Task-Rate-Related Neural Dynamics Using Wireless EEG to Assist Diagnosis and Intervention Planning for Preschoolers with ADHD Exhibiting Heterogeneous Cognitive Proficiency
Abstract
:1. Introduction
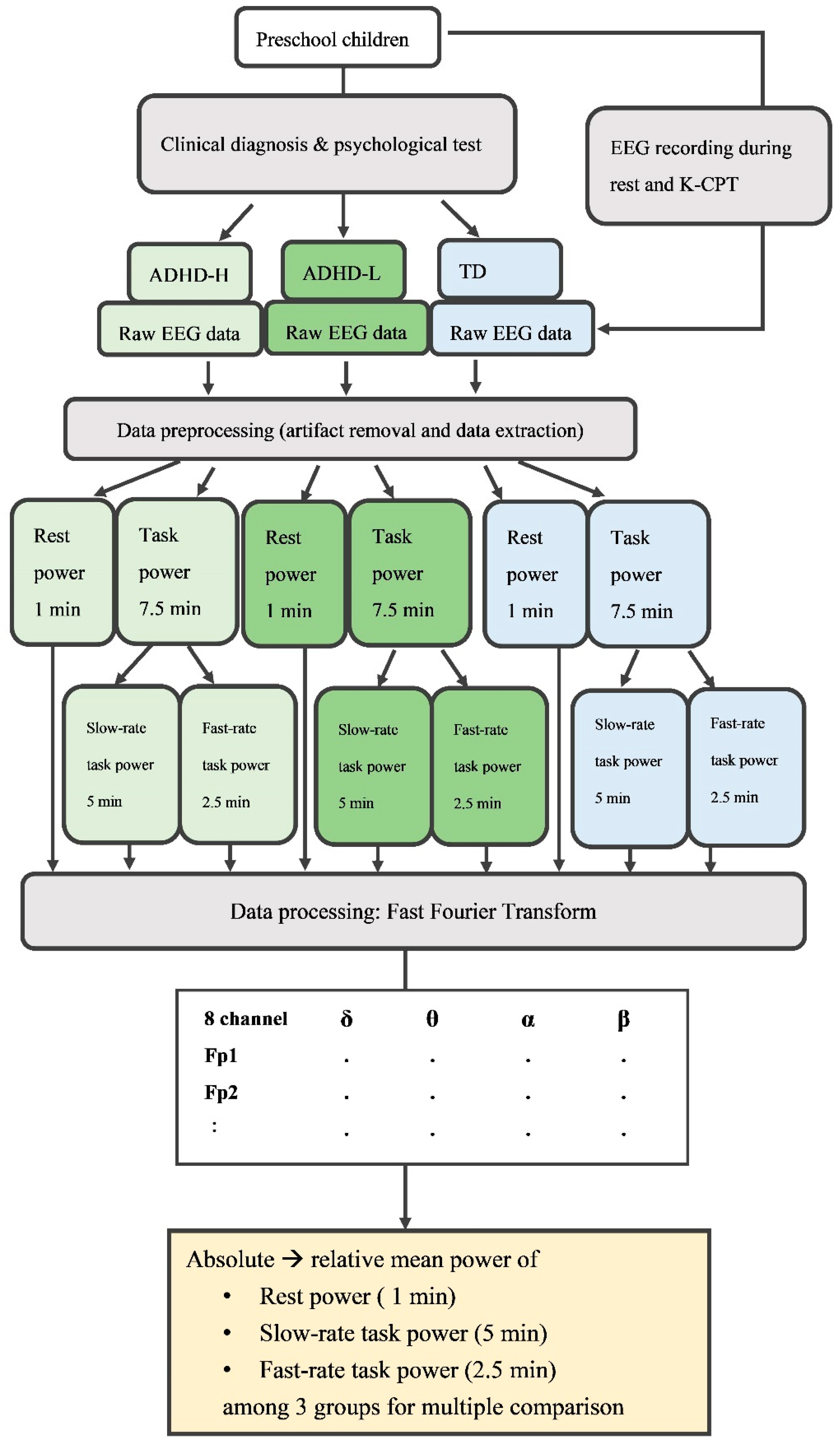
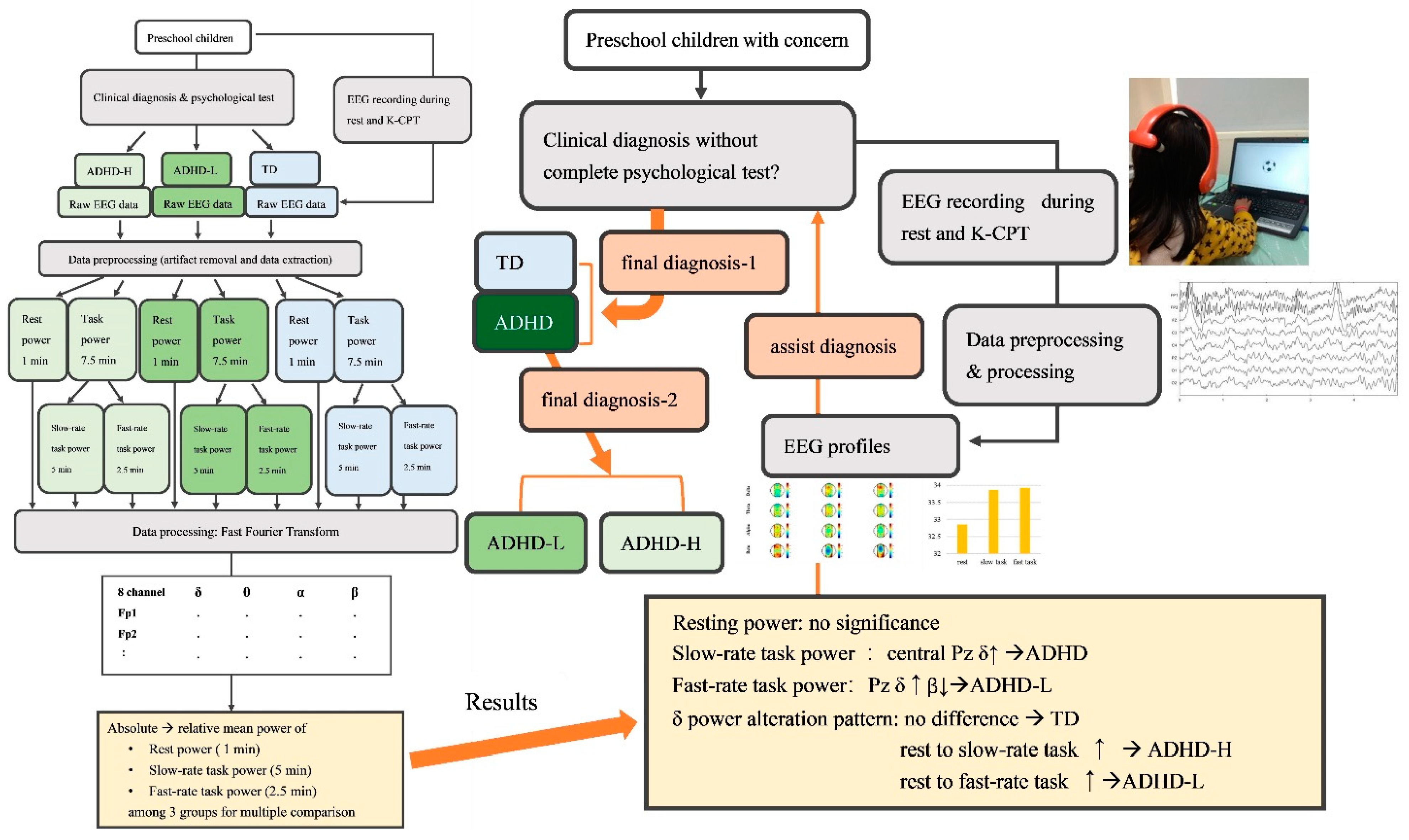
2. Materials and Methods
2.1. Participants
2.2. Apparatus
2.2.1. Neuropsychological Measurements
Intelligence Quotient Test and Behavioral Rating Scales
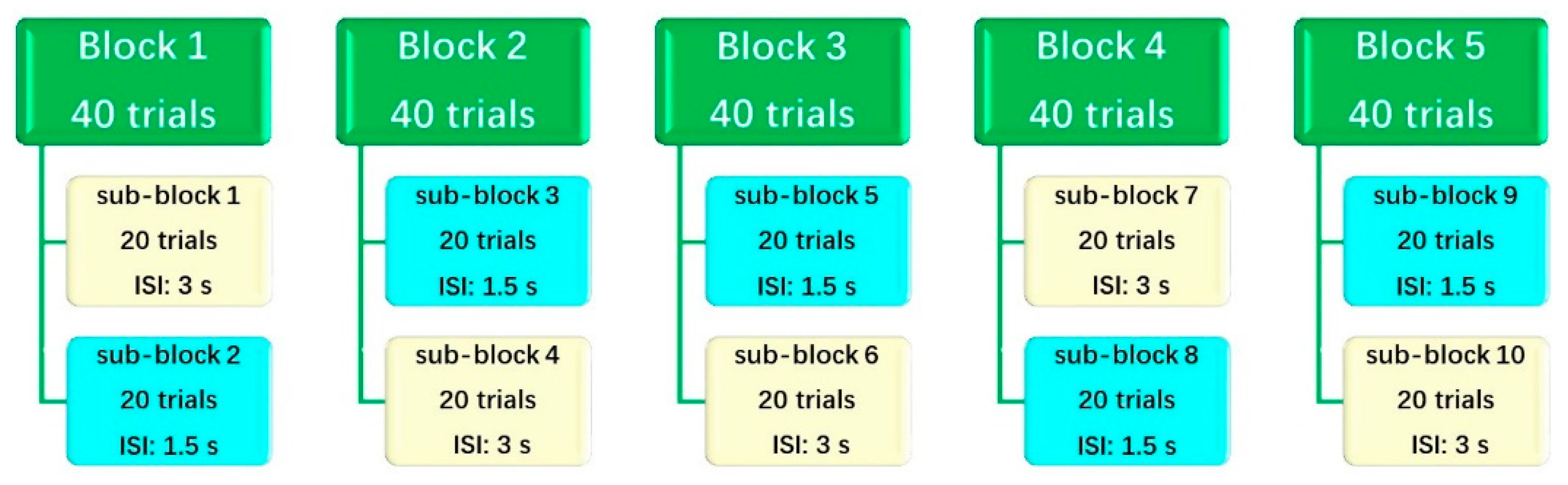
Conners Kiddie Continuous Performance Test 2nd Edition
2.2.2. Wireless and Wearable EEG
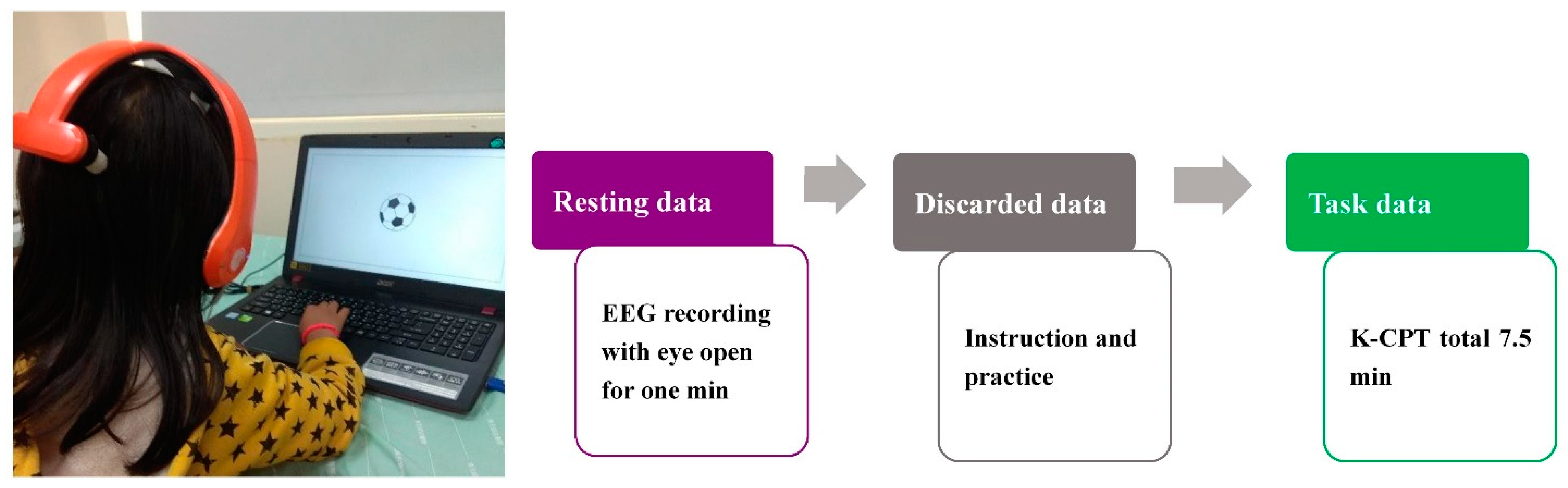
2.3. Procedure and EEG Recording
2.4. Data Preprocessing and Processing
2.5. Statistical Analysis
3. Results
3.1. Neuropsychological Measurements
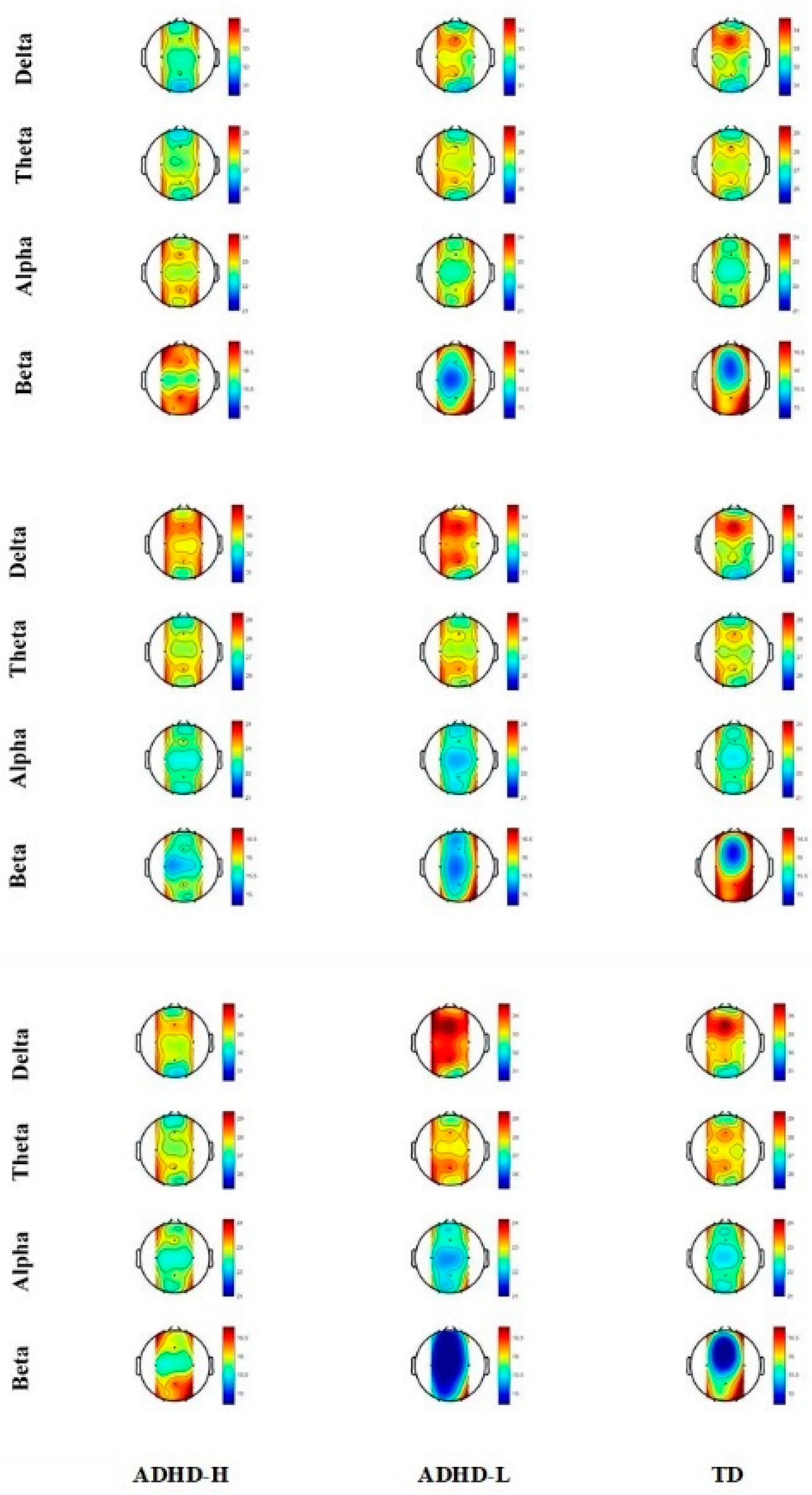
3.2. Resting Relative Spectral Power
3.3. Slow-Task-Related Relative Spectral Power
3.4. Fast-Task-Related Relative Spectral Power
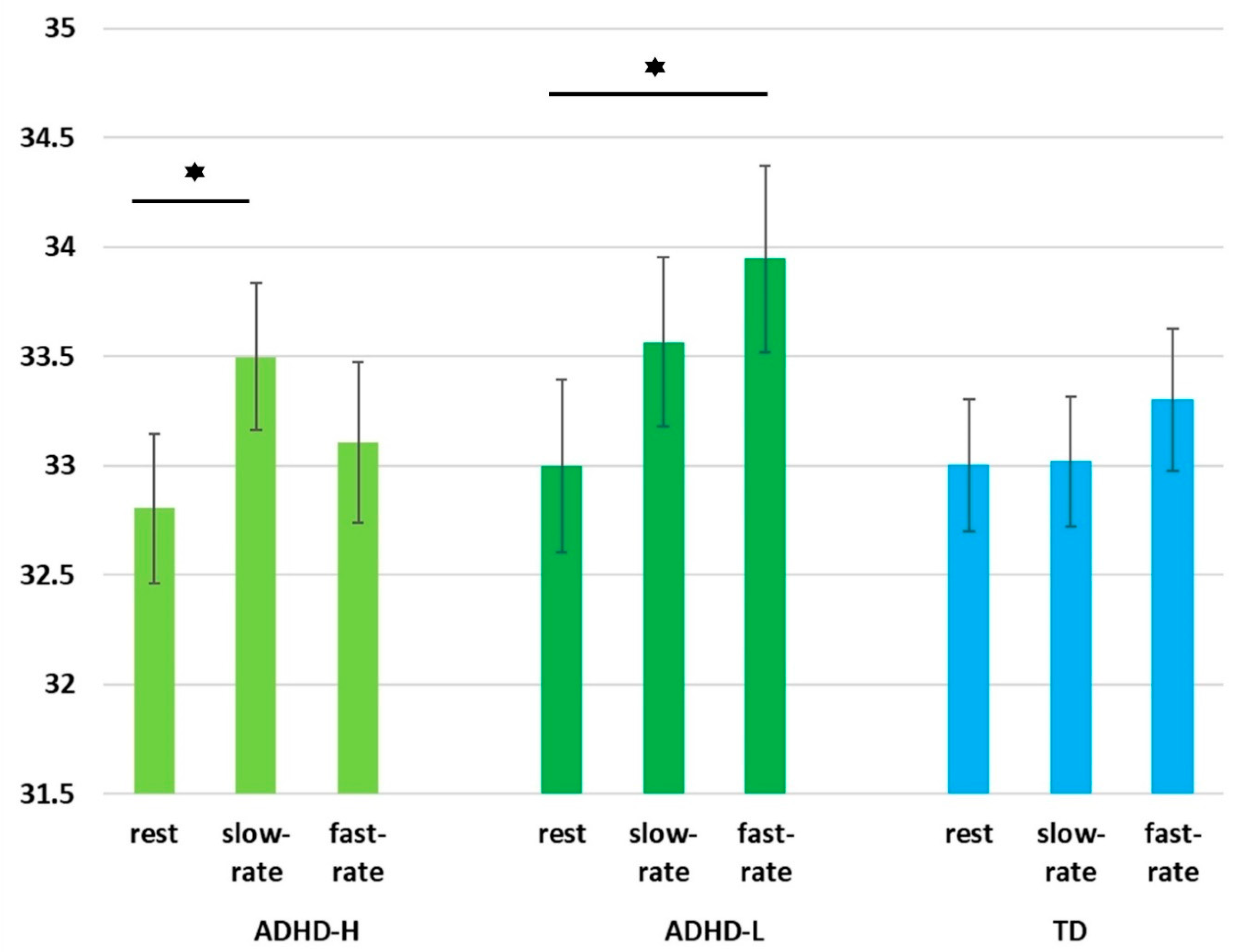
3.5. EEG Spectral Power Alterations of Groups under Three Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM-5; American Psychiatric Press: Washington, DC, USA, 2013. [Google Scholar]
- Tandon, M.; Pergjika, A. Attention Deficit Hyperactivity Disorder in Preschool-Age Children. Child Adolesc. Psychiatr. Clin. N. Am. 2017, 26, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Halperin, J.M.; Marks, D.J. Practitioner Review: Assessment and treatment of preschool children with attention-deficit/hyperactivity disorder. J. Child Psychol. Psychiatry 2019, 60, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Wolraich, M.; Brown, L.; Brown, R.T.; DuPaul, G.; Earls, M.; Feldman, H.M.; Ganiats, T.G.; Kaplanek, B.; Meyer, B.; Perrin, J.; et al. ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011, 128, 1007–1022. [Google Scholar] [PubMed] [Green Version]
- Charach, A.; Carson, P.; Fox, S.; Ali, M.U.; Beckett, J.; Lim, C.G. Interventions for preschool children at high risk for ADHD: A comparative effectiveness review. Pediatrics 2013, 131, e1584–e1604. [Google Scholar] [CrossRef] [PubMed]
- Fiks, A.G.; Ross, M.E.; Mayne, S.L.; Song, L.; Liu, W.; Steffes, J.; McCarn, B.; Grundmeier, R.W.; Localio, A.R.; Wasserman, R. Preschool ADHD Diagnosis and Stimulant Use Before and After the 2011 AAP Practice Guideline. Pediatrics 2016, 138, e20162025. [Google Scholar] [CrossRef] [Green Version]
- Takayanagi, N.; Yoshida, S.; Yasuda, S.; Adachi, M.; Kaneda-Osato, A.; Tanaka, M.; Masuda, T.; Kuribayashi, M.; Saito, M.; Nakamura, K. Psychometric properties of the Japanese ADHD-RS in preschool children. Res. Dev. Disabil. 2016, 55, 268–278. [Google Scholar] [CrossRef]
- Merkt, J.; Siniatchkin, M.; Petermann, F. Neuropsychological Measures in the Diagnosis of ADHD in Preschool: Can Developmental Research Inform Diagnostic Practice? J. Atten. Disord. 2016, 24, 1588–1604. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, S.; Schneiderman, R.L.; Rajendran, K.; Marks, D.J.; Halperin, J.M. Reliable ratings or reading tea leaves: Can parent, teacher, and clinician behavioral ratings of preschoolers predict ADHD at age six? J. Abnorm. Child Psychol. 2014, 42, 623–634. [Google Scholar] [CrossRef] [Green Version]
- Jeste, S.S.; Frohlich, J.; Loo, S.K. Electrophysiological biomarkers of diagnosis and outcome in neurodevelopmental disorders. Curr. Opin. Neurol. 2015, 28, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Lenartowicz, A.; Loo, S.K. Use of EEG to diagnose ADHD. Curr. Psychiatry Rep. 2014, 16, 498. [Google Scholar] [CrossRef] [Green Version]
- Alba, G.; Pereda, E.; Mañas, S.; Méndez, L.D.; González, A.; González, J.J. Electroencephalography signatures of attention-deficit/hyperactivity disorder: Clinical utility. Neuropsychiatr. Dis. Treat. 2015, 11, 2755–2769. [Google Scholar] [PubMed] [Green Version]
- Olbrich, S.; van Dinteren, R.; Arns, M. Personalized Medicine: Review and Perspectives of Promising Baseline EEG Biomarkers in Major Depressive Disorder and Attention Deficit Hyperactivity Disorder. Neuropsychobiology 2015, 72, 229–240. [Google Scholar] [CrossRef] [PubMed]
- McVoy, M.; Lytle, S.; Fulchiero, E.; Aebi, M.E.; Adeleye, O.; Sajatovic, M. A systematic review of quantitative EEG as a possible biomarker in child psychiatric disorders. Psychiatry Res. 2019, 279, 331–344. [Google Scholar] [CrossRef]
- Hall, C.L.; Valentine, A.Z.; Groom, M.; Walker, G.; Sayal, K.; Daley, D.; Hollis, C. The clinical utility of the continuous performance test and objective measures of activity for diagnosing and monitoring ADHD in children: A systematic review. Eur. Child Adolesc. Psychiatry 2016, 25, 677–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, S.K.; Makeig, S. Clinical utility of EEG in attention-deficit/hyperactivity disorder: A research update. Neurotherapeutics 2012, 9, 569–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newson, J.J.; Thiagarajan, T.C. EEG Frequency Bands in Psychiatric Disorders: A Review of Resting State Studies. Front. Hum. Neurosci. 2018, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.M.; Rugino, T.A.; Hornig, M.; Stein, M.A. Integration of an EEG biomarker with a clinician’s ADHD evaluation. Brain Behav. 2015, 5, e00330. [Google Scholar] [CrossRef]
- Lansbergen, M.M.; Arns, M.; van Dongen-Boomsma, M.; Spronk, D.; Buitelaar, J.K. The increase in theta/beta ratio on resting-state EEG in boys with attention-deficit/hyperactivity disorder is mediated by slow alpha peak frequency. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 47–52. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; Johnstone, S. Resting state EEG power research in Attention-Deficit/Hyperactivity Disorder: A review update. Clin. Neurophysiol. 2020, 131, 1463–1479. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; McCarthy, R.; Selikowitz, M. EEG-defined subtypes of children with attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 2001, 112, 2098–2105. [Google Scholar] [CrossRef]
- Arns, M.; Conners, C.K.; Kraemer, H.C. A decade of EEG Theta/Beta Ratio Research in ADHD: A meta-analysis. J. Atten. Disord. 2013, 17, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Barry, R.J.; McCarthy, R.; Selikowitz, M. Age and sex effects in the EEG: Differences in two subtypes of attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 2001, 112, 815–826. [Google Scholar] [PubMed]
- Kim, J.W.; Lee, J.; Kim, B.-N.; Kang, T.; Min, K.J.; Han, D.H.; Lee, Y.S. Theta-phase gamma-amplitude coupling as a neurophysiological marker of attention deficit/hyperactivity disorder in children. Neurosci. Lett. 2015, 603, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Markovska-Simoska, S.; Pop-Jordanova, N. Quantitative EEG in Children and Adults With Attention Deficit Hyperactivity Disorder: Comparison of Absolute and Relative Power Spectra and Theta/Beta Ratio. Clin. EEG. Neurosci. 2017, 48, 20–32. [Google Scholar] [CrossRef]
- Wechsler, D. WPPSI-IV: Wechsler Preschool and Primary Scale of Intelligence—Fourth Edition Manual; Pearson Education: San Antonio, CA, USA, 2014. [Google Scholar]
- Areces, D.; Dockrell, J.; García, T.; González-Castro, P.; Rodríguez, C. Analysis of cognitive and attentional profiles in children with and without ADHD using an innovative virtual reality tool. PLoS ONE 2018, 13, e0201039. [Google Scholar] [CrossRef]
- Fried, R.; Chan, J.; Feinberg, L.; Pope, A.; Woodworth, K.Y.; Faraone, S.V.; Biederman, J. Clinical correlates of working memory deficits in youth with and without ADHD: A controlled study. J. Clin. Exp. Neuropsychol. 2016, 38, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Mayes, S.D.; Calhoun, S.L. WISC-IV and WISC-III Profiles in Children With ADHD. J. Atten. Disord. 2006, 9, 486–493. [Google Scholar] [CrossRef]
- Jacobson, L.A.; Ryan, M.; Martin, R.B.; Ewen, J.; Mostofsky, S.H.; Denckla, M.B.; Mahone, E.M. Working memory influences processing speed and reading fluency in ADHD. Child Neuropsychol. 2011, 17, 209–224. [Google Scholar] [CrossRef] [Green Version]
- Martinussen, R.; Hayden, J.; Hogg-Johnson, S.; Tannock, R. A Meta-Analysis of Working Memory Impairments in Children With Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 377–384. [Google Scholar] [CrossRef]
- Coghill, D.R.; Seth, S.; Matthews, K. A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: Advancing beyond the three-pathway models. Psychol. Med. 2014, 44, 1989–2001. [Google Scholar] [CrossRef]
- Otterman, D.L.; Koopman-Verhoeff, M.E.; White, T.J.; Tiemeier, H.; Bolhuis, K.; Jansen, P.W. Executive functioning and neurodevelopmental disorders in early childhood: A prospective population-based study. Child Adolesc. Psychiatry Ment. Health 2019, 13, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjöwall, D.; Thorell, L.B. A critical appraisal of the role of neuropsychological deficits in preschool ADHD. Child Neuropsychol. 2019, 25, 60–80. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, K.; Mulder, H.; Deković, M.; Matthys, W. Executive functions in preschool children with externalizing behavior problems: A meta-analysis. J. Abnorm. Child Psychol. 2013, 41, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Pauli-Pott, U.; Becker, K. Neuropsychological basic deficits in preschoolers at risk for ADHD: A meta-analysis. Clin. Psychol. Rev. 2011, 31, 626–637. [Google Scholar] [CrossRef]
- Hoza, B.; Shoulberg, E.K.; Tompkins, C.L.; Martin, C.P.; Krasner, A.; Dennis, M.; Meyer, L.E.; Cook, H. Moderate-to-vigorous physical activity and processing speed: Predicting adaptive change in ADHD levels and related impairments in preschoolers. J. Child Psychol. Psychiatry 2020, 61, 1380–1387. [Google Scholar] [CrossRef]
- Gooch, D.; Sears, C.; Maydew, H.; Vamvakas, G.; Norbury, C.F. Does Inattention and Hyperactivity Moderate the Relation Between Speed of Processing and Language Skills? Child Dev. 2019, 90, e565–e583. [Google Scholar] [CrossRef]
- Sjöwall, D.; Backman, A.; Thorell, L.B. Neuropsychological heterogeneity in preschool ADHD: Investigating the interplay between cognitive, affective and motivation-based forms of regulation. J. Abnorm. Child Psychol. 2015, 43, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Clarke, A.R.; Barry, R.; Dupuy, F.E.; Heckel, L.D.; McCarthy, R.; Selikowitz, M.; Johnstone, S.J. Behavioural differences between EEG-defined subgroups of children with Attention-Deficit/Hyperactivity Disorder. Clin. Neurophysiol. 2011, 122, 1333–1341. [Google Scholar] [CrossRef]
- Mazaheri, A.; Fassbender, C.; Coffey-Corina, S.; Hartanto, T.A.; Schweitzer, J.B.; Mangun, G.R. Differential oscillatory electroencephalogram between attention-deficit/hyperactivity disorder subtypes and typically developing adolescents. Biol. Psychiatry 2014, 76, 422–429. [Google Scholar] [CrossRef] [Green Version]
- Ogrim, G.; Kropotov, J.; Hestad, K. The quantitative EEG theta/beta ratio in attention deficit/hyperactivity disorder and normal controls: Sensitivity, specificity, and behavioral correlates. Psychiatry Res. 2012, 198, 482–488. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.T. Manual for the Wechsler Preschool and Primary Scale of Intelligence-IV; Chinese Behavioral Science Corporation: Taiwan, China, 2013. [Google Scholar]
- Barkley, R.A.; Murphy, K.R. Attention-Deficit Hyperactivity Disorder: A Clinical Workbook; Guilford Publications: New York, NY, USA, 2005. [Google Scholar]
- Conners, C.K. Conners’ Kiddie Continuous Performance Test, 2nd ed.; Multi-Health Systems Inc.: New York, NY, USA, 2015. [Google Scholar]
- Chen, I.C.; Chang, C.H.; Chang, Y.; Lin, D.S.; Lin, C.H.; Ko, L.W. Neural Dynamics for Facilitating ADHD Diagnosis in Preschoolers: Central and Parietal Delta Synchronization in the Kiddie Continuous Performance Test. IEEE Trans. Neural. Syst. Rehabil. Eng. 2021, 29, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Ko, L.-W.; Chang, Y.; Wu, P.-L.; Tzou, H.-A.; Chen, S.-F.; Tang, S.-C.; Yeh, C.-L.; Chen, Y.-J. Development of a Smart Helmet for Strategical BCI Applications. Sensors 2019, 19, 1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferree, T.C.; Luu, P.; Russell, G.S.; Tucker, D.M. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001, 112, 536–544. [Google Scholar] [CrossRef]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; Mckeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef]
- Pion-Tonachini, L.; Kreutz-Delgado, K.; Makeig, S. ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. NeuroImage 2019, 198, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin. Neurophysiol. 2003, 114, 171–183. [Google Scholar] [CrossRef]
- Nazari, M.A.; Wallois, F.; Aarabi, A.; Berquin, P. Dynamic changes in quantitative electroencephalogram during continuous performance test in children with attention-deficit/hyperactivity disorder. Int. J. Psychophysiol. 2011, 81, 230–236. [Google Scholar] [CrossRef]
- Matsuura, M.; Okubo, Y.; Toru, M.; Kojima, T.; He, Y.; Shen, Y.; Lee, C.K. A cross-national EEG study of children with emotional and behavioral problems: A WHO collaborative study in the Western Pacific region. Biol. Psychiatry 1993, 34, 59–65. [Google Scholar] [CrossRef]
- Burke, A.; Edge, A. Neurodevelopmental Pathways of Childhood ADHD into Adulthood: Maturational Lag, Deviation, or Both? Intech 2013. [Google Scholar] [CrossRef] [Green Version]
- Sohn, H.; Kim, I.; Lee, W.; Peterson, B.S.; Hong, H.; Chae, J.-H.; Hong, S.; Jeong, J. Linear and non-linear EEG analysis of adolescents with attention-deficit/hyperactivity disorder during a cognitive task. Clin. Neurophysiol. 2010, 121, 1863–1870. [Google Scholar] [CrossRef]
- Pliszka, S.R.; McCracken, J.T.; Maas, J.W. Catecholamines in Attention-Deficit Hyperactivity Disorder: Current Perspectives. J. Am. Acad. Child Adolesc. Psychiatry 1996, 35, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Cherkasova, M.V.; Hechtman, L. Neuroimaging in attention-deficit hyperactivity disorder: Beyond the frontostriatal circuitry. Can. J. Psychiatry 2009, 54, 651–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonuga-Barke, E.J.S.; Barton, J.; Daley, D.; Hutchings, J.; Maishman, T.; Raftery, J.; Stanton, L.; Laver-Bradbury, C.; Chorozoglou, M.; Coghill, D.; et al. A comparison of the clinical effectiveness and cost of specialised individually delivered parent training for preschool attention-deficit/hyperactivity disorder and a generic, group-based programme: A multi-centre, randomised controlled trial of the New Forest Parenting Programme versus Incredible Years. Eur. Child Adolesc. Psychiatry 2018, 27, 797–809. [Google Scholar] [PubMed] [Green Version]
- Tamm, L.; Nakonezny, P.A.; Hughes, C.W. An open trial of a metacognitive executive function training for young children with ADHD. J. Atten. Disord. 2014, 18, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Re, A.M.; Capodieci, A.; Cornoldi, C. Effect of training focused on executive functions (attention, inhibition, and working memory) in preschoolers exhibiting ADHD symptoms. Front. Psychol. 2015, 6, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capodieci, A.; Gola, M.L.; Cornoldi, C.; Re, A.M. Effects of a working memory training program in preschoolers with symptoms of attention-deficit/hyperactivity disorder. J. Clin. Exp. Neuropsychol. 2018, 40, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Maeir, A.; Fisher, O.; Bar-Ilan, R.T.; Boas, N.; Berger, I.; Landau, Y.E. Effectiveness of Cognitive-Functional (Cog-Fun) occupational therapy intervention for young children with attention deficit hyperactivity disorder: A controlled study. Am. J. Occup. Ther. 2014, 68, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Pauli-Pott, U.; Mann, C.; Becker, K. Do cognitive interventions for preschoolers improve executive functions and reduce ADHD and externalizing symptoms? A meta-analysis of randomized controlled trials. Eur. Child Adolesc. Psychiatry 2020, 30, 1503–1521. [Google Scholar] [CrossRef]
- Peñuelas-Calvo, I.; Jiang-Lin, L.K.; Girela-Serrano, B.; Delgado-Gomez, D.; Navarro-Jimenez, R.; Baca-Garcia, E.; Porras-Segovia, A. Video games for the assessment and treatment of attention-deficit/hyperactivity disorder: A systematic review. Eur. Child Adolesc. Psychiatry 2022, 31, 5–20. [Google Scholar] [CrossRef]
- Aubrey, H.F.; Ronald, A.K. Therapist’s Guide to Learning and Attention Disorders; Academic Press: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Rommel, A.-S.; James, S.-N.; McLoughlin, G.; Brandeis, D.; Banaschewski, T.; Asherson, P.; Kuntsi, J. Altered EEG spectral power during rest and cognitive performance: A comparison of preterm-born adolescents to adolescents with ADHD. Eur. Child Adolesc. Psychiatry 2017, 26, 1511–1522. [Google Scholar] [CrossRef] [Green Version]
- Buyck, I.; Wiersema, J.R. Resting electroencephalogram in attention deficit hyperactivity disorder: Developmental course and diagnostic value. Psychiatry Res. 2014, 216, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.K.; Cho, A.; Hale, T.S.; McGough, J.; McCracken, J.; Smalley, S.L. Characterization of the theta to beta ratio in ADHD: Identifying potential sources of heterogeneity. J. Atten. Disord. 2013, 17, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Liechti, M.; Valko, L.; Müller, U.C.; Döhnert, M.; Drechsler, R.; Steinhausen, H.-C.; Brandeis, D. Diagnostic value of resting electroencephalogram in attention-deficit/hyperactivity disorder across the lifespan. Brain Topogr. 2013, 26, 135–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Mean ± S.D. | ADHD-H (n = 24) | ADHD-L (n = 18) | TD (n = 31) | p | ADHD-H vs. TD (p) | ADHD-L vs. TD (p) | ADHD-H vs. ADHD-L (p) |
|---|---|---|---|---|---|---|---|
| Age | 67.21 ± 5.79 | 69.00 ± 6.80 | 67.81 ± 5.09 | 0.607 | |||
| Sex (male: female) | 18:6 | 16:2 | 23:8 | 0.482 | |||
| FSIQ | 99.83 ± 12.24 | 87.94 ± 13.84 | 97.66 ± 14.06 | 0.015 * | 0.558 | 0.019 * | 0.006 ** |
| VCI | 99.88 ± 12.01 | 96.11 ± 14.07 | 98.34 ± 14.21 | 0.670 | 0.682 | 0.582 | 0.373 |
| CPI | 99.76 ± 9.90 | 75.55 ± 6.96 | 97.18 ± 13.38 | <0.001 ** | 0.465 | <0.001 ** | <0.001 ** |
| DBRS-P-i | 12.88 ± 3.89 | 14.89 ± 4.85 | 10.48 ± 4.68 | 0.005 ** | 0.054 | 0.001 ** | 0.154 |
| DBRS-P-h | 13.38 ± 5.51 | 12.50 ± 6.51 | 8.90 ± 5.34 | 0.012 * | 0.005 ** | 0.037 * | 0.624 |
| DBRS-T-i | 13.92 ± 5.37 | 17.72 ± 4.28 | 8.47 ± 5.43 | <0.001 ** | <0.001 ** | <0.001 * | 0.021 * |
| DBRS-T-h | 12.63 ± 5.88 | 14.00 ± 5.89 | 6.60 ± 7.24 | <0.001 ** | 0.001 ** | <0.001 ** | 0.499 |
| d’ | 50.00 ± 6.43 | 54.61 ± 7.92 | 48.39 ± 6.08 | 0.009 ** | 0.378 | 0.002 ** | 0.030 * |
| omission | 48.58 ± 6.11 | 54.56 ± 9.26 | 48.13 ± 7.92 | 0.016 * | 0.830 | 0.007 ** | 0.016 * |
| commission | 48.88 ± 7.74 | 52.61 ± 9.68 | 46.94 ± 7.94 | 0.078 | 0.395 | 0.025 * | 0.155 |
| perseveration | 48.63 ± 5.34 | 49.89 ± 7.44 | 46.97 ± 3.83 | 0.179 | 0.262 | 0.072 | 0.455 |
| HRT | 54.58 ± 7.19 | 63.17 ± 7.31 | 55.32 ± 6.46 | <0.001 ** | 0.695 | <0.001 ** | <0.001 ** |
| HRT SD | 49.96 ± 6.05 | 54.78 ± 10.62 | 47.19 ± 6.13 | 0.004 ** | 0.177 | 0.001 ** | 0.042 * |
| variability | 52.29 ± 10.15 | 51.22 ± 9.05 | 47.65 ± 6.70 | 0.116 | 0.049 * | 0.162 | 0.689 |
| HRT block change | 50.08 ± 6.46 | 51.11 ± 11.04 | 48.48 ± 5.80 | 0.428 | 0.442 | 0.247 | 0.666 |
| HRT ISI change | 50.08 ± 6.59 | 55.67 ± 10.84 | 47.55 ± 7.03 | 0.004 * | 0.248 | 0.001 ** | 0.028 * |
| Mean | SD | Overall (p) | ADHD-H vs. TD (p) | ADHD-L vs. TD (p) | ADHD-H vs. ADHD-L (p) | |||
| Delta | Ctrl | ADHD-H | 33.73 | 1.38 | 0.026 | 0.008 * | 0.145 | 0.325 |
| ADHD-L | 33.33 | 1.16 | ||||||
| TD | 32.77 | 1.28 | ||||||
| Pz | ADHD-H | 33.32 | 0.81 | 0.022 | 0.281 | 0.006 * | 0.083 | |
| ADHD-L | 33.97 | 1.31 | ||||||
| TD | 32.97 | 1.35 | ||||||
| Alpha | Pz | ADHD-H | 22.65 | 0.51 | 0.054 | 0.895 | 0.031 | 0.030 |
| ADHD-L | 22.30 | 0.45 | ||||||
| TD | 22.63 | 0.55 | ||||||
| Beta | Ctrl | ADHD-H | 15.60 | 1.72 | 0.059 | 0.018 * | 0.340 | 0.233 |
| ADHD-L | 16.20 | 1.64 | ||||||
| TD | 16.66 | 1.48 | ||||||
| Pz | ADHD-H | 15.96 | 1.28 | 0.101 | 0.286 | 0.034 | 0.267 | |
| ADHD-L | 15.41 | 1.57 | ||||||
| TD | 16.41 | 1.73 |
| Mean | SD | Overall (p) | ADHD-H vs. TD (p) | ADHD-L vs. TD (p) | ADHD-H vs. ADHD-L (p) | |||
|---|---|---|---|---|---|---|---|---|
| Delta | Pz | ADHD-H | 32.92 | 1.30 | 0.002 | 0.208 | 0.011 * | 0.001 * |
| ADHD-L | 34.27 | 0.96 | ||||||
| TD | 33.34 | 1.25 | ||||||
| Theta | Pz | ADHD-H | 27.95 | 1.09 | 0.090 | 0.154 | 0.327 | 0.031 * |
| ADHD-L | 28.57 | 0.80 | ||||||
| TD | 28.30 | 0.79 | ||||||
| Alpha | Pz | ADHD-H | 22.76 | 0.79 | 0.030 | 0.195 | 0.102 | 0.008 * |
| ADHD-L | 22.26 | 0.39 | ||||||
| TD | 22.55 | 0.50 | ||||||
| Beta | Pz | ADHD-H | 16.37 | 1.81 | 0.007 | 0.161 | 0.039 | 0.002 * |
| ADHD-L | 14.90 | 0.66 | ||||||
| TD | 15.81 | 1.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, I.-C.; Chen, C.-L.; Chang, C.-H.; Fan, Z.-C.; Chang, Y.; Lin, C.-H.; Ko, L.-W. Task-Rate-Related Neural Dynamics Using Wireless EEG to Assist Diagnosis and Intervention Planning for Preschoolers with ADHD Exhibiting Heterogeneous Cognitive Proficiency. J. Pers. Med. 2022, 12, 731. https://doi.org/10.3390/jpm12050731
Chen I-C, Chen C-L, Chang C-H, Fan Z-C, Chang Y, Lin C-H, Ko L-W. Task-Rate-Related Neural Dynamics Using Wireless EEG to Assist Diagnosis and Intervention Planning for Preschoolers with ADHD Exhibiting Heterogeneous Cognitive Proficiency. Journal of Personalized Medicine. 2022; 12(5):731. https://doi.org/10.3390/jpm12050731
Chicago/Turabian StyleChen, I-Chun, Chia-Ling Chen, Chih-Hao Chang, Zuo-Cian Fan, Yang Chang, Cheng-Hsiu Lin, and Li-Wei Ko. 2022. "Task-Rate-Related Neural Dynamics Using Wireless EEG to Assist Diagnosis and Intervention Planning for Preschoolers with ADHD Exhibiting Heterogeneous Cognitive Proficiency" Journal of Personalized Medicine 12, no. 5: 731. https://doi.org/10.3390/jpm12050731







