Cone-Beam Navigation Can Reduce the Radiation Exposure and Save Fusion Length-Dependent Operation Time in Comparison to Conventional Fluoroscopy in Pedicle-Screw-Based Lumbar Interbody Fusion
Abstract
:1. Introduction
2. Materials and Methods
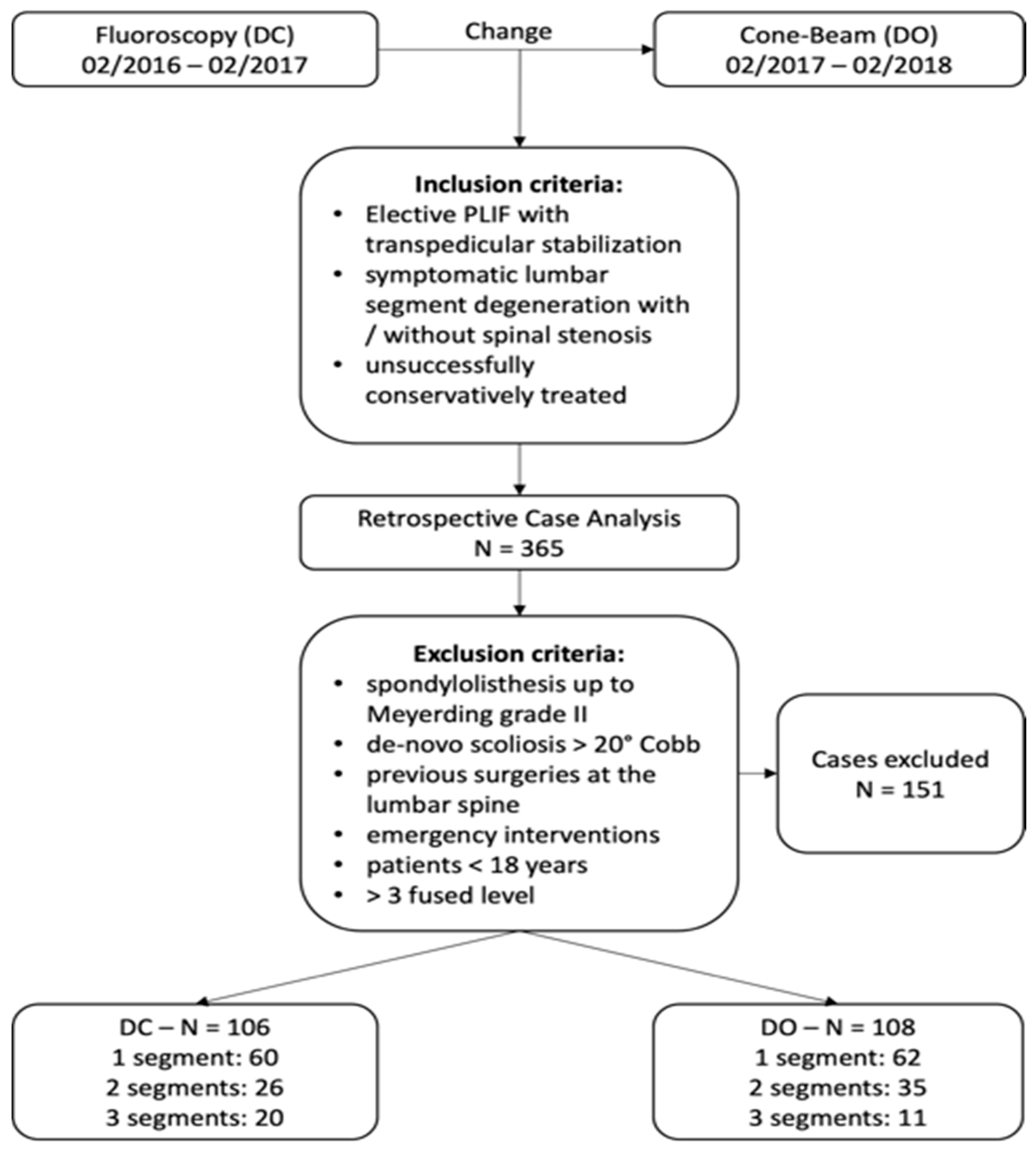
2.1. Study Design, Patients and Groups
2.2. Surgical Procedure
2.3. Radiation Exposure
2.4. Quantification and Comparison of the Radiation Dose
2.5. Statistical Analysis
3. Results
3.1. Demographics
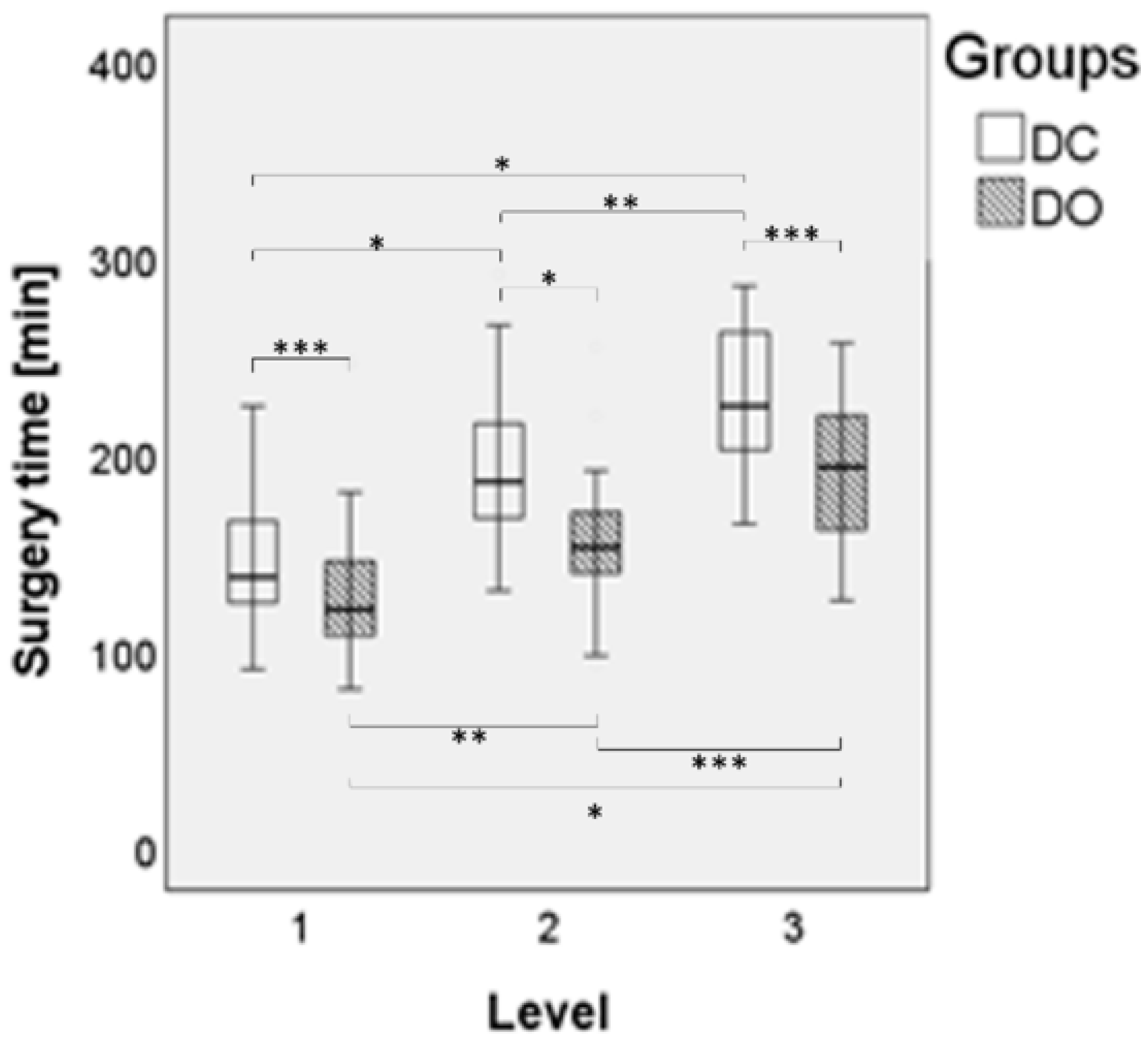
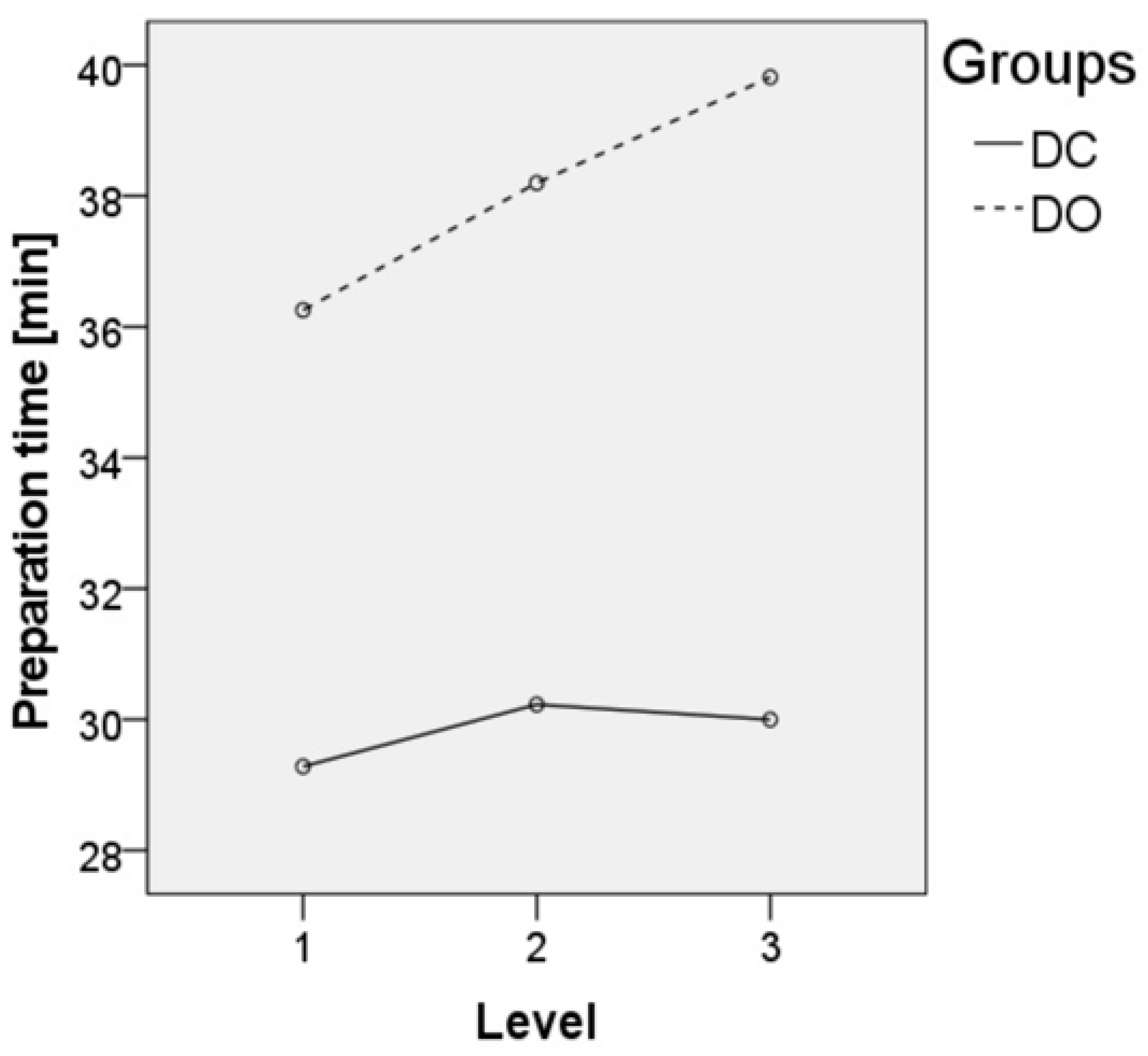
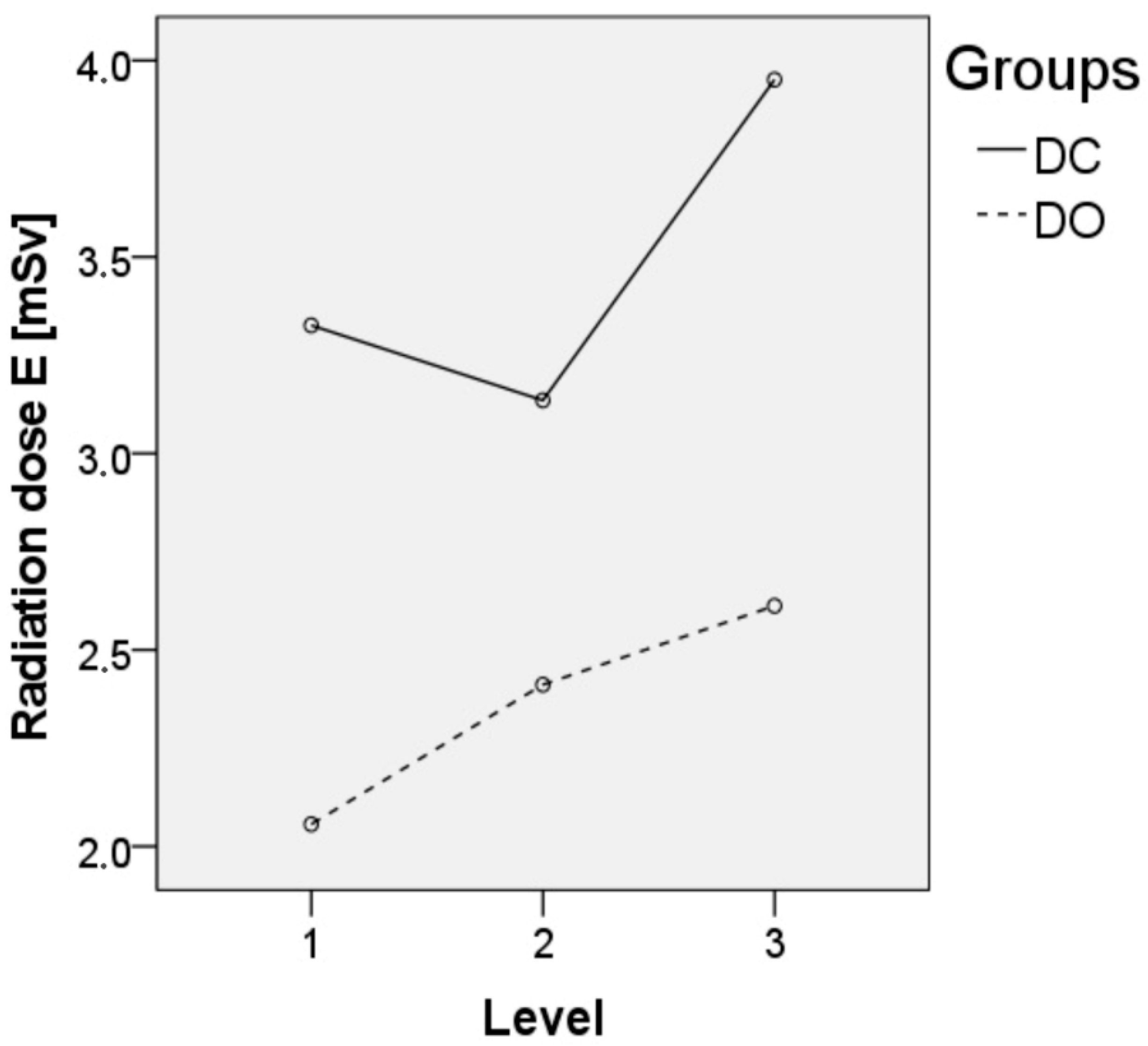
3.2. Surgical Data
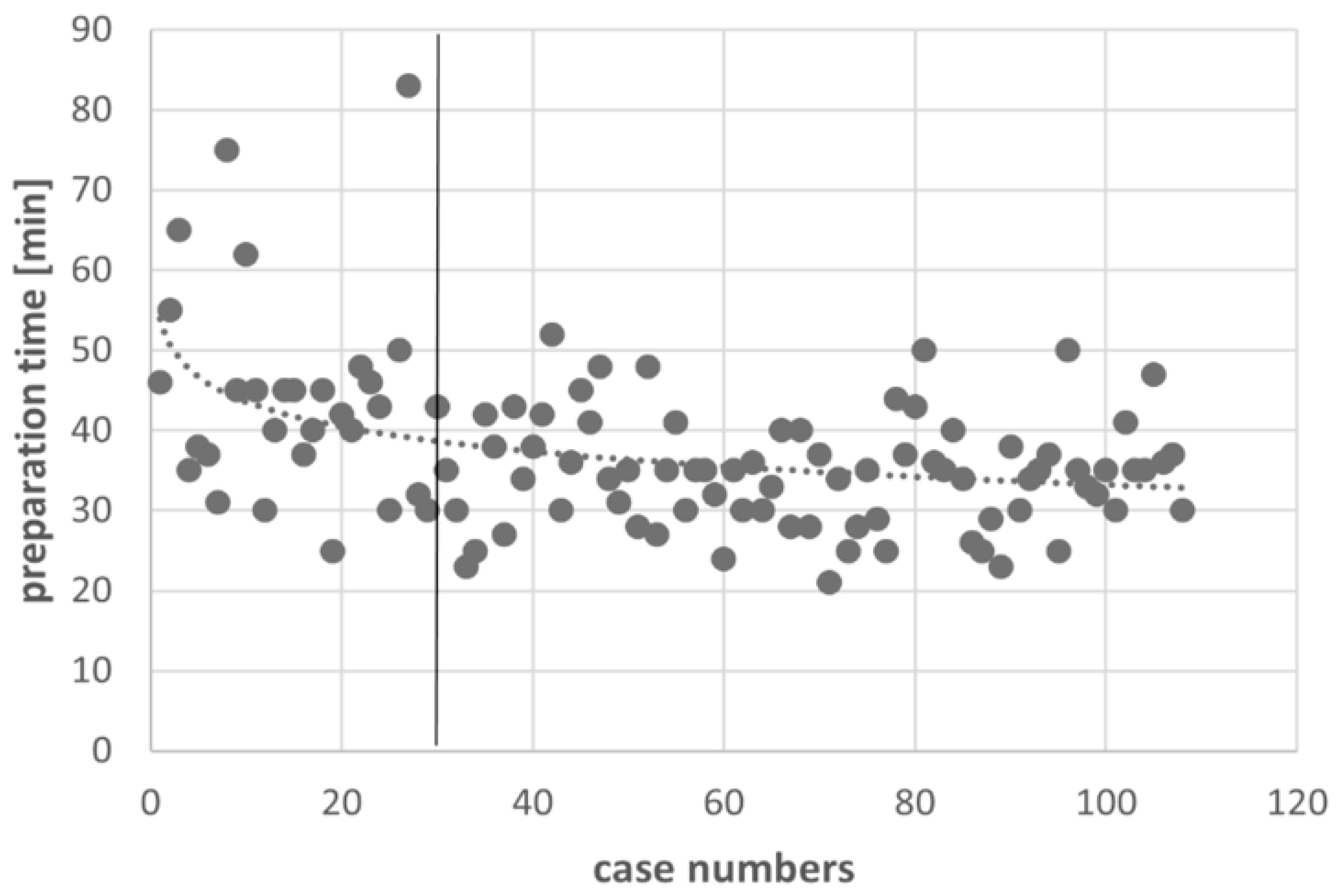
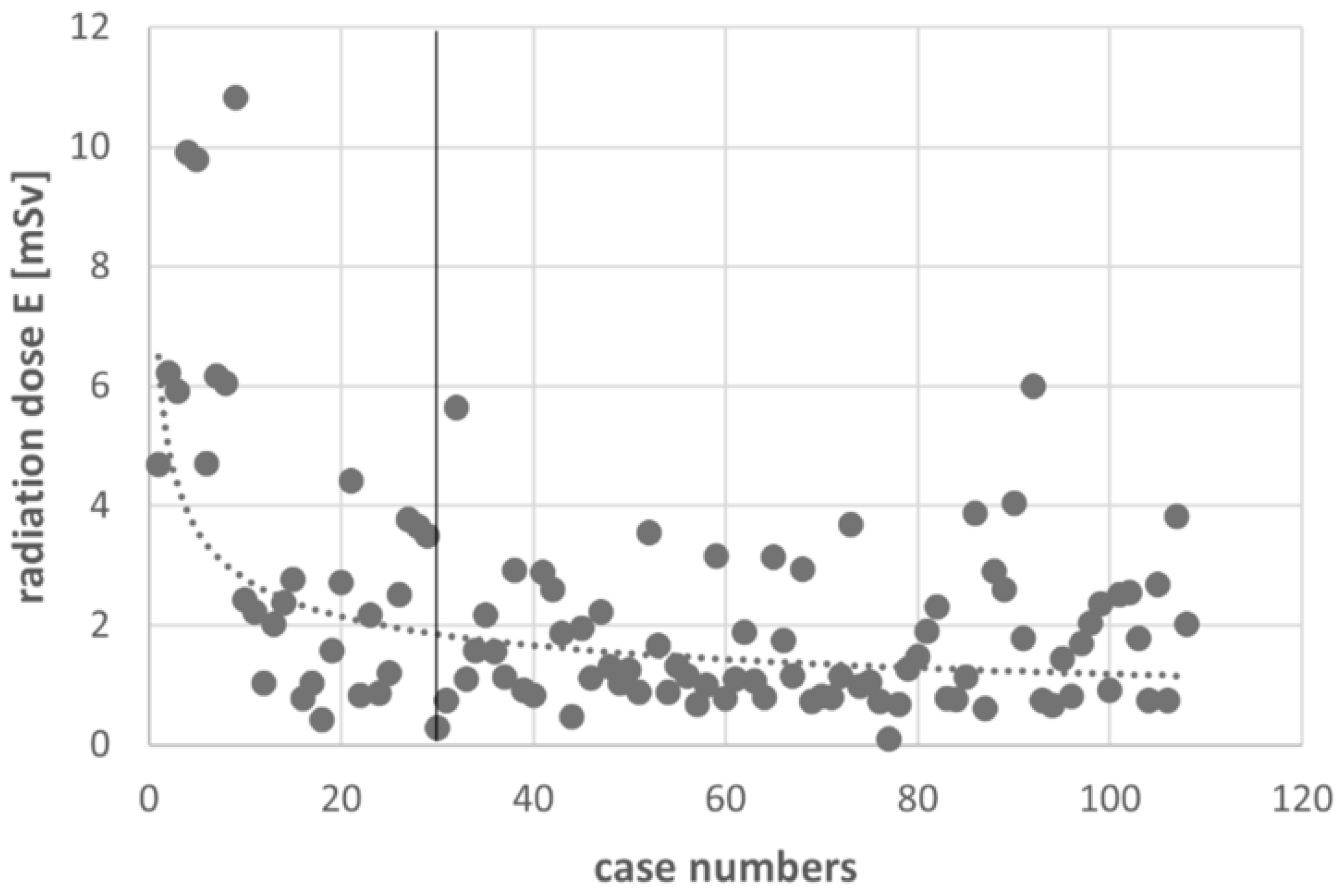
3.3. Learning Curve
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esses, S.I.; Sachs, B.L.; Dreyzin, V. Complications associated with the technique of pedicle screw fixation. A selected survey of ABS members. Spine 1993, 18, 2231–2238; discussion 2238–2239. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Hur, J.W.; Ryu, K.S.; Park, C.K. Prospective Comparison Study between the Fluoroscopy-guided and Navigation Coupled with O-arm-guided Pedicle Screw Placement in the Thoracic and Lumbosacral Spines. J. Spinal Disord. Tech. 2015, 28, E347–E351. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.F.; Huang, Q.S.; Zhou, P.; Zhou, Y.; Wu, R.K.; Lou, Y.; Xu, H.Z. Pedicle screw insertion accuracy with different assisted methods: A systematic review and meta-analysis of comparative studies. Eur. Spine J. 2011, 20, 846–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, R.; Miller, J.A.; Sabharwal, N.C.; Lubelski, D.; Alentado, V.J.; Healy, A.T.; Mroz, T.E.; Benzel, E.C. Clinical outcomes following spinal fusion using an intraoperative computed tomographic 3D imaging system. J. Neurosurg. Spine 2017, 26, 628–637. [Google Scholar] [CrossRef] [Green Version]
- Tabaraee, E.; Gibson, A.G.; Karahalios, D.G.; Potts, E.A.; Mobasser, J.P.; Burch, S. Intraoperative cone beam-computed tomography with navigation (O-ARM) versus conventional fluoroscopy (C-ARM): A cadaveric study comparing accuracy, efficiency, and safety for spinal instrumentation. Spine 2013, 38, 1953–1958. [Google Scholar] [CrossRef]
- Kaminski, L.; Cordemans, V.; Cartiaux, O.; Van Cauter, M. Radiation exposure to the patients in thoracic and lumbar spine fusion using a new intraoperative cone-beam computed tomography imaging technique: A preliminary study. Eur. Spine J. 2017, 26, 2811–2817. [Google Scholar] [CrossRef]
- Nelson, E.M.; Monazzam, S.M.; Kim, K.D.; Seibert, J.A.; Klineberg, E.O. Intraoperative fluoroscopy, portable X-ray, and CT: Patient and operating room personnel radiation exposure in spinal surgery. Spine J. 2014, 14, 2985–2991. [Google Scholar] [CrossRef]
- The 2007 Recommendations of the International Commission on Radiological Protection ICRP publication 103. Ann. ICRP 2007, 37, 1–332. [CrossRef]
- Deak, P.D.; Smal, Y.; Kalender, W.A. Multisection CT protocols: Sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 2010, 257, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Chang, H.K.; Wu, J.C.; Tu, T.H.; Cheng, H.; Huang, W.C. Comparison of Radiation Exposure Between O-Arm Navigated and C-Arm Guided Screw Placement in Minimally Invasive Transforaminal Lumbar Interbody Fusion. World Neurosurg. 2020, 139, e489–e495. [Google Scholar] [CrossRef]
- Gebhard, F.T.; Kraus, M.D.; Schneider, E.; Liener, U.C.; Kinzl, L.; Arand, M. Does computer-assisted spine surgery reduce intraoperative radiation doses? Spine 2006, 31, 2024–2027. [Google Scholar] [CrossRef]
- Mendelsohn, D.; Strelzow, J.; Dea, N.; Ford, N.L.; Batke, J.; Pennington, A.; Yang, K.; Ailon, T.; Boyd, M.; Dvorak, M.; et al. Patient and surgeon radiation exposure during spinal instrumentation using intraoperative computed tomography-based navigation. Spine J. 2016, 16, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.E.; Welsch, M.D.; Sasso, R.C.; Vaccaro, A.R. Comparison of radiation exposure in lumbar pedicle screw placement with fluoroscopy vs computer-assisted image guidance with intraoperative three-dimensional imaging. J. Spinal Cord Med. 2008, 31, 532–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grelat, M.; Zairi, F.; Quidet, M.; Marinho, P.; Allaoui, M.; Assaker, R. Assessment of the surgeon radiation exposure during a minimally invasive TLIF: Comparison between fluoroscopy and O-arm system. Neurochirurgie 2015, 61, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Lee, Y.P.; Taylor, W.; Oygar, A.; Kim, W.K. Use of navigation-assisted fluoroscopy to decrease radiation exposure during minimally invasive spine surgery. Spine J. 2008, 8, 584–590. [Google Scholar] [CrossRef]
- Liu, X.; Joseph, J.R.; Smith, B.W.; Saadeh, Y.; Park, P. Analysis of Intraoperative Cone-Beam Computed Tomography Combined with Image Guidance for Lateral Lumbar Interbody Fusion. Oper. Neurosurg. 2018, 14, 620–626. [Google Scholar] [CrossRef]
- Schauer, D.A.; Linton, O.W. NCRP Report No. 160, Ionizing Radiation Exposure of the Population of the United States, medical exposure--are we doing less with more, and is there a role for health physicists? Health Phys. 2009, 97, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Lemburg, S.P.; Peters, S.A.; Roggenland, D.; Nicolas, V.; Heyer, C.M. Cumulative effective dose associated with radiography and CT of adolescents with spinal injuries. Am. J. Roentgenol. 2010, 195, 1411–1417. [Google Scholar] [CrossRef]
- Tien, H.C.; Tremblay, L.N.; Rizoli, S.B.; Gelberg, J.; Spencer, F.; Caldwell, C.; Brenneman, F.D. Radiation exposure from diagnostic imaging in severely injured trauma patients. J. Trauma Acute Care Surg. 2007, 62, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Ling, J.M.; Dinesh, S.K.; Pang, B.C.; Chen, M.W.; Lim, H.L.; Louange, D.T.; Yu, C.S.; Wang, C.M. Routine spinal navigation for thoraco-lumbar pedicle screw insertion using the O-arm three-dimensional imaging system improves placement accuracy. J. Clin. Neurosci. 2014, 21, 493–498. [Google Scholar] [CrossRef]
- Balling, H. Learning curve analysis of 3D-fluoroscopy image-guided pedicle screw insertions in lumbar single-level fusion procedures. Arch Orthop Trauma Surg. 2018, 138, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Ryang, Y.M.; Villard, J.; Obermuller, T.; Friedrich, B.; Wolf, P.; Gempt, J.; Ringel, F.; Meyer, B. Learning curve of 3D fluoroscopy image-guided pedicle screw placement in the thoracolumbar spine. Spine J. 2015, 15, 467–476. [Google Scholar] [CrossRef] [PubMed]
| Total | DC Group | DO Group | p-Value * | |
|---|---|---|---|---|
| Age [y] ± SD | 61.7 ± 12.2 | 60.8 ± 10.3 | 62.8 ± 13.9 | 0.291 |
| Sex f:m, n | 123:91 | 53:53 | 70:38 | 0.028 |
| Mean BMI [kg/m2] ± [95%-CI] | 29.4 ± 5.8 [28.6–30.1] | 29.4 ± 6.4 [28.2–30.6] | 29.3 ± 5.1 [28.4–30.3] | 0.911 |
| Number of patients | 214 | 106 | 108 | |
| 1-level-fusion | 122 | 60 | 62 | 0.986 |
| 2-level-fusion | 61 | 26 | 35 | 0.202 |
| 3-level-fusion | 31 | 20 | 11 | 0.071 |
| DC Group | DO Group (Complete) | p-Value * | DO Group (Learning Excluded) | p-Value * | |
|---|---|---|---|---|---|
| Surgery time [min] ** | 171.10 ± 48.91 | 143.62 ± 43.87 | <0.001 | 138.51 ± 36.03 | <0.001 |
| Surgery time 1-level [min] ** | 144.10 ± 30.73 | 128.66 ± 42.05 | 122.43 ± 22.63 | ||
| Surgery time 2-level [min] ** | 190.88 ± 47.16 | 154.77 ± 31.70 | 152.62 ± 34.31 | ||
| Surgery time 3-level [min] ** | 226.40 ± 36.10 | 192.45 ± 44.11 | 187.14 ± 48.49 | ||
| Preparation time [min] ** | 29.65 ± 7.69 | 37.25 ± 09.99 | <0.001 | 34.68 ± 6.86 | 0.001 |
| Preparation time 1-level [min] ** | 29.28 ± 7.54 | 36.26 ± 07.92 | 34.98 ± 6.60 | ||
| Preparation time 2-level [min] ** | 30.23 ± 8.85 | 38.20 ± 11.69 | 34.38 ± 6.87 | ||
| Preparation time 3-level [min] ** | 30.00 ± 6.83 | 39.82 ± 14.28 | 33.86 ± 9.24 | ||
| Effective dose [mSv] ** | 3.39 ± 2.32 | 2.23 ± 1.96 | 0.002 | 1.76 ± 1.13 | <0.001 |
| Effective dose 1-level [mSv] ** | 3.33 ± 2.79 | 2.06 ± 1.97 | 1.64 ± 1.07 | ||
| Effective dose 2-level [mSv] ** | 3.14 ± 1.50 | 2.41 ± 2.08 | 1.90 ± 1.28 | ||
| Effective dose 3-level [mSv] ** | 3.95 ± 1.52 | 2.61 ± 1.51 | 2.01 ± 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohe, S.; Strube, P.; Hölzl, A.; Böhle, S.; Zippelius, T.; Lindemann, C. Cone-Beam Navigation Can Reduce the Radiation Exposure and Save Fusion Length-Dependent Operation Time in Comparison to Conventional Fluoroscopy in Pedicle-Screw-Based Lumbar Interbody Fusion. J. Pers. Med. 2022, 12, 736. https://doi.org/10.3390/jpm12050736
Rohe S, Strube P, Hölzl A, Böhle S, Zippelius T, Lindemann C. Cone-Beam Navigation Can Reduce the Radiation Exposure and Save Fusion Length-Dependent Operation Time in Comparison to Conventional Fluoroscopy in Pedicle-Screw-Based Lumbar Interbody Fusion. Journal of Personalized Medicine. 2022; 12(5):736. https://doi.org/10.3390/jpm12050736
Chicago/Turabian StyleRohe, Sebastian, Patrick Strube, Alexander Hölzl, Sabrina Böhle, Timo Zippelius, and Chris Lindemann. 2022. "Cone-Beam Navigation Can Reduce the Radiation Exposure and Save Fusion Length-Dependent Operation Time in Comparison to Conventional Fluoroscopy in Pedicle-Screw-Based Lumbar Interbody Fusion" Journal of Personalized Medicine 12, no. 5: 736. https://doi.org/10.3390/jpm12050736






