Impact of Third-Generation Cephalosporin Resistance on Recurrence in Children with Febrile Urinary Tract Infections
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Ethical Considerations
2.2. Study Cohort
2.3. Definitions
2.4. Management Protocol
2.5. Protocol for Renal Imaging Studies
2.6. Covariates
2.7. Statistical Analysis
3. Results
3.1. Baseline Demographic and Clinical Characteristics
3.2. Antibiotic Susceptibilities
3.3. Risk Factors for Early and Late Recurrence of UTIs: A Multivariable Analysis
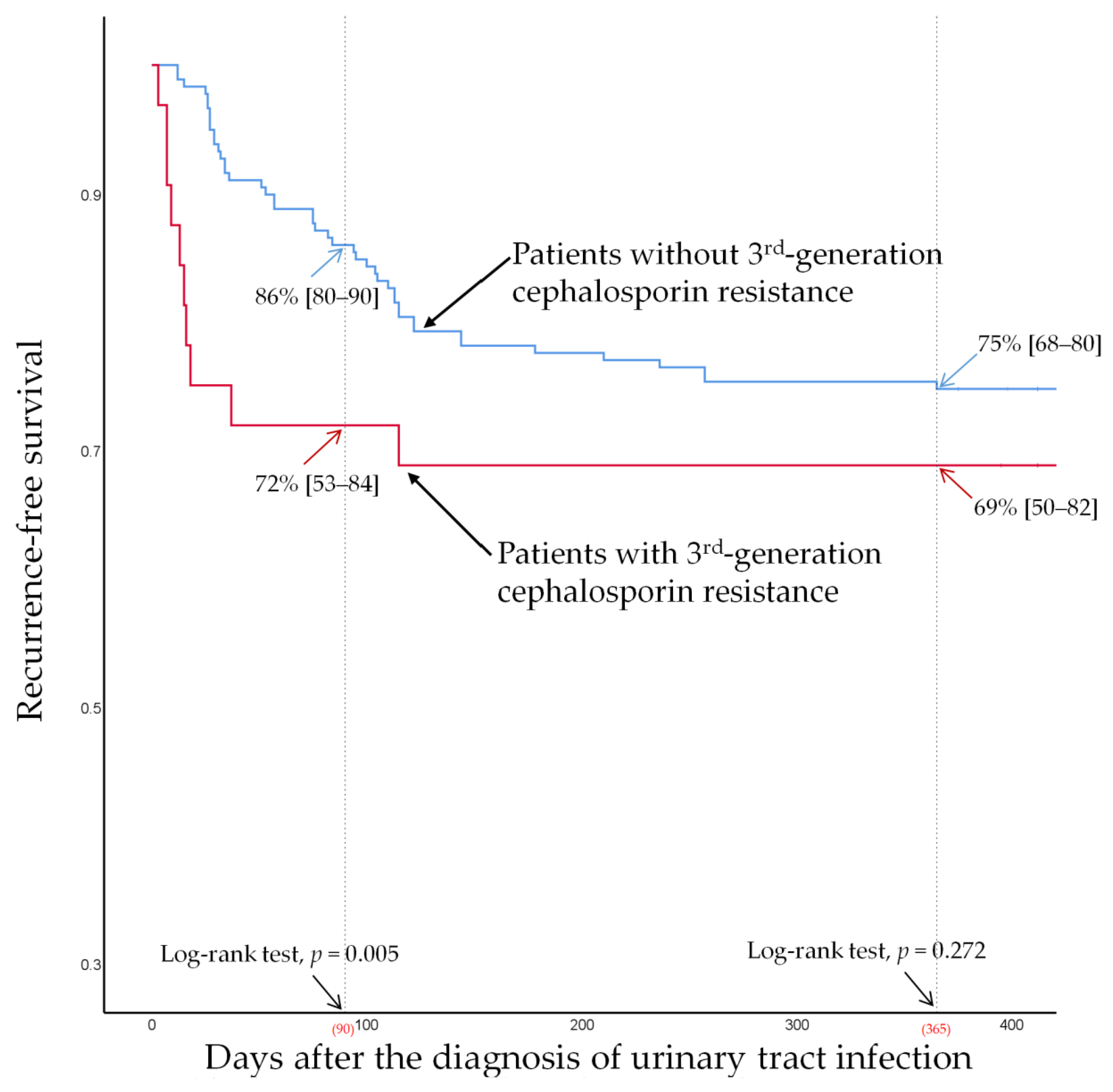
3.4. Recurrence-Free Survival According to VUR (Grade ≥ 3) and Third-Generation Cephalosporin Resistance
3.5. Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Roberts, K.B. Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management; Urinary tract infection: Clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011, 128, 595–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logan, L.K.; Braykov, N.P.; Weinstein, R.A.; Laxminarayan, R. Extended-Spectrum β-Lactamase-Producing and Third-Generation Cephalosporin-Resistant Enterobacteriaceae in Children: Trends in the United States, 1999–2011. J. Pediatric Infect. Dis. Soc. 2014, 3, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Dayan, N.; Dabbah, H.; Weissman, I.; Aga, I.; Even, L.; Glikman, D. Urinary Tract Infections Caused by Community-Acquired Extended-Spectrum β-Lactamase-Producing and Nonproducing Bacteria: A Comparative Study. J. Pediatrics 2013, 163, 1417–1421. [Google Scholar] [CrossRef]
- Flokas, M.E.; Detsis, M.; Alevizakos, M.; Mylonakis, E. Prevalence of ESBL-producing Enterobacteriaceae in paediatric urinary tract infections: A systematic review and meta-analysis. J. Infect. 2016, 73, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Lukac, P.J.; Bonomo, R.A.; Logan, L.K. Extended-spectrum β-lactamase-producing Enterobacteriaceae in children: Old foe, emerging threat. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 60, 1389–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, N.C.; Chen, H.H.; Chen, C.L.; Ou, L.S.; Lin, T.Y.; Tsai, M.H.; Chiu, C.H. Rise of community-onset urinary tract infection caused by extended-spectrum β-lactamase-producing Escherichia coli in children. J. Microbiol. Immunol. Infect. 2014, 47, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Paterson, D.L. Resistance in gram-negative bacteria: Enterobacteriaceae. Am. J. Infect. Control 2006, 34, S20–S28. [Google Scholar] [CrossRef]
- Bidell, M.R.; Palchak, M.; Mohr, J.; Lodise, T.P. Fluoroquinolone and Third-Generation-Cephalosporin Resistance among Hospitalized Patients with Urinary Tract Infections Due to Escherichia coli: Do Rates Vary by Hospital Characteristics and Geographic Region? Antimicrob. Agents Chemother. 2016, 60, 3170–3173. [Google Scholar] [CrossRef] [Green Version]
- Bevan, E.R.; Jones, A.M.; Hawkey, P.M. Global epidemiology of CTX-M β-lactamases: Temporal and geographical shifts in genotype. J. Antimicrob. Chemother. 2017, 72, 2145–2155. [Google Scholar] [CrossRef] [Green Version]
- Peirano, G.; Pitout, J.D.D. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: Update on Molecular Epidemiology and Treatment Options. Drugs 2019, 79, 1529–1541. [Google Scholar] [CrossRef]
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community: An Update. Infect. Dis. Clin. N. Am. 2020, 34, 709–722. [Google Scholar] [CrossRef]
- Megged, O. Extended-spectrum β-lactamase-producing bacteria causing community-acquired urinary tract infections in children. Pediatric Nephrol. 2014, 29, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.T.; Ng, K.; Kendrick, J.; Tilley, P.; Ting, J.; Rassekh, S.; Murthy, S.; Roberts, A. Third-generation cephalosporin-resistant urinary tract infections in children presenting to the paediatric emergency department. Paediatr. Child Health 2020, 25, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.E.; Greenhow, T.L.; Lee, V.; Beck, J.; Bendel-Stenzel, M.; Hames, N.; McDaniel, C.E.; King, E.E.; Sherry, W.; Parmar, D.; et al. Management and Outcomes in Children with Third-Generation Cephalosporin-Resistant Urinary Tract Infections. J. Pediatric Infect. Dis. Soc. 2021, 10, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Doern, C.D.; Richardson, S.E. Diagnosis of Urinary Tract Infections in Children. J. Clin. Microbiol. 2016, 54, 2233–2242. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; p. 332. [Google Scholar]
- Keren, R.; Shaikh, N.; Pohl, H.; Gravens-Mueller, L.; Ivanova, A.; Zaoutis, L.; Patel, M.; de Berardinis, R.; Parker, A.; Bhatnagar, S.; et al. Risk Factors for Recurrent Urinary Tract Infection and Renal Scarring. Pediatrics 2015, 136, e13–e21. [Google Scholar] [CrossRef] [Green Version]
- Medical versus surgical treatment of primary vesicoureteral reflux: Report of the International Reflux Study Committee. Pediatrics 1981, 67, 392–400. [CrossRef]
- Shaikh, N.; Hoberman, A.; Keren, R.; Gotman, N.; Docimo, S.G.; Mathews, R.; Bhatnagar, S.; Ivanova, A.; Mattoo, T.K.; Moxey-Mims, M.; et al. Recurrent Urinary Tract Infections in Children with Bladder and Bowel Dysfunction. Pediatrics 2016, 137, e20152982. [Google Scholar] [CrossRef] [Green Version]
- Dias, C.S.; Silva, J.M.; Diniz, J.S.; Lima, E.M.; Marciano, R.C.; Lana, L.G.; Trivelato, A.L.; Lima, M.S.; Simöes e Silva, A.C.; Oliveira, E.A. Risk factors for recurrent urinary tract infections in a cohort of patients with primary vesicoureteral reflux. Pediatric Infect. Dis. J. 2010, 29, 139–144. [Google Scholar] [CrossRef]
- Larcombe, J. Urinary tract infection in children: Recurrent infections. BMJ Clin. Evid. 2015, 2015, 0306. [Google Scholar]
- Dotis, J.; Printza, N.; Marneri, A.; Gidaris, D.; Papachristou, F. Urinary tract infections caused by extended-spectrum betalactamase-producing bacteria in children: A matched casecontrol study. Turk. J. Pediatrics 2013, 55, 571–574. [Google Scholar]
- Pérez Heras, I.; Sanchez-Gomez, J.C.; Beneyto-Martin, P.; Ruano-de-Pablo, L.; Losada-Pinedo, B. Community-onset extended-spectrum β-lactamase producing Escherichia coli in urinary tract infections in children from 2015 to 2016: Prevalence, risk factors, and resistances. Medicine 2017, 96, e8571. [Google Scholar] [CrossRef] [PubMed]
- Topaloglu, R.; Er, I.; Dogan, B.G.; Bilginer, Y.; Ozaltin, F.; Besbas, N.; Ozen, S.; Bakkaloglu, A.; Gur, D. Risk factors in community-acquired urinary tract infections caused by ESBL-producing bacteria in children. Pediatric Nephrol. 2010, 25, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.E.; Lee, V.; Greenhow, T.L.; Beck, J.; Bendel-Stenzel, M.; Hames, N.; McDaniel, C.E.; King, E.E.; Sherry, W.; Parmar, D.; et al. Clinical Response to Discordant Therapy in Third-Generation Cephalosporin-Resistant UTIs. Pediatrics 2020, 145, e20191608. [Google Scholar] [CrossRef] [PubMed]
- Jerardi, K.E.; Auger, K.A.; Shah, S.S.; Hall, M.; Hain, P.D.; Myers, A.L.; Williams, D.J.; Tieder, J.S. Discordant antibiotic therapy and length of stay in children hospitalized for urinary tract infection. J. Hosp. Med. 2012, 7, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Madhi, F.; Jung, C.; Timsit, S.; Levy, C.; Biscardi, S.; Lorrot, M.; Grimprel, E.; Hees, L.; Craiu, I.; Galerne, A.; et al. Febrile urinary-tract infection due to extended-spectrum beta-lactamase-producing Enterobacteriaceae in children: A French prospective multicenter study. PLoS ONE 2018, 13, e0190910. [Google Scholar] [CrossRef] [Green Version]
- Katsuta, T.; Shoji, K.; Watanabe, Y.; Saitoh, A. Treatment of Pyelonephritis Caused By Extended-Spectrum Β-Lactamase–Producing Enterobacteriaceae in Children. Pediatric Infect. Dis. J. 2013, 32, 417–419. [Google Scholar] [CrossRef]
- Hyun, H.S.; Kim, J.H.; Cho, M.H.; Park, E.; Ha, I.-S.; Cheong, H.I.; Kang, H.G. Low relapse rate of urinary tract infections from extended-spectrum beta-lactamase-producing bacteria in young children. Pediatric Nephrol. 2019, 34, 2399–2407. [Google Scholar] [CrossRef]
- Lee, B.; Kang, S.Y.; Kang, H.M.; Yang, N.R.; Kang, H.G.; Ha, I.S.; Cheong, H.I.; Lee, H.J.; Choi, E.H. Outcome of Antimicrobial Therapy of Pediatric Urinary Tract Infections Caused by Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae. Infect. Chemother. 2013, 45, 415–421. [Google Scholar] [CrossRef]
- Tratselas, A.; Iosifidis, E.; Ioannidou, M.; Saoulidis, S.; Kollios, K.; Antachopoulos, C.; Sofianou, D.; Roilides, E.J. Outcome of urinary tract infections caused by extended spectrum β-lactamase-producing Enterobacteriaceae in children. Pediatric Infect. Dis. J. 2011, 30, 707–710. [Google Scholar] [CrossRef]
- Bundtzen, R.W.; Toothaker, R.D.; Nielson, O.S.; Madsen, P.O.; Welling, P.G.; Craig, W.A. Pharmacokinetics of cefuroxime in normal and impaired renal function: Comparison of high-pressure liquid chromatography and microbiological assays. Antimicrob. Agents Chemother. 1981, 19, 443–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Variables | Category | All | UTI with Recurrence within 1 Year | UTI without Recurrence within 1 Year | Unadjusted Odds Ratios for 1-Year Recurrence | p-Value |
|---|---|---|---|---|---|---|
| Cases, n (%) | 210 (100) | 55 (26.2) | 155 (73.8) | |||
| Age, Median (years) | 0.4 (0.3–0.7) | 0.3 (0.1–0.5) | 0.4 (0.3–0.7) | 0.64 (0.38–1.06) | 0.081 | |
| Category, n (%) | ≤3 mo | 103 (49.0) | 33 (60.0) | 70 (45.2) | 3.78 (0.82–17.37) | 0.088 |
| 4–24 mo | 89 (42.4) | 20 (36.4) | 69 (44.5) | 2.32 (0.19–10.95) | 0.288 | |
| 2–17 y | 18 (8.6) | 2 (3.6) | 16 (10.3) | Reference | - | |
| Sex, male | 149 (71.0) | 38 (69.1) | 111 (71.6) | 0.89 (0.45–1.73) | 0.723 | |
| Previous history of antibiotics | 27 (12.9) | 10 (18.2) | 17 (11.0) | 1.80 (0.77–4.22) | 0.174 | |
| Duration of fever, days | At admission | 1.7 ± 1.8 | 1.8 ± 2.0 | 1.7 ± 1.7 | 1.05 (0.89–1.23) | 0.576 |
| After admission | 1.0 ± 0.9 | 0.9 ± 0.9 | 1.0 ± 0.9 | 0.97 (0.70–1.35) | 0.850 | |
| Total | 2.7 ± 2.1 | 2.8 ± 2.1 | 2.6 ± 2.0 | 1.03 (0.89–1.19) | 0.684 | |
| Fever over 39 °C | 102 (48.6) | 26 (47.3) | 76 (49.0) | 0.93 (0.50–1.73) | 0.823 | |
| Length of stay, days | 6.8 ± 2.2 | 6.9 ± 2.6 | 6.7 ± 2.1 | 1.03 (0.90–1.18) | 0.625 | |
| Initial inflammatory markers | WBC * 103/µL | 1.7 ± 0.7 | 1.7 ± 0.6 | 1.8 ± 0.7 | 1.00 (1.00–1.00) | 0.366 |
| Neutrophils (%) | 55.1 ± 14.3 | 55.9 ± 14.0 | 54.9 ± 14.5 | 1.01 (0.98–1.03) | 0.650 | |
| Lymphocytes (%) | 32.4 ± 13.0 | 31.4 ± 13.6 | 32.8 ± 12.7 | 0.99 (0.97–1.02) | 0.478 | |
| N/L ratio | 2.8 ± 4.7 | 2.3 ± 1.6 | 3.0 ± 5.3 | 0.96 (0.88–1.05) | 0.391 | |
| Platelet * 103/µL | 428.9 ± 143.3 | 421.9 ± 169.5 | 437.6 ± 126.2 | 1.00 (0.99–1.00) | 0.144 | |
| Erythrocyte Sedimentation Rate (mm/h) | 37.4 ± 23.2 | 41.1 ± 25.6 | 36.1 ± 22.3 | 1.10 (1.00–1.02) | 0.183 | |
| C-Reactive Protein (mg/L) | 65.0 ± 56.7 | 70.7 ± 52.2 | 62.9 ± 58.3 | 1.00 (1.00–1.01) | 0.385 | |
| Duration of pyuria ≥ 3 days | 108 (51.4) | 30 (54.5) | 78 (50.3) | 1.19 (0.64–2.20) | 0.591 | |
| Resistance to 3rd cephalosporins | 32 (15.2) | 10 (18.2) | 22 (14.2) | 1.34 (0.59–3.05) | 0.481 | |
| Imaging studies | ||||||
| Kidney US | Acute pyelonephritis | 82 (39.0) | 26 (47.3) | 56 (36.1) | 1.59 (0.85–2.95) | 0.147 |
| Hydronephrosis | 108 (51.4) | 31 (56.4) | 77 (49.7) | 1.31 (0.70–2.43) | 0.395 | |
| DMSA renal scan | Cortical defect | 148 (70.5) | 42 (76.4) | 106 (68.4) | 1.49 (0.74–3.03) | 0.267 |
| Other urinary tract abnormalities * | 15 (7.1) | 8 (14.5) | 7 (4.5) | 3.60 (1.24–10.45) | 0.019 | |
| VCUG | VUR grade ≥ 3 | 50 (23.8) | 28 (50.9) | 22 (14.2) | 3.27 (3.13–12.56) | <0.001 |
| Treatment with non-susceptible antibiotics in vitro | 27 (12.9) | 8 (14.5) | 19 (12.3) | 1.22 (0.50–2.97) | 0.664 |
| Outcome | Variables | Univariable | Multivariable | Multivariable (Bootstrap Adjusted) | |||
|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | Relative Bias (%) | ||
| Early (90-day) recurrence | |||||||
| Sex, female | 2.48 (1.12–5.46) | 0.025 | 2.37 (1.03–5.44) | 0.042 | 2.32 (0.88—6.13) | 2.2 | |
| Resistance to 3rd cephalosporins | 2.93 (1.20–7.16) | 0.019 | 2.79 (1.08–7.20) | 0.034 | 2.71 (1.02–8.25) | 3.0 | |
| Other urinary tract abnormalities * | 3.40 (1.07–10.77) | 0.037 | 3.13 (0.90–10.86) | 0.072 | 3.78 (0.57–11.64) | −16.4 | |
| VUR grade ≥ 3 on VCUG | 2.49 (1.10–5.62) | 0.028 | 2.45 (1.04–5.81) | 0.041 | 2.47 (1.07–5.97) | −0.7 | |
| Late (1-year) recurrence | |||||||
| Resistance to 3rd cephalosporins | 1.34 (0.59–3.05) | 0.481 | |||||
| Other urinary tract abnormalities * | 3.60 (1.24–10.45) | 0.019 | 3.14 (0.98–10.06) | 0.054 | 3.14 (1.01–10.17) | <0.1 | |
| VUR grade ≥ 3 on VCUG | 6.27 (3.13–12.56) | <0.001 | 6.06 (3.00–12.25) | <0.001 | 6.01 (3.18–12.01) | 0.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.Y.; Jang, M.S.; Kim, J. Impact of Third-Generation Cephalosporin Resistance on Recurrence in Children with Febrile Urinary Tract Infections. J. Pers. Med. 2022, 12, 773. https://doi.org/10.3390/jpm12050773
Kim SY, Jang MS, Kim J. Impact of Third-Generation Cephalosporin Resistance on Recurrence in Children with Febrile Urinary Tract Infections. Journal of Personalized Medicine. 2022; 12(5):773. https://doi.org/10.3390/jpm12050773
Chicago/Turabian StyleKim, Sin Young, Min Sik Jang, and Jihye Kim. 2022. "Impact of Third-Generation Cephalosporin Resistance on Recurrence in Children with Febrile Urinary Tract Infections" Journal of Personalized Medicine 12, no. 5: 773. https://doi.org/10.3390/jpm12050773






