Goal-Directed Fluid Therapy Enhances Gastrointestinal Recovery after Laparoscopic Surgery: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Registration and Protocol
2.2. Eligibility Criteria
2.3. Data Items
2.4. Search Strategy and Information Sources
2.5. Selection Process
2.6. Data Collection Process
2.7. Synthesis of Results and Effect Measures
2.8. Study Risk of Bias Assessment and Reporting Bias Assessment
2.9. Certainty Assessment
3. Results
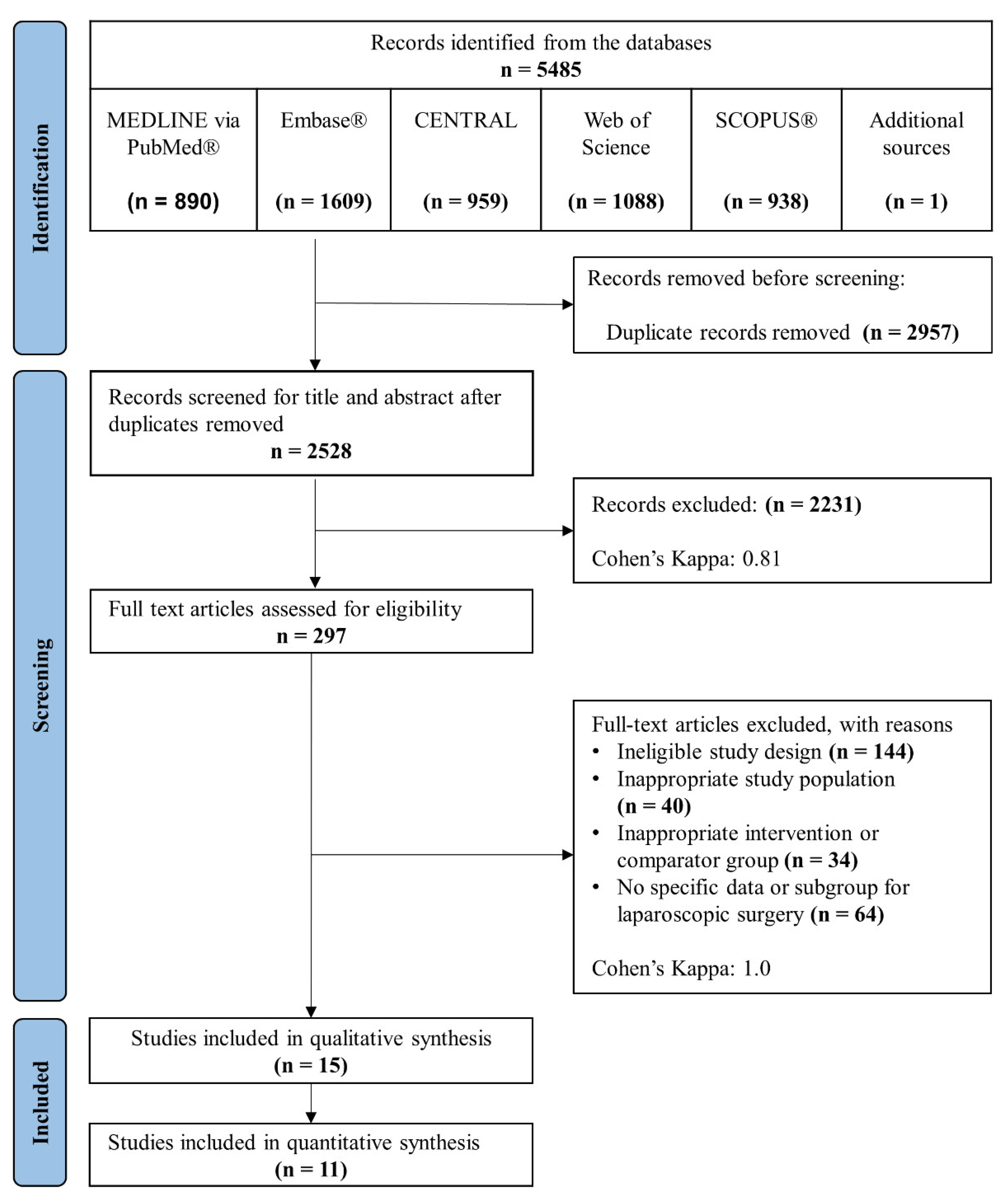
3.1. Study Selection
3.2. Study Characteristics
3.3. Results of Syntheses and Individual Studies
3.3.1. Length of Hospital Stay
3.3.2. Readmission and Reoperation Rate
3.3.3. Overall Complications within 30 Days
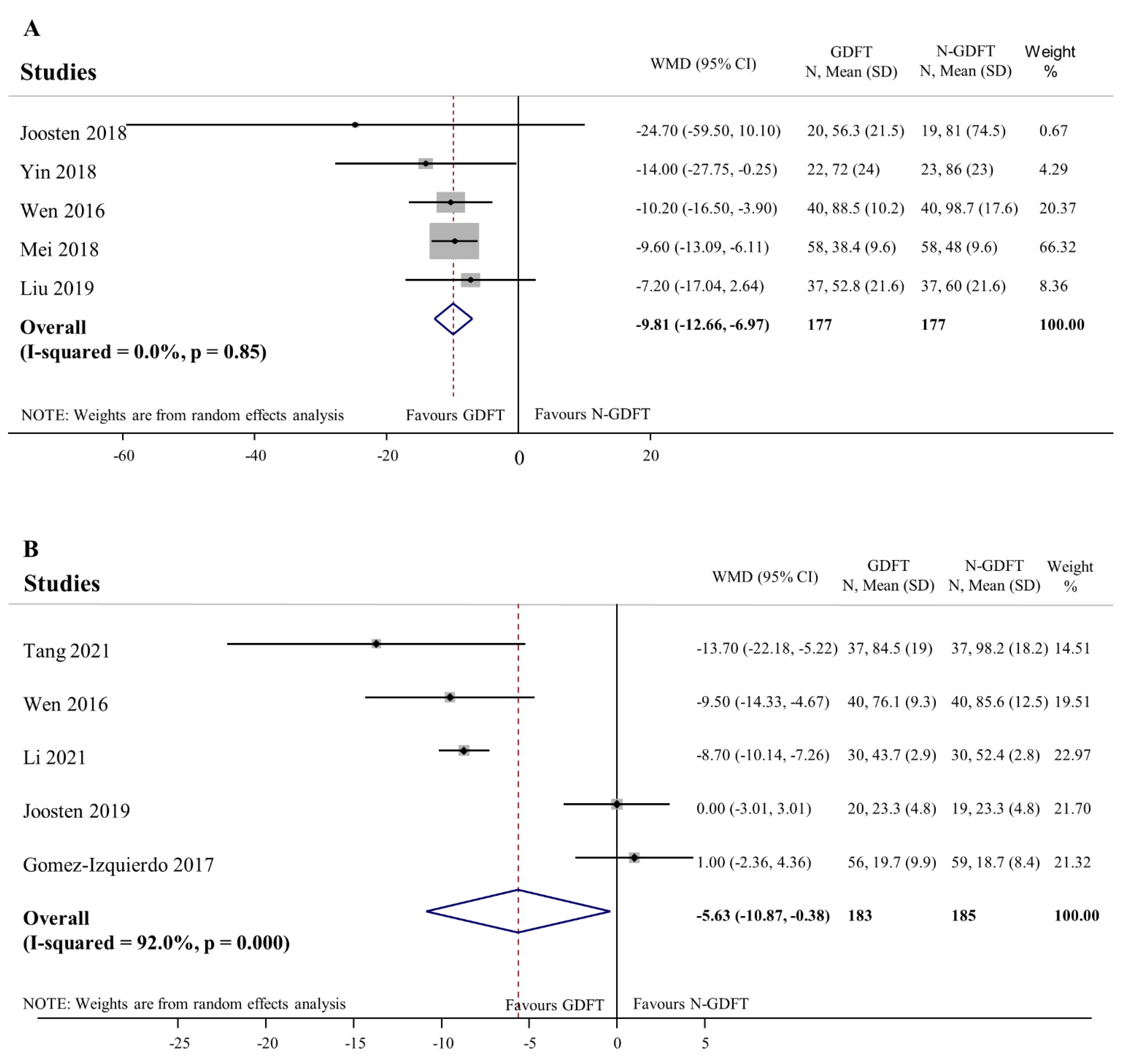
3.3.4. Recovery of Gastrointestinal Function as Indicated by Time to Firs Flatus and Time to First Stool
3.3.5. Intraoperative Clinical Outcomes: Intraoperative Fluid and Vasopressor Requirement, Standardised Intraoperative Urinary Output and Lactate Levels at the End of the Operation
3.4. Risk of Bias in Studies and Certainty of Evidence
4. Discussion
4.1. Summary of Evidence
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buia, A.; Stockhausen, F.; Hanisch, E. Laparoscopic surgery: A qualified systematic review. World J. Methodol. 2015, 5, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Salvans, S.; Pera, M. Laparoscopic colorectal surgery: Current status and implementation of the latest technological innovations. World J. Gastroenterol. 2016, 22, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Concha, M.R.; Mertz, V.F.; Cortinez, L.I.; Gonzalez, K.A.; Butte, J.M.; Lopez, F.; Pinedo, G.; Zuniga, A. The Volume of Lactated Ringer’s Solution Required to Maintain Preload and Cardiac Index during Open and Laparoscopic Surgery. Anesth. Analg. 2009, 108, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Safran, D.B.; Orlando, R., III. Physiologic effects of pneumoperitoneum. Am. J. Surg. 1994, 167, 281–286. [Google Scholar] [CrossRef]
- Atkinson, T.M.; Giraud, G.D.; Togioka, B.M.; Jones, D.B.; Cigarroa, J.E. Cardiovascular and Ventilatory Consequences of Laparoscopic Surgery. Circulation 2017, 135, 700–710. [Google Scholar] [CrossRef]
- Oti, C.; Mahendran, M.; Sabir, N. Anaesthesia for laparoscopic surgery. Br. J. Hosp. Med. 2016, 77, 24–28. [Google Scholar] [CrossRef]
- Cecconi, M.; Corredor, C.; Arulkumaran, N.; Abuella, G.; Ball, J.; Grounds, R.M.; Hamilton, M.; Rhodes, A. Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit. Care 2013, 17, 209. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations: 2018. World J. Surg. 2018, 43, 659–695. [Google Scholar] [CrossRef] [Green Version]
- Melloul, E.; Hübner, M.; Scott, M.; Snowden, C.; Prentis, J.; Dejong, C.H.; Garden, O.J.; Farges, O.; Kokudo, N.; Vauthey, J.N.; et al. Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J. Surg. 2016, 40, 2425–2440. [Google Scholar] [CrossRef] [Green Version]
- Nygren, J.; Thacker, J.; Carli, F.; Fearon, K.C.H.; Norderval, S.; Lobo, D.N.; Ljungqvist, O.; Soop, M.; Ramirez, J. Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery after Surgery (ERAS®) Society recommendations. Clin. Nutr. 2012, 31, 801–816. [Google Scholar] [CrossRef]
- Larsen, J.F.; Svendsen, F.M.; Pedersen, V. Randomized clinical trial of the effect of pneumoperitoneum on cardiac function and haemodynamics during laparoscopic cholecystectomy. Br. J. Surg. 2004, 91, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Odeberg, S.; Ljungqvist, O.; Svenberg, T.; Gannedahl, P.; Bäckdahl, M.; von Rosen, A.; Sollevi, A. Haemodynamic effects of pneumoperitoneum and the influence of posture during anaesthesia for laparoscopic surgery. Acta Anaesthesiol. Scand. 1994, 38, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Portera, C.A.; Compton Rp Fau-Walters, D.N.; Walters Dn Fau-Browder, I.W.; Browder, I.W. Benefits of pulmonary artery catheter and transesophageal echocardiographic monitoring in laparoscopic cholecystectomy patients with cardiac disease. Am. J. Surg. 1995, 169, 202–206. [Google Scholar] [CrossRef]
- Hein, H.A.T.; Joshi, G.P.; Ramsay, M.A.E.; Fox, L.G.; Gawey, B.J.; Hellman, C.L.; Arnold, J.C. Hemodynamic changes during laparoscopic cholecystectomy in patients with severe cardiac disease. J. Clin. Anesth. 1997, 9, 261–265. [Google Scholar] [CrossRef]
- Miller Te Fau-Roche, A.M.; Roche Am Fau-Gan, T.J.; Gan, T.J. Poor adoption of hemodynamic optimization during major surgery: Are we practicing substandard care? Anesth. Analg. 2011, 112, 1274–1276. [Google Scholar] [CrossRef]
- Gurgel, S.T.; do Nascimento, P. Maintaining Tissue Perfusion in High-Risk Surgical Patients: A Systematic Review of Randomized Clinical Trials. Anesth. Analg. 2011, 112, 1384–1391. [Google Scholar] [CrossRef]
- Hamilton, M.A.; Cecconi, M.; Rhodes, A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth. Analg. 2011, 112, 1392–1402. [Google Scholar] [CrossRef]
- Srinivasa, S.; Taylor, M.H.; Singh, P.P.; Yu, T.C.; Soop, M.; Hill, A.G. Randomized clinical trial of goal-directed fluid therapy within an enhanced recovery protocol for elective colectomy. Br. J. Surg. 2013, 100, 66–74. [Google Scholar] [CrossRef]
- Miller, T.E.; Roche, A.M.; Mythen, M. Fluid management and goal-directed therapy as an adjunct to Enhanced Recovery After Surgery (ERAS). Can. J. Anesth-J. Can. D Anesth. 2015, 62, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Wrzosek, A.; Jakowicka-Wordliczek, J.; Zajaczkowska, R.; Serednicki, W.T.; Jankowski, M.; Bala, M.M.; Swierz, M.J.; Polak, M.; Wordliczek, J. Perioperative restrictive versus goal-directed fluid therapy for adults undergoing major non-cardiac surgery. Cochrane Database Syst. Rev. 2019, 12, Cd012767. [Google Scholar] [CrossRef]
- Xu, C.; Peng, J.; Liu, S.; Huang, Y.; Guo, X.; Xiao, H.; Qi, D. Goal-directed fluid therapy versus conventional fluid therapy in colorectal surgery: A meta analysis of randomized controlled trials. Int. J. Surg. 2018, 56, 264–273. [Google Scholar] [CrossRef]
- Chong, M.A.; Wang, Y.; Berbenetz, N.M.; McConachie, I. Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes?: A systematic review and meta-analysis. Eur. J. Anaesthesiol. 2018, 35, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.A.-O.; Robba, C.; Calabrò, L.; Zambelli, D.; Iannuzzi, F.; Molinari, E.; Scarano, S.; Battaglini, D.; Baggiani, M.; de Mattei, G.; et al. Association between perioperative fluid administration and postoperative outcomes: A 20-year systematic review and a meta-analysis of randomized goal-directed trials in major visceral/noncardiac surgery. Crit. Care 2021, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Izquierdo, J.C.; Feldman, L.S.; Carli, F.; Baldini, G. Meta-analysis of the effect of goal-directed therapy on bowel function after abdominal surgery. Br. J. Surg. 2015, 102, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Rollins, K.E.; Mathias, N.C.; Lobo, D.N. Meta-analysis of goal-directed fluid therapy using transoesophageal Doppler monitoring in patients undergoing elective colorectal surgery. BJS Open 2019, 3, 606–616. [Google Scholar] [CrossRef] [Green Version]
- Arulkumaran, N.; Corredor, C.; Hamilton, M.A.; Ball, J.; Grounds, R.M.; Rhodes, A.; Cecconi, M. Cardiac complications associated with goal-directed therapy in high-risk surgical patients: A meta-analysis. Br. J. Anaesth. 2014, 112, 648–659. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, T.A.-O.; Clement, R.P.; Scheeren, T.W.L.; Saugel, B.; Keus, F.; van der Horst, I.C.C. Perioperative goal-directed therapy: A systematic review without meta-analysis. Acta Anaesthesiol. Scand. 2018, 62, 1340–1355. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Tian, L.; Brackett, A.; Dai, F.; Xu, J.; Meng, L. Classification and differential effectiveness of goal-directed hemodynamic therapies in surgical patients: A network meta-analysis of randomized controlled trials. J. Crit. Care 2021, 61, 152–161. [Google Scholar] [CrossRef]
- Feldheiser, A.; Aziz, O.; Baldini, G.; Cox, B.P.; Fearon, K.C.; Feldman, L.S.; Gan, T.J.; Kennedy, R.H.; Ljungqvist, O.; Lobo, D.N.; et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: Consensus statement for anaesthesia practice. Acta Anaesthesiol. Scand. 2016, 60, 289–334. [Google Scholar] [CrossRef] [Green Version]
- McEvoy, M.D.; Scott, M.J.; Gordon, D.B.; Grant, S.A.; Thacker, J.K.M.; Wu, C.L.; Gan, T.J.; Mythen, M.G.; Shaw, A.D.; Miller, T.E. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on optimal analgesia within an enhanced recovery pathway for colorectal surgery: Part 1-from the preoperative period to PACU. Perioper. Med. 2017, 6, 8. [Google Scholar] [CrossRef]
- Thiele, R.H.; Raghunathan, K.; Brudney, C.S.; Lobo, D.N.; Martin, D.; Senagore, A.; Cannesson, M.; Gan, T.J.; Mythen, M.M.; Shaw, A.D.; et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on perioperative fluid management within an enhanced recovery pathway for colorectal surgery. Perioper. Med. 2016, 17, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.A.-O.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Heerdt, P.M. Perioperative goal-directed haemodynamic therapy based on flow parameters: A concept in evolution. Br. J. Anaesth. 2016, 117, iii3–iii17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bundgaard-Nielsen, M.; Holte, K.; Secher, N.H.; Kehlet, H. Monitoring of peri-operative fluid administration by individualized goal-directed therapy. Acta Anaesthesiol. Scand. 2007, 51, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- DerSimonian, R.; Fau-Laird, N.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, T.J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane. 2021. Available online: www.training.cochrane.org/handbook (accessed on 26 April 2022).
- Sterne, J.A.-O.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Sutton Aj Fau-Ioannidis, J.P.A.; Ioannidis Jp Fau-Terrin, N.; Terrin, N.; Fau-Jones, D.R.; Jones Dr Fau-Lau, J.; Lau, J.; Fau-Carpenter, J.; Carpenter, J.; Fau-Rücker, G.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman Ad Fau-Vist, G.E.; Vist Ge Fau-Kunz, R.; Kunz, R.; Fau-Falck-Ytter, Y.; Falck-Ytter Y Fau-Alonso-Coello, P.; Alonso-Coello, P.; Fau-Schünemann, H.J.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924. [Google Scholar] [CrossRef] [Green Version]
- Brozek, J.L.; Akl Ea Fau-Jaeschke, R.; Jaeschke, R.; Fau-Lang, D.M.; Lang Dm Fau-Bossuyt, P.; Bossuyt, P.; Fau-Glasziou, P.; Glasziou, P.; Fau-Helfand, M.; Helfand, M.; et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines: Part 2 of 3. The GRADE approach to grading quality of evidence about diagnostic tests and strategies. Allergy 2009, 64, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; Higgins, J.P.T.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H.; on behalf of the Cochrane GRADEing Methods Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations Updated October 2013: The GRADE Working Group. Available online: https://training.cochrane.org/handbook/current/chapter-14 (accessed on 9 June 2021).
- Brandstrup, B.; Svendsen, P.E.; Rasmussen, M.; Belhage, B.; Rodt, S.; Hansen, B.; Møller, D.R.; Lundbech, L.B.; Andersen, N.; Berg, V.; et al. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: Near-maximal stroke volume or zero fluid balance? Br. J. Anaesth. 2012, 109, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo-Vecino, J.M.; Ripollés-Melchor, J.; Mythen, M.G.; Casans-Francés, R.; Balik, A.; Artacho, J.P.; Martínez-Hurtado, E.; Serrano Romero, A.; Fernández Pérez, C.; Asuero de Lis, S. Effect of goal-directed haemodynamic therapy on postoperative complications in low-moderate risk surgical patients: A multicentre randomised controlled trial (FEDORA trial). Br. J. Anaesth. 2018, 120, 734–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.J.; Huang, Y.H.; Poon, K.S.; Chen, K.B.; Liao, K.H. Perioperative hemodynamic optimization in laparoscopic sleeve gastrectomy using stroke volume variation to reduce postoperative nausea and vomiting. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2021, 17, 1549–1557. [Google Scholar] [CrossRef]
- Demirel, I.; Bolat, E.; Altun, A.Y.; Ozdemir, M.; Bestas, A. Efficacy of Goal-Directed Fluid Therapy via Pleth Variability Index During Laparoscopic Roux-en-Y Gastric Bypass Surgery in Morbidly Obese Patients. Obes. Surg. 2017, 28, 358–363. [Google Scholar] [CrossRef]
- Gómez-Izquierdo, J.C.; Trainito, A.; Mirzakandov, D.; Stein, B.L.; Liberman, S.; Charlebois, P.; Pecorelli, N.; Feldman, L.S.; Carli, F.; Baldini, G. Goal-directed Fluid Therapy Does Not Reduce Primary Postoperative Ileus after Elective Laparoscopic Colorectal Surgery: A Randomized Controlled Trial. Anesthesiology 2017, 127, 36–49. [Google Scholar] [CrossRef]
- Joosten, A.; Raj Lawrence, S.; Colesnicenco, A.; Coeckelenbergh, S.; Vincent, J.L.; van der Linden, P.; Cannesson, M.; Rinehart, J. Personalized Versus Protocolized Fluid Management Using Noninvasive Hemodynamic Monitoring (Clearsight System) in Patients Undergoing Moderate-Risk Abdominal Surgery. Anesth. Analg. 2019, 129, e8–e12. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Liu, Y.; Zhang, J.; Zhang, W.; Guo, F. Effect of goal-directed fluid therapy on gastrointestinal function of patients after laparoscopic radical resection of cervical cancer. Cancer Res. Clin. 2021, 33, 204–208. [Google Scholar] [CrossRef]
- Liu, F.; Lv, J.; Zhang, W.; Liu, Z.; Dong, L.; Wang, Y. Randomized controlled trial of regional tissue oxygenation following goal-directed fluid therapy during laparoscopic colorectal surgery. Int. J. Clin. Exp. Pathol. 2019, 12, 4390–4399. [Google Scholar]
- Mei, X.; Liu, J.; Wang, Y.; Wei, L.; Tan, S. Application of stroke volume variation-guided liquid therapy in laparoscopic precision hepatectomy. Zhong Nan Da Xue Xue Bao Yi Xue Ban J. Cent. South Univ. Med. Sci. 2019, 44, 1163–1168. [Google Scholar] [CrossRef]
- Muhlbacher, J.; Luf, F.; Zotti, O.; Herkner, H.; Fleischmann, E.; Kabon, B. Effect of Intraoperative Goal-Directed Fluid Management on Tissue Oxygen Tension in Obese Patients: A Randomized Controlled Trial. Obes. Surg. 2021, 31, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Ratti, F.; Cipriani, F.; Reineke, R.; Catena, M.; Paganelli, M.; Comotti, L.; Beretta, L.; Aldrighetti, L. Intraoperative monitoring of stroke volume variation versus central venous pressure in laparoscopic liver surgery: A randomized prospective comparative trial. HPB 2016, 18, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senagore, A.J.; Emery, T.; Luchtefeld, M.; Kim, D.; Dujovny, N.; Hoedema, R. Fluid management for laparoscopic colectomy: A prospective, randomized assessment of goal-directed administration of balanced salt solution or hetastarch coupled with an enhanced recovery program. Dis. Colon Rectum 2009, 52, 1935–1940. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Zhou, S. Analysis on the application value of goal-directed fluid therapy in patients undergoing laparoscopy-assisted radical gastrectomy with fast-track anesthesia. Am. J. Transl. Res. 2021, 13, 5174–5182. [Google Scholar] [PubMed]
- Wen, X.L.; Jing, G.X.; He, P.; Hou, J.R. Clinical study on the capacity management guided by stroke volume variation in elderly patients with laparoscopic radical gastrectomy for gastric cancer. J. Xi’an Jiaotong Univ. (Med. Sci.) 2016, 37, 851–856. [Google Scholar] [CrossRef]
- Yin, K.; Ding, J.; Wu, Y.; Peng, M. Goal-directed fluid therapy based on noninvasive cardiac output monitor reduces postoperative complications in elderly patients after gastrointestinal surgery: A randomized controlled trial. Pak. J. Med. Sci. 2018, 34, 1320–1325. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Wolfe, B.M. The physiologic effects of pneumoperitoneum in the morbidly obese. Ann. Surg. 2005, 241, 219–226. [Google Scholar] [CrossRef]
- Maddison, L.; Starkopf, J.; Reintam Blaser, A. Mild to moderate intra-abdominal hypertension: Does it matter? World J. Crit. Care Med. 2016, 5, 96–102. [Google Scholar] [CrossRef]
- Saugel, B.; Vincent, J.L. Protocolised personalised peri-operative haemodynamic management. Eur. J. Anaesthesiol. 2019, 36, 551–554. [Google Scholar] [CrossRef]
- Molnar, Z.; Benes, J.; Saugel, B. Intraoperative hypotension is just the tip of the iceberg: A call for multimodal, individualised, contextualised management of intraoperative cardiovascular dynamics. Br. J. Anaesth. 2020, 125, 419–423. [Google Scholar] [CrossRef]
- Moore-Olufemi, S.D.; Xue, H.; Fau-Attuwaybi, B.O.; Attuwaybi Bo Fau-Fischer, U.; Fischer, U.; Fau-Harari, Y.; Harari, Y.; Fau-Oliver, D.H.; Oliver Dh Fau-Weisbrodt, N.; Weisbrodt, N.; et al. Resuscitation-induced gut edema and intestinal dysfunction. J. Trauma 2005, 58, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.H.; Lobo, D.N. Fluids and gastrointestinal function. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 469–476. [Google Scholar] [CrossRef]
- Mythen, M.G.; Webb, A.R. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg. 1995, 130, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Bellomo, R.; Corcoran, T.; Forbes, A.; Peyton, P.; Story, D.; Christophi, C.; Leslie, K.; McGuinness, S.; Parke, R.; et al. Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N. Engl. J. Med. 2018, 378, 2263–2274. [Google Scholar] [CrossRef]
- Holte, K.; Kehlet, H. Fluid therapy and surgical outcomes in elective surgery: A need for reassessment in fast-track surgery. J. Am. Coll. Surg. 2006, 202, 971–989. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Zimmermann, J.B.; Schmidt, T.; Koch, M.; Weigand, M.A.; Weitz, J. Meta-analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. Br. J. Surg. 2009, 96, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Saugel, B.; Fau-Thiele, R.H.; Thiele Rh Fau-Hapfelmeier, A.; Hapfelmeier, A.; Fau-Cannesson, M.; Cannesson, M. Technological Assessment and Objective Evaluation of Minimally Invasive and Noninvasive Cardiac Output Monitoring Systems. Anesthesiology 2020, 133, 921–928. [Google Scholar] [CrossRef]
- Heming, N.; Moine, P.; Coscas, R.; Annane, D. Perioperative fluid management for major elective surgery. Br. J. Surg. 2020, 107, e56–e62. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Soppitt, A.; Maroof, M.; el-Moalem, H.; Robertson, K.M.; Moretti, E.; Dwane, P.; Glass, P.S. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 2002, 97, 820–826. [Google Scholar] [CrossRef]
- Grocott, M.P.W.; Dushianthan, A.; Hamilton, M.A.; Mythen, M.G.; Harrison, D.; Rowan, K.; Optimisation Systematic Review Steering Group. Perioperative increase in global blood flow to explicit defined goals and outcomes after surgery: A Cochrane Systematic Review. Br. J. Anaesth. 2013, 111, 535–548. [Google Scholar] [CrossRef] [Green Version]
- Wakeling, H.G.; McFall, M.R.; Jenkins, C.S.; Woods, W.G.; Miles, W.F.; Barclay, G.R.; Fleming, S.C. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br. J. Anaesth. 2005, 95, 634–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Malley, C.; Cunningham, A.J. Physiologic Changes during Laparoscopy. Anesthesiol. Clin. N. Am. 2001, 19, 1–19. [Google Scholar] [CrossRef]
- Meregalli, A.; Oliveira Rp Fau-Friedman, G.; Friedman, G. Occult hypoperfusion is associated with increased mortality in hemodynamically stable, high-risk, surgical patients. Crit. Care 2004, 8, R60–R65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forget, P.; Lois, F.; de Kock, M. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth. Analg. 2010, 111, 910–914. [Google Scholar] [CrossRef] [PubMed]


| Author (Year) | Type of Surgery | Preoperative Fluid Protocol | Intraoperative Fluid Protocol | Postoperative Fluid Protocol | Primary Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Haemodyamic Technology | Primary Goal | Bolus | Type of Fluid | Basis | Type of Fluid | |||||
| Brandstrup (2012) [44] | Elective laparoscopic colorectal resection | 0.9% saline UD * | Oesophageal Doppler | SV < 10% | 200 mL | VOLUVEN® | Replacement of lost blood volume only | VOLUVEN® | daily 2000 mL | Overall postoperative complications |
| N-GDFT | 200 mL | VOLUVEN® | Replacement of lost blood volume only | VOLUVEN® | ||||||
| Calvo-Vecino (2018) [45] | Laparoscopic gastrointestinal, urological, gynaecological | N/A | Oesophageal Doppler | SV < 10% | 250 mL | VOLUVEN®, Lactated Ringer | 0 mL kg−1 bw−1 | None | N/A | Moderate or severe postoperative complications |
| N-GDFT | AAE | VOLUVEN®, Lactated Ringer | 3–5 mL kg−1 bw−1 | Lactated Ringer | ||||||
| Cho (2021) [46] | Laparoscopic sleeve gastrectomy | 4/2/1 | Arterial waveform-derived | SVV < 10% | 100 mL | 6% hydroxyethyl starch 130/0.4 | 4 mL kg−1 bw−1 | Lactated Ringer or Saline 0.9% | N/A | Postoperative nausea and vomiting |
| Arterial waveform-derived | SVV < 10% | 100 mL | Lactated Ringer | 4 mL kg−1 bw−1 | Lactated Ringer or Saline 0.9% | |||||
| N-GDFT | AAE | 6% hydroxyethyl starch 130/0.4 | 4 mL kg−1 bw−1 | Lactated Ringer or Saline 0.9% | ||||||
| Demirel (2017) [47] | Laparoscopic RYGB surgery | N/A | Pulse oximetry | PVI < 14% | 250 mL | Gelofusine® | 2 mL kg−1 bw−1 | 0.9% NaCl or Lactated Ringer | N/A | Perioperative lactate, creatinine levels, hemodynamic variables |
| N-GDFT | 250 mL | Gelofusine® | 4–8 mL kg−1 bw−1 | 0.9% NaCl or Lactated Ringer | ||||||
| Gomez-Izquierdo (2017) [48] | Laparoscopic colorectal | 4/2/1 | Oesophageal Doppler | SV < 10% | 200 mL | VOLUVEN® | 1.5 mL kg−1 bw−1 | Lactated Ringer | 1.5 mL kg−1/bw−1/h−1 In PACU 15 mL h−1 in Surgical Department | Primary postoperative ileus |
| N-GDFT | 5 mL kg−1 bw−1 | VOLUVEN® | 4/2/1 Rule | Lactated Ringer | ||||||
| Joosten (2018) [49] | Laparoscopic colorectal, gynaecological, urological | N/A | Arterial waveform-derived | SVV < 13% | 100 mL | PlasmaLyte® | 0 mL kg−1 bw−1 | None | N/A | Percentage of intraoperative time spent within defined haemodynamic targets (CI ≥2.5 L/min/m2 and/or an SVV <13%) |
| N-GDFT | AAE | 6% hydroxyethyl starch 130/0.4 | 4 mL kg−1 bw−1 | PlasmaLyte® | ||||||
| Li (2021) [50] | Laparoscopic radical resection of lower cervical cancer | N/A | Arterial waveform-derived | SVV < 13% | 250 mL | 6% hydroxyethyl starch 130/0.4 | 500 mL | Lactated Ringer | N/A | Appearance of first bowel sounds, time to first flatus, lengths of hospital stay, incidence of postoperative nausea and vomiting |
| N-GDFT | AAE | 6% hydroxyethyl starch 130/0.4 | N/A | Lactated Ringer | ||||||
| Liu (2019) [51] | Laparoscopic colorectal | 5 mL kg−1 bw−1 before anaesthesia | Arterial waveform-derived | SVV < 13% | 200 mL | Colloid solution UD | 2 mL kg−1 bw−1 | Lactated Ringer | N/A | Haemodynamic variables and tissue oxygen saturations intraoperatively and at the end of operation |
| N-GDFT | AAE | Colloid solution UD | 5 mL kg−1 bw−1 | Lactated Ringer | ||||||
| Mei (2018) [52] | Laparoscopic precision hepatectomy | N/A | Arterial waveform-derived | SVV < 13% | 3 mL kg−1 bw−1 | Colloid solution UD | 6–10 mL kg−1 bw−1 | Crystalloid UD | N/A | MAP, SVV, CVP, and lactate levels through the intraoperative period and at the end of surgery |
| N-GDFT | 10 mL kg−1 bw−1 | Crystalloid UD | 6–10 mL kg−1 bw−1 | Crystalloid UD | ||||||
| Mühlbacher (2021) [53] | Laparoscopic gastric bypass | 500 mL Lactated-Ringer | Oesophageal Doppler | SV < 10% | 250 mL | Lactated Ringer | 2 mL kg−1 bw−1 | Lactated Ringer | AAE in PACU | Perioperative subcutaneous tissue oxygen tension (upper arm) |
| N-GDFT | AAE | Lactated Ringer | N/A | Lactated Ringer | ||||||
| Ratti (2016) [54] | Laparoscopic liver resection | ERAS ** | Arterial waveform-derived | SVV < 12% | N/A | Crystalloid UD | N/A | Crystalloid UD | ERAS ** | Rate and reasons of conversion |
| N-GDFT | N/A | Crystalloid UD | N/A | Crystalloid UD | ||||||
| Senagore (2009) [55] | Laparoscopic colorectal | N/A | Oesophageal Doppler | SV < 10% | 300 mL | Lactated Ringer | 5 mL kg−1 bw−1 | Lactated Ringer | N/A | Length of hospital stay |
| N-GDFT | AAE | 6% hydroxyethyl starch 130/0.4/, Lactated Ringer | 5 mL kg−1 bw−1 | Lactated Ringer | ||||||
| Tang (2021) [56] | Laparoscopic radical gastectomy | 250 mL warm sugar water per os | Arterial waveform-derived | SVV < 13% | 250 mL | 6% hydroxyethyl starch 130/0.4 | N/A | Crystalloid UD | N/A | Incidence of postoperative complications |
| N-GDFT | AAE | 6% hydroxyethyl starch 130/0.4 | N/A | Crystalloid UD | ||||||
| Wen (2016) [57] | Laparoscopic gastrectomy | N/A | Arterial waveform-derived | SVV < 13% | 3 mL kg−1 bw−1 | 6% hydroxyethyl starch 130/0.4 | 5 mL kg−1 bw−1 | Lactated Ringer | N/A | Changes of haemodynamic variables and application of vasoactive drugs |
| N-GDFT | 5 mL kg−1 bw−1 | 6% hydroxyethyl starch 130/0.4 | 7 mL kg−1 bw−1 | Lactated Ringer | ||||||
| Yin (2018) [58] | Laparoscopic colorectal | N/A | Bioreactance | SVV < 13% | 250 mL | 6% hydroxyethyl starch 130/0.4 | 8 mL kg−1 bw−1 | Saline UD | N/A | Moderate or severe postoperative complications within 30 days |
| N-GDFT | 250 mL | 6% hydroxyethyl starch 130/0.4 | 8 mL kg−1 bw−1 | Saline UD | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virág, M.; Rottler, M.; Gede, N.; Ocskay, K.; Leiner, T.; Tuba, M.; Ábrahám, S.; Farkas, N.; Hegyi, P.; Molnár, Z. Goal-Directed Fluid Therapy Enhances Gastrointestinal Recovery after Laparoscopic Surgery: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 734. https://doi.org/10.3390/jpm12050734
Virág M, Rottler M, Gede N, Ocskay K, Leiner T, Tuba M, Ábrahám S, Farkas N, Hegyi P, Molnár Z. Goal-Directed Fluid Therapy Enhances Gastrointestinal Recovery after Laparoscopic Surgery: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2022; 12(5):734. https://doi.org/10.3390/jpm12050734
Chicago/Turabian StyleVirág, Marcell, Máté Rottler, Noémi Gede, Klementina Ocskay, Tamás Leiner, Máté Tuba, Szabolcs Ábrahám, Nelli Farkas, Péter Hegyi, and Zsolt Molnár. 2022. "Goal-Directed Fluid Therapy Enhances Gastrointestinal Recovery after Laparoscopic Surgery: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 12, no. 5: 734. https://doi.org/10.3390/jpm12050734






