Validation of a Novel Three-Dimensional (3D Fusion) Gross Sampling Protocol for Clear Cell Renal Cell Carcinoma to Overcome Intratumoral Heterogeneity: The Meet-Uro 18 Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
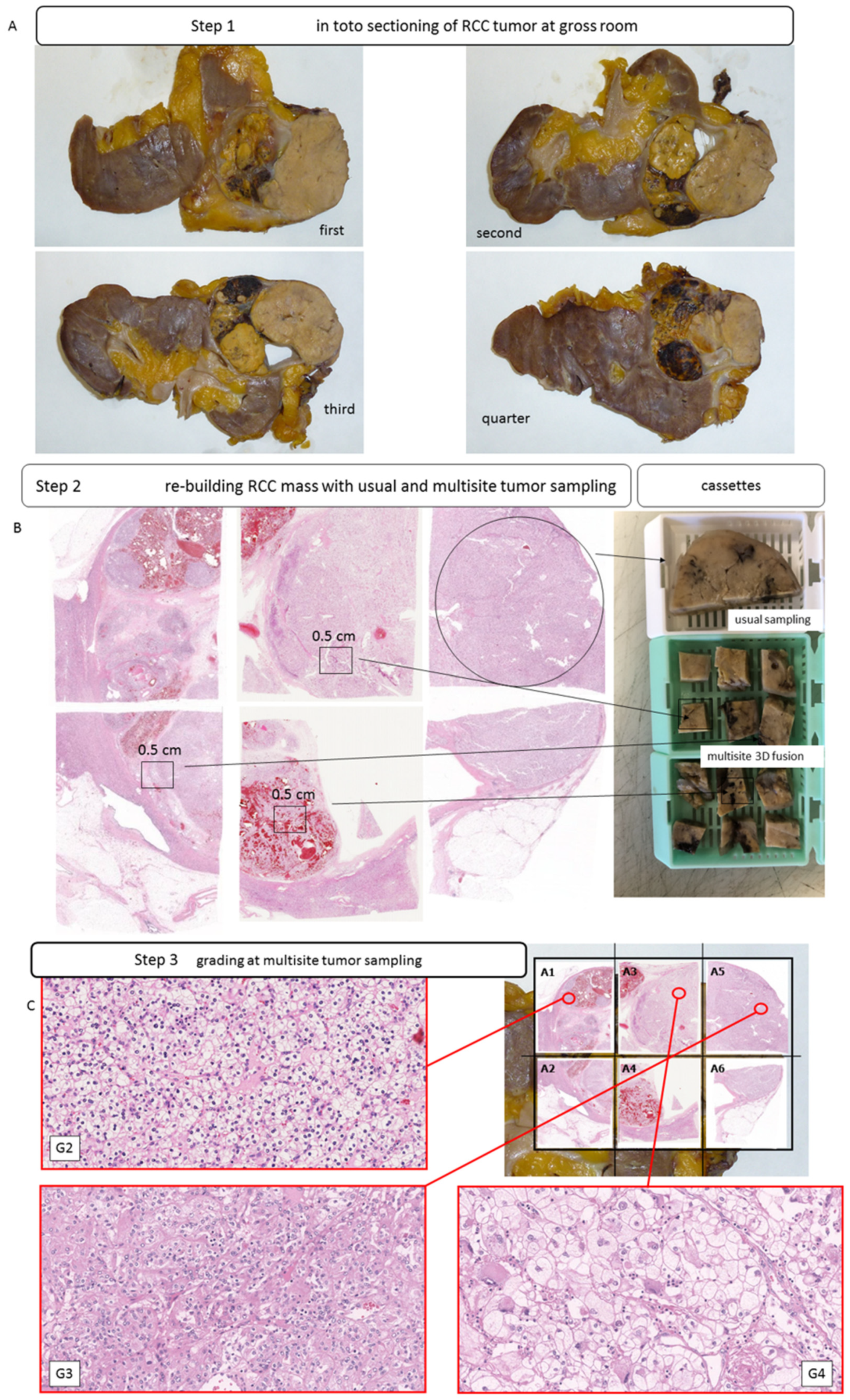
2.2. Case Selection and Sampling Methodology
2.3. TCGA Transcriptome Investigation
2.4. Immunohistochemical Staining
2.5. Digital Slides Image Capture and Evaluation
2.6. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics of Patients
3.2. TCGA Findings—CD31 and CD34 mRNA Levels in clearRCC
3.3. Immunohistochemical Findings
3.4. Evaluation of 3D Fusion Sections
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López, J.I. Intratumor heterogeneity in clear cell renal cell carcinoma: A review for the practicing pathologist. APMIS 2016, 124, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L. Tumor Heterogeneity and Personalized Medicine. N. Engl. J. Med. 2012, 366, 956–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGranahan, N.; Burrell, R.A.; Endesfelder, D.; Novelli, M.R.; Swanton, C. Cancer chromosomal instability: Therapeutic and diagnostic challenges. EMBO Rep. 2012, 13, 528–538. [Google Scholar] [CrossRef] [Green Version]
- Gerlinger, M.; Horswell, S.; Larkin, J.; Rowan, A.J.; Salm, M.; Varela, I.; Fisher, R.; McGranahan, N.; Matthews, N.; Santos, C.R.; et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat. Genet. 2014, 46, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Malouf, G.G.; Su, X.; Yao, H.; Tripathi, D.; Soeung, M.; Gao, J.; Rao, P.; Coarfa, C.; Creighton, C.J.; et al. Comprehensive Molecular Characterization Identifies Distinct Genomic and Immune Hallmarks of Renal Medullary Carcinoma. Cancer Cell 2020, 37, 720–734.e13. [Google Scholar] [CrossRef]
- Algaba, F.; Delahunt, B.; Berney, D.M.; Camparo, P.; Compérat, E.; Griffiths, D.; Kristiansen, G.; Lopez-Beltran, A.; Martignoni, G.; Moch, H.; et al. Handling and reporting of nephrectomy specimens for adult renal tumours: A survey by the European Network of Uropathology. J. Clin. Pathol. 2012, 65, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Trpkov, K.; Grignon, D.J.; Bonsib, S.M.; Amin, M.B.; Billis, A.; Lopez-Beltran, A.; Samaratunga, H.; Tamboli, P.; Delahunt, B.; Egevad, L.; et al. Handling and staging of renal cell carcinoma: The International Society of Urological Pathology Consensus (ISUP) conference recommendations. Am. J. Surg. Pathol. 2013, 37, 1505–1517. [Google Scholar] [CrossRef]
- MacLennan, G.T.; Bostwick, D.H. Microvesel density in renal cell carcinoma: Lack of prognostic significance. Urology 1995, 46, 27–30. [Google Scholar] [CrossRef]
- Delahunt, B.; Bethwaite, P.; Thornton, A. Prognostic significance of microscopic vascularity for clear cell renal cell carcinoma. Br. J. Urol. 1997, 80, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Nativ, O.; Sabo, E.; Reiss, A.; Wald, M.; Madjar, S.; Moskovitz, B. Clinical significance of tumor angiogenesis in patients wit localized renal cell carcinoma. Urology 1998, 51, 693–696. [Google Scholar] [CrossRef]
- Berndt, A.; Eiselt, M.; Katenkamp, D.; Herbst, C.; Kosmehl, H. Evaluation of microvessel density by computerised image analysis in human renal cell carcinoma. Correlation to pT category, nuclear grade, proliferative activity and occurance of metastasis. J. Cancer Res. Clin. Oncol. 1998, 124, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Sabo, E.; Boltenko, A.; Sova, Y.; Stein, A.; Kleinhaus, S.; Resnick, M.B. Microscopic analysis and significance of vascular architectural complexity in renal cell carcinoma. Clin. Cancer Res. 2001, 7, 533–537. [Google Scholar] [PubMed]
- Rioux-Leclercq, N.; Epstein, J.I.; Bansard, J.-Y.; Turlin, B.; Patard, J.-J.; Manunta, A.; Chan, T.; Ramee, M.-P.; Lobel, B.; Moulinoux, J.-P. Clinical significance of cell proliferation, microvessel density, and CD44 adhesion molecule expression in renal cell carcinoma. Hum. Pathol. 2001, 32, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Dekel, Y.; Koren, R.; Kugel, V.; Livne, P.M. Significance of angiogenesis and microvascular invasion in renal cell carcinoma. Pathol. Oncol. Res. 2002, 8, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Mertz, K.D.; Demichelis, F.; Kim, R.; Schraml, P.; Storz, M.; Diener, P.A.; Moch, H.; Rubin, M.A. Automatdimmunofluoresence analysis defines microvessel area asa a prognostic parameter in clear cell renal cell carcinoma. Hum. Pathol. 2007, 38, 1454–1462. [Google Scholar] [CrossRef]
- Yao, X.; Qian, C.-N.; Zhang, Z.-F.; Tan, M.-H.; Kort, E.J.; Yang, X.J.; Resau, J.H.; Teh, B.T. Two Distinct Types of Blood Vessels in Clear Cell Renal Cell Carcinoma Have Contrasting Prognostic Implications. Clin. Cancer Res. 2007, 13, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Aren Frontera, O.; Hammers, H.J.; Carducci, M.A.; Salman, P.; Escudier, B.; Beuselinck, B.; Amin, A.; et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1370–1385. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Plimack, E.R.; Procopio, G.; McDermott, D.F.; et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 2020, 126, 4156–4167. [Google Scholar] [CrossRef]
- Motzer, R.J.; Banchereau, R.; Hamidi, H.; Powles, T.; McDermott, D.; Atkins, M.B.; Escudier, B.; Liu, L.F.; Leng, N.; Abbas, A.R.; et al. Molecular Subsets in Renal Cancer Determine Outcome to Checkpoint and Angiogenesis Blockade. Cancer Cell 2020, 20, 324–334. [Google Scholar] [CrossRef]
- McDermott, D.F.; Huseni, M.A.; Atkins, M.B.; Motzer, R.J.; Rini, B.I.; Escudier, B.; Fong, L.; Joseph, R.W.; Pal, S.K.; Reeves, J.A.; et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 2018, 24, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.L.; et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019, 393, 2404–2415. [Google Scholar] [CrossRef]
- Escudier, B. Combination Therapy as First-Line Treatment in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.; Motzer, R.; Rini, B.; Haanen, J.; Campbell, M.; Venugopal, B.; Kollmannsberger, C.; Gravis-Mescam, G.; Uemura, M.; Lee, J.; et al. Updated efficacy results from the JAVELIN Renal 101 trial: First-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann. Oncol. 2020, 31, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Curti, B.D. Immunotherapy in Advanced Renal Cancer—Is Cure Possible? N. Engl. J. Med. 2018, 378, 1344–1345. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.E.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e296. [Google Scholar] [CrossRef] [Green Version]
- Delahunt, B.; Eble, J.N.; Egevad, L.; Samaratunga, H. Grading of renal cell carcinoma. Histopathology 2018, 74, 4–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiello, N.M.; Maddipati, R.; Norgard, R.J.; Balli, D.; Li, J.; Yuan, S.; Yamazoe, T.; Black, T.; Sahmoud, A.; Furth, E.E.; et al. EMT Subtype Influences Epithelial Plasticity and Mode of Cell Migration. Dev. Cell 2018, 45, 681–695.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choueiri, T.K.; Duh, M.S.; Clement, J.; Brick, A.J.; Rogers, M.J.; Kwabi, C.; Shah, K.; Percy, A.G.; Antràs, L.; Jayawant, S.S.; et al. Angiogenesis inhibitor therapies for metastatic renal cell carcinoma: Effectiveness, safety and treatment patterns in clinical practice-based on medical chart review. Br. J. Urol. 2010, 105, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.I.; Cortes, J.M. A divide-and-conquer strategy in tumor sampling enhances detection of intratumor heterogeneity in routine pathology: A modeling approach in clear cell renal cell carcinoma. F1000Research 2016, 5, 385. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, M.; Beccari, S.; Colombari, R.; Gobbo, S.; Giobelli, L.; Pellegrini, A.; Chilosi, M.; Lunardi, M.; Martignoni, G.; Scarpa, A.; et al. iPathology cockpit diagnostic station: Validation according to College of American Pathologists Pathology and Laboratory Quality Center recommendation at the Hospital Trust and University of Verona. Diagn. Pathol. 2014, 9 (Suppl. 1), S12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trpkov, K.; Williamson, S.R.; Gill, A.J.; Adeniran, A.J.; Agaimy, A.; Alaghehbandan, R.; Amin, M.B.; Argani, P.; Chen, Y.B.; Cheng, L.; et al. Novel, emerging and provisional renal entities: The Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod. Pathol. 2021, 34, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Cossu-Rocca, P.; Contini, M.; Brunelli, M.; Festa, A.; Pili, F.; Gobbo, S.; Eccher, A.; Mura, A.; Massarelli, G.; Martignoni, G. S-100A1 Is a Reliable Marker in Distinguishing Nephrogenic Adenoma From Prostatic Adenocarcinoma. Am. J. Surg. Pathol. 2009, 33, 1031–1036. [Google Scholar] [CrossRef]





| Males | 69 |
| females | 31 |
| right kidney | 54 |
| left kidney | 46 |
| pT3a | 68 |
| pT3b | 28 |
| pT4 | 6 |
| grading sec. ISUP/WHO 2016 | |
| G1 | 0 |
| G2 | 9 |
| G3 | 61 |
| G4 | 30 |
| rhabdoid differentiation | 9 |
| sarcomatoid differentiation | 8 |
| gross images collected | 656 |
| paraffin blocks embedded | 5231 |
| Tissue microarray array (TMA) sections | 3093 |
| Size Samples | CD31 | CD34 | Vessels/mm2 | ||
|---|---|---|---|---|---|
| Routine sampling | one sample (3 × 1.5 cm) per cm single block | 5–389 | 7–410 | 816 | manual count |
| 13–408 | 21–478 | 899 | digital count | ||
| Tissue microarray (TMAs) sampling | 0.6 mm tissue core sample | 41–480 | 49–393 | 432 | manual count |
| 524 | digital count | ||||
| Fusion 3D multisite tissue sampling | six samples (0.5 × 0.5 cm) per cm single block | 21–465 | 15–691 | 995 | manual count |
| 19–510 | 32–680 | 1001 | digital count |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunelli, M.; Martignoni, G.; Malpeli, G.; Volpe, A.; Cima, L.; Raspollini, M.R.; Barbareschi, M.; Tafuri, A.; Masi, G.; Barzon, L.; et al. Validation of a Novel Three-Dimensional (3D Fusion) Gross Sampling Protocol for Clear Cell Renal Cell Carcinoma to Overcome Intratumoral Heterogeneity: The Meet-Uro 18 Study. J. Pers. Med. 2022, 12, 727. https://doi.org/10.3390/jpm12050727
Brunelli M, Martignoni G, Malpeli G, Volpe A, Cima L, Raspollini MR, Barbareschi M, Tafuri A, Masi G, Barzon L, et al. Validation of a Novel Three-Dimensional (3D Fusion) Gross Sampling Protocol for Clear Cell Renal Cell Carcinoma to Overcome Intratumoral Heterogeneity: The Meet-Uro 18 Study. Journal of Personalized Medicine. 2022; 12(5):727. https://doi.org/10.3390/jpm12050727
Chicago/Turabian StyleBrunelli, Matteo, Guido Martignoni, Giorgio Malpeli, Alessandro Volpe, Luca Cima, Maria Rosaria Raspollini, Mattia Barbareschi, Alessandro Tafuri, Giulia Masi, Luisa Barzon, and et al. 2022. "Validation of a Novel Three-Dimensional (3D Fusion) Gross Sampling Protocol for Clear Cell Renal Cell Carcinoma to Overcome Intratumoral Heterogeneity: The Meet-Uro 18 Study" Journal of Personalized Medicine 12, no. 5: 727. https://doi.org/10.3390/jpm12050727












