failure to Consider the Menstrual Cycle Phase May Cause Misinterpretation of Clinical and Research Findings of Cardiometabolic Biomarkers in Premenopausal Women. Epidemiol. Rev. 2014, 36, 71–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biegon, A.; Franschesci, D.; Schweitzer, M. Nuclear medicine in women: Unappreciated risks to reproductive organs? Radiology 2018, 289, 25–27. [Google Scholar] [CrossRef] [Green Version]
- Brady, E.; Nielsen, M.W.; Andersen, J.P.; Oertelt-Prigione, S. Lack of consideration of sex and gender in COVID-19 clinical studies. Nat. Commun. 2021, 12, 4015. [Google Scholar] [CrossRef]
- Streffer, C. The ICRP 2007 recommendations. Radiat. Prot. Dosim. 2007, 127, 2–7. [Google Scholar] [CrossRef]
- Harrison, J.D.; Balonov, M.; Bochud, F.; Martin, C.J.; Menzel, H.G.; Smith-Bindman, R.; Ortiz-López, P.; Simmonds, J.; Wakeford, R. The use of dose quantities in radiological protection: ICRP publication 147 Ann ICRP 50(1) 2021. J. Radiol. Prot. 2021, 41, 410. [Google Scholar] [CrossRef]
- Giammarile, F.; Chiti, A.; Lassmann, M.; Brans, B.; Flux, G. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1039–1047. [Google Scholar] [CrossRef]
- Treves, S.T.; Gelfand, M.J.; Fahey, F.H.; Parisi, M.T. 2016 Update of the North American Consensus Guidelines for Pediatric Administered Radiopharmaceutical Activities. J. Nucl. Med. 2016, 57, 15N–18N. [Google Scholar] [PubMed]
- American College of Radiology Practice Parameters. 2018. Available online: https://www.acr.org/Clinical-Resources/Practice-Parameters-and-Technical-Standards/Practice-Parameters-by-Modality (accessed on 26 January 2022).
- FDA. Code of Federal Regulations Title 21 Section 361.1. Radioactive Drugs for Certain Research Use. 2020. Available online: https://www.accessdata.fda.gov/scripts (accessed on 6 February 2022).
- Zanotti-Fregonara, P.; Innis, R.B. Suggested Pathway to Assess Radiation Safety of 11C-labeled PET Tracers for First-in-Human Studies. Eur. Med. Mol. Imaging 2012, 39, 544–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
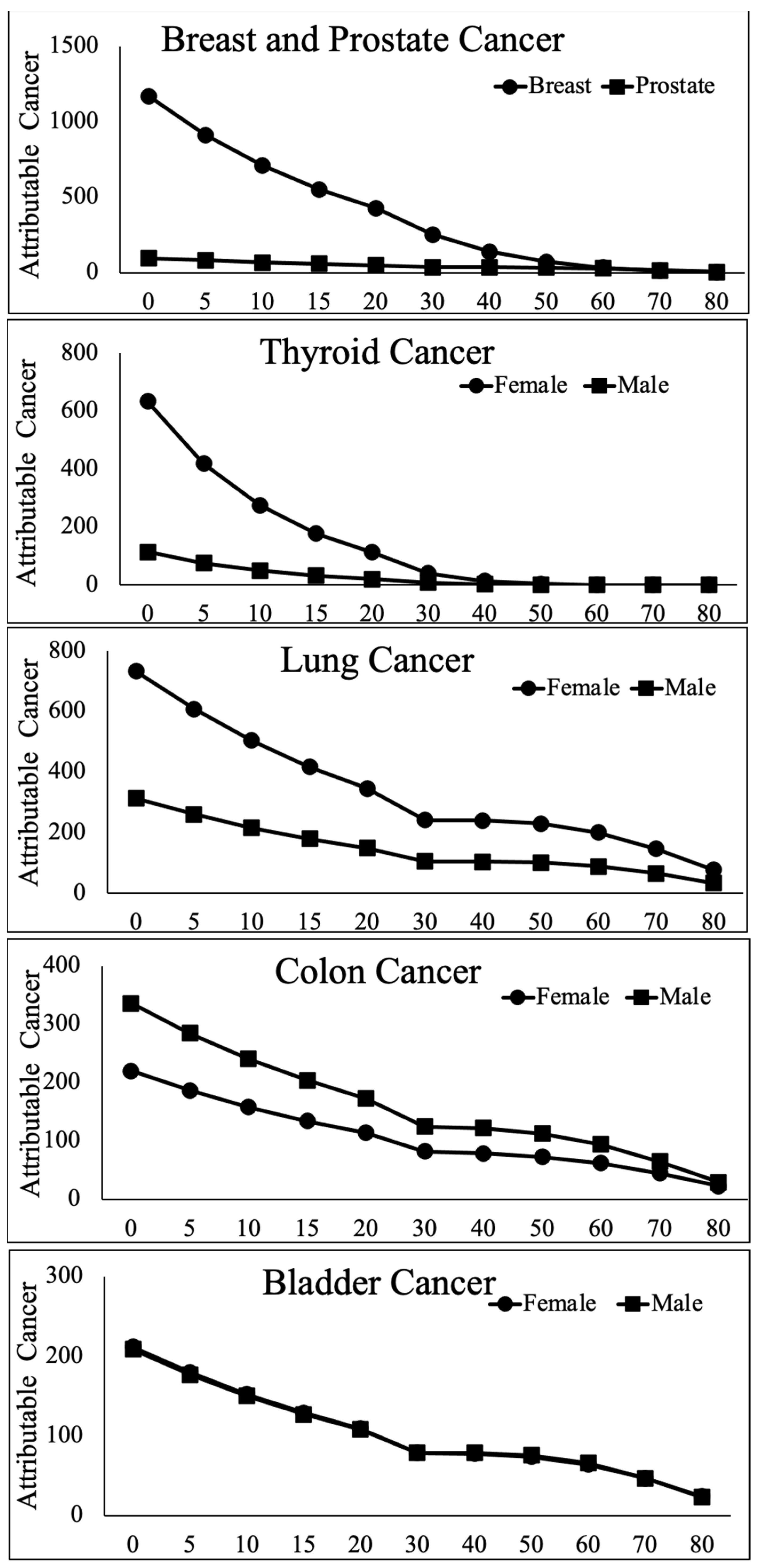
| Exposure Age (Hormonal Stage) | Rank | Females Organ | Cases/100 K | Rank | Males Organ | Cases/100 K |
|---|---|---|---|---|---|---|
| Birth (0) | 1 | Breast | 1171 | 1 | Colon | 336 |
| Pre | 2 | Lung | 733 | 2 | Lung | 314 |
| (Pre-pubertal) | 3 | Thyroid | 634 | 3 | Leukemia | 237 |
| 4 | Colon | 220 | 4 | Bladder | 209 | |
| 5 | Bladder | 212 | 5 | Thyroid | 115 | |
| 6 | Leukemia | 185 | 6 | Prostate | 93 | |
| 7 | Ovary | 104 | 7 | Stomach | 76 | |
| 8 | Stomach | 101 | 8 | Liver | 61 | |
| 9 | Uterus | 50 | ||||
| 10 | Liver | 28 | ||||
| All Cancers | 4777 | All Cancers | 2563 | |||
| 15 years | 1 | Breast | 553 | 1 | Colon | 204 |
| (pubertal) | 2 | Lung | 417 | 2 | Lung | 180 |
| 3 | Thyroid | 178 | 3 | Bladder | 127 | |
| 4 | Colon | 134 | 4 | Leukemia | 105 | |
| 5 | Bladder | 129 | 5 | Prostate | 57 | |
| 6 | Leukemia | 76 | 6 | Stomach | 40 | |
| 7 | Stomach | 61 | 7 | Liver | 36 | |
| 8 | Ovary | 60 | 8 | Thyroid | 33 | |
| 9 | Uterus | 30 | ||||
| 10 | Liver | 16 | ||||
| All Cancers | 2064 | All Cancers | 1182 | |||
| 60 years | 1 | Lung | 201 | 1 | Colon | 94 |
| (Menopausal) | 2 | Bladder | 64 | 2 | Leukemia | 82 |
| 3 | Colon | 62 | 3 | Lung | 81 | |
| 4 | Leukemia | 57 | 4 | Bladder | 66 | |
| 5 | Breast | 31 | 5 | Prostate | 26 | |
| 6 | Stomach | 27 | 6 | Stomach | 20 | |
| 7 | Ovary | 18 | 7 | Liver | 14 | |
| 8 | Uterus | 9 | 8 | Thyroid | 0.3 | |
| 9 | Liver | 7 | ||||
| 10 | Thyroid | 1 | ||||
| All cancers | 586 | All cancers | 489 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biegon, A.; Cohen, S.; Franceschi, D. Modulation of Secondary Cancer Risks from Radiation Exposure by Sex, Age and Gonadal Hormone Status: Progress, Opportunities and Challenges. J. Pers. Med. 2022, 12, 725. https://doi.org/10.3390/jpm12050725
Biegon A, Cohen S, Franceschi D. Modulation of Secondary Cancer Risks from Radiation Exposure by Sex, Age and Gonadal Hormone Status: Progress, Opportunities and Challenges. Journal of Personalized Medicine. 2022; 12(5):725. https://doi.org/10.3390/jpm12050725
Chicago/Turabian StyleBiegon, Anat, Siobhan Cohen, and Dinko Franceschi. 2022. "Modulation of Secondary Cancer Risks from Radiation Exposure by Sex, Age and Gonadal Hormone Status: Progress, Opportunities and Challenges" Journal of Personalized Medicine 12, no. 5: 725. https://doi.org/10.3390/jpm12050725
APA StyleBiegon, A., Cohen, S., & Franceschi, D. (2022). Modulation of Secondary Cancer Risks from Radiation Exposure by Sex, Age and Gonadal Hormone Status: Progress, Opportunities and Challenges. Journal of Personalized Medicine, 12(5), 725. https://doi.org/10.3390/jpm12050725






