Clinical Phenotypes of Atrial Fibrillation and Mortality Risk—A Cluster Analysis from the Nationwide Italian START Registry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Patient and Public Involvement Statement
2.3. Statistical Analysis
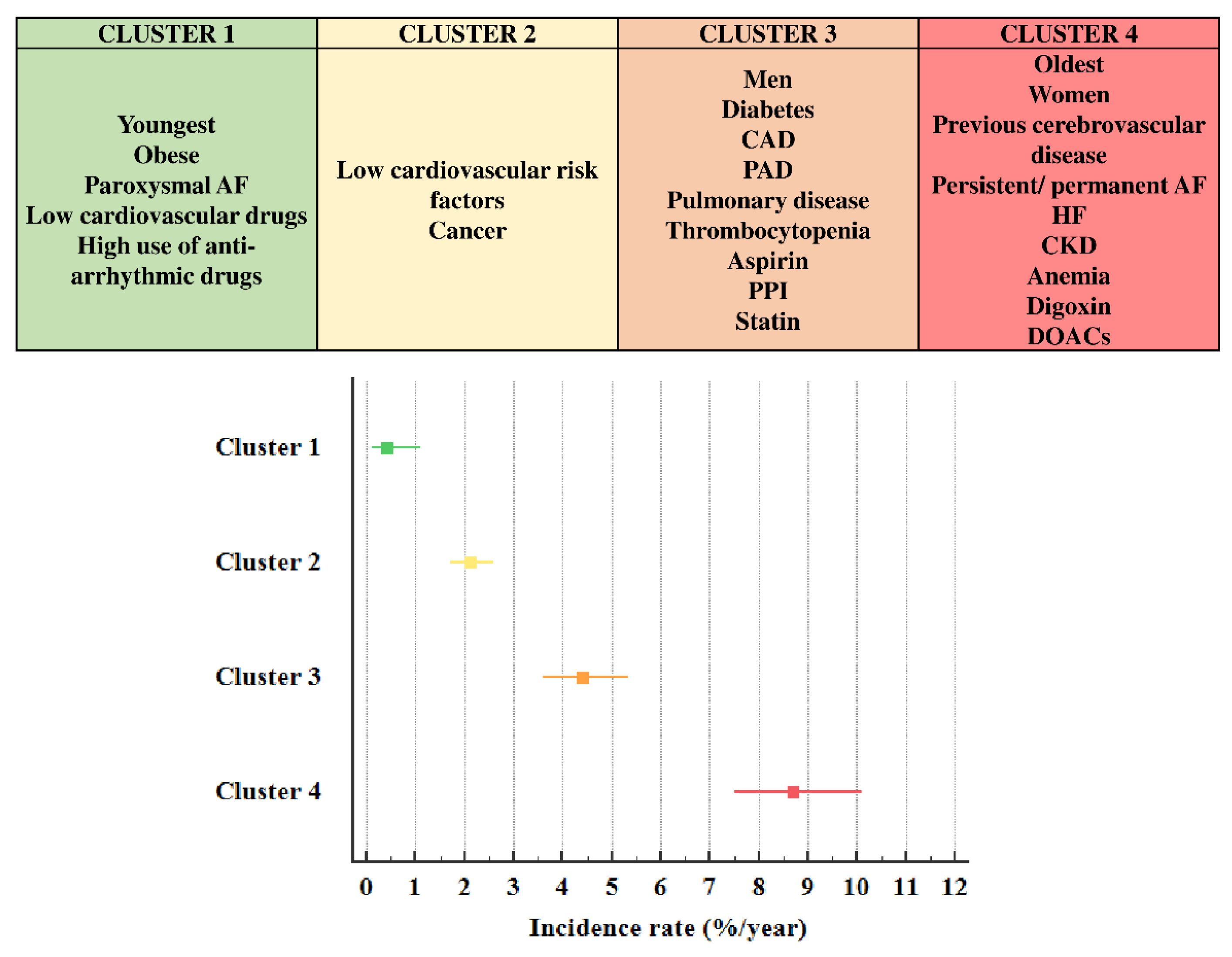
3. Results
3.1. Description of Clusters
3.2. Clusters and Mortality Risk
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Pastori, D.; Menichelli, D.; Del Sole, F.; Pignatelli, P.; Violi, F.; ATHERO-AF study group. Long-Term Risk of Major Adverse Cardiac Events in Atrial Fibrillation Patients on Direct Oral Anticoagulants. Mayo Clin. Proc. 2020, 96, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Antonucci, E.; Violi, F.; Palareti, G.; Pignatelli, P. Thrombocytopenia and Mortality Risk in Patients With Atrial Fibrillation: An Analysis From the START Registry. J. Am. Heart Assoc. 2019, 8, e012596. [Google Scholar] [CrossRef] [PubMed]
- Perera, K.S.; Pearce, L.A.; Sharma, M.; Benavente, O.; Connolly, S.J.; Hart, R.G.; ACTIVE A (Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events) Steering Committee and Investigators. Predictors of Mortality in Patients With Atrial Fibrillation (from the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events [ACTIVE A]). Am. J. Cardiol. 2018, 121, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, L.; Villejoubert, O.; Clementy, N.; Bernard, A.; Pierre, B.; Angoulvant, D.; Ivanes, F.; Babuty, D.; Lip, G.Y. Causes of Death and Influencing Factors in Patients with Atrial Fibrillation. Am. J. Med. 2016, 129, 1278–1287. [Google Scholar] [CrossRef]
- Gomez-Outes, A.; Lagunar-Ruiz, J.; Terleira-Fernandez, A.I.; Calvo-Rojas, G.; Suarez-Gea, M.L.; Vargas-Castrillon, E. Causes of Death in Anticoagulated Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2016, 68, 2508–2521. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Farcomeni, A.; Romiti, G.F.; Di Rocco, A.; Placentino, F.; Diemberger, I.; Lip, G.Y.; Boriani, G. Association between clinical risk scores and mortality in atrial fibrillation: Systematic review and network meta-regression of 669,000 patients. Eur. J. Prev. Cardiol. 2020, 27, 633–644. [Google Scholar] [CrossRef]
- Fox, K.A.A.; Lucas, J.E.; Pieper, K.S.; Bassand, J.P.; Camm, A.J.; Fitzmaurice, D.A.; Goldhaber, S.Z.; Goto, S.; Haas, S.; Hacke, W.; et al. Improved risk stratification of patients with atrial fibrillation: An integrated GARFIELD-AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation. BMJ Open 2017, 7, e017157. [Google Scholar] [CrossRef] [Green Version]
- Horne, B.D.; May, H.T.; Kfoury, A.G.; Renlund, D.G.; Muhlestein, J.B.; Lappe, D.L.; Rasmusson, K.D.; Bunch, T.J.; Carlquist, J.F.; Bair, T.L.; et al. The Intermountain Risk Score (including the red cell distribution width) predicts heart failure and other morbidity endpoints. Eur. J. Heart Fail. 2010, 12, 1203–1213. [Google Scholar] [CrossRef]
- Samaras, A.; Kartas, A.; Akrivos, E.; Fotos, G.; Dividis, G.; Vasdeki, D.; Vrana, E.; Rampidis, G.; Karvounis, H.; Giannakoulas, G.; et al. A novel prognostic tool to predict mortality in patients with atrial fibrillation: The BASIC-AF risk score. Hellenic. J. Cardiol. 2021, 62, 339–348. [Google Scholar] [CrossRef]
- Ahmad, T.; Pencina, M.J.; Schulte, P.J.; O’Brien, E.; Whellan, D.J.; Pina, I.L.; Kitzman, D.W.; Lee, K.L.; O’Connor, C.M.; Felker, G.M. Clinical implications of chronic heart failure phenotypes defined by cluster analysis. J. Am. Coll. Cardiol. 2014, 64, 1765–1774. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.; Desai, N.; Wilson, F.; Schulte, P.; Dunning, A.; Jacoby, D.; Allen, L.; Fiuzat, M.; Rogers, J.; Felker, G.M.; et al. Clinical Implications of Cluster Analysis-Based Classification of Acute Decompensated Heart Failure and Correlation with Bedside Hemodynamic Profiles. PLoS ONE 2016, 11, e0145881. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.R.; Paillasseur, J.L.; Caillaud, D.; Tillie-Leblond, I.; Chanez, P.; Escamilla, R.; Court-Fortune, I.; Perez, T.; Carre, P.; Roche, N.; et al. Clinical COPD phenotypes: A novel approach using principal component and cluster analyses. Eur. Respir. J. 2010, 36, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonucci, E.; Poli, D.; Tosetto, A.; Pengo, V.; Tripodi, A.; Magrini, N.; Marongiu, F.; Palareti, G.; Register, S. The Italian START-Register on Anticoagulation with Focus on Atrial Fibrillation. PLoS ONE 2015, 10, e0124719. [Google Scholar] [CrossRef] [PubMed]
- Hennig, C.; Liao, T.F. How to find an appropriate clustering for mixed-type variables with application to socio-economic stratification. J. R. Stat. Soc. 2013, 62, 309–369. [Google Scholar] [CrossRef] [Green Version]
- Link, M.S.; Giugliano, R.P.; Ruff, C.T.; Scirica, B.M.; Huikuri, H.; Oto, A.; Crompton, A.E.; Murphy, S.A.; Lanz, H.; Mercuri, M.F.; et al. Stroke and Mortality Risk in Patients With Various Patterns of Atrial Fibrillation: Results From the ENGAGE AF-TIMI 48 Trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48). Circ. Arrhythm Electrophysiol. 2017, 10, e004267. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, L.; Xiao, K.; Zhu, W.; Liu, M.; Wan, R.; Hong, K. The obesity paradox for outcomes in atrial fibrillation: Evidence from an exposure-effect analysis of prospective studies. Obes. Rev. 2019. [CrossRef]
- Pastori, D.; Marang, A.; Bisson, A.; Menichelli, D.; Herbert, J.; Lip, G.Y.H.; Fauchier, L. Thromboembolism, mortality, and bleeding in 2,435,541 atrial fibrillation patients with and without cancer: A nationwide cohort study. Cancer 2021, 127, 2122–2129. [Google Scholar] [CrossRef]
- Menichelli, D.; Vicario, T.; Ameri, P.; Toma, M.; Violi, F.; Pignatelli, P.; Pastori, D. Cancer and atrial fibrillation: Epidemiology, mechanisms, and anticoagulation treatment. Prog. Cardiovasc. Dis. 2021, 66, 28–36. [Google Scholar] [CrossRef]
- Pastori, D.; Pignatelli, P.; Sciacqua, A.; Perticone, M.; Violi, F.; Lip, G.Y.H. Relationship of peripheral and coronary artery disease to cardiovascular events in patients with atrial fibrillation. Int. J. Cardiol. 2018, 255, 69–73. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2017, 39, 763–816. [Google Scholar] [CrossRef] [Green Version]
- Ntaios, G.; Papavasileiou, V.; Makaritsis, K.; Milionis, H.; Manios, E.; Michel, P.; Lip, G.Y.; Vemmos, K. Statin treatment is associated with improved prognosis in patients with AF-related stroke. Int. J. Cardiol. 2014, 177, 129–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Y.; Ogawa, H.; Yamashita, Y.; Ishii, M.; Iguchi, M.; Masunaga, N.; Esato, M.; Tsuji, H.; Wada, H.; Hasegawa, K.; et al. Causes of death in Japanese patients with atrial fibrillation: The Fushimi Atrial Fibrillation Registry. Eur. Heart J. Qual. Care Clin. Outcomes 2019, 5, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokorney, S.D.; Piccini, J.P.; Stevens, S.R.; Patel, M.R.; Pieper, K.S.; Halperin, J.L.; Breithardt, G.; Singer, D.E.; Hankey, G.J.; Hacke, W.; et al. Cause of Death and Predictors of All-Cause Mortality in Anticoagulated Patients With Nonvalvular Atrial Fibrillation: Data From ROCKET AF. J. Am. Heart Assoc. 2016, 5, e002197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inohara, T.; Piccini, J.P.; Mahaffey, K.W.; Kimura, T.; Katsumata, Y.; Tanimoto, K.; Inagawa, K.; Ikemura, N.; Ueda, I.; Fukuda, K.; et al. A Cluster Analysis of the Japanese Multicenter Outpatient Registry of Patients With Atrial Fibrillation. Am. J. Cardiol. 2019, 124, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Inohara, T.; Shrader, P.; Pieper, K.; Blanco, R.G.; Thomas, L.; Singer, D.E.; Freeman, J.V.; Allen, L.A.; Fonarow, G.C.; Gersh, B.; et al. Association of of Atrial Fibrillation Clinical Phenotypes With Treatment Patterns and Outcomes: A Multicenter Registry Study. JAMA Cardiol. 2018, 3, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Menichelli, D.; Violi, F.; Pignatelli, P.; Gregory, Y.H.L.; ATHERO-AF study group. The Atrial fibrillation Better Care (ABC) pathway and cardiac complications in atrial fibrillation: A potential sex-based difference. The ATHERO-AF study. Eur. J. Intern. Med. 2021, 85, 80–85. [Google Scholar] [CrossRef]
- Windgassen, S.; Moss-Morris, R.; Goldsmith, K.; Chalder, T. The importance of cluster analysis for enhancing clinical practice: An example from irritable bowel syndrome. J. Ment. Health 2018, 27, 94–96. [Google Scholar] [CrossRef]

| Cluster Denomination | Whole Cohort | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | p-Value (among Groups) |
|---|---|---|---|---|---|---|
| Youngest and Low Comorbidities | Low Cardiovascular Risk and High Cancer | High Cardiovascular Risk and More Men | Oldest, More Women and Cerebrovascular Disease | |||
| Cluster size n | 5171 | 512 | 2201 | 1268 | 1190 | |
| Variables used to define clusters | ||||||
| Age (years) | 75.0 ± 9.6 | 55.6 ± 7.9 | 75.0 ± 6.0 | 74.6 ± 7.0 | 83.7 ± 4.2 | <0.001 |
| Women (%) | 45.3 | 23.6 | 54.0 | 8.1 | 78.2 | <0.001 |
| Diabetes (%) | 20.2 | 10.7 | 16.5 | 35.0 | 15.1 | <0.001 |
| Previous cerebrovascular events (%) | 16.5 | 14.6 | 12.5 | 17.2 | 23.9 | <0.001 |
| Previous cardiovascular disease (%) | 18.6 | 6.8 | 1.5 | 53.5 | 18.1 | <0.001 |
| Heart failure (%) | 15.5 | 7.0 | 1.1 | 29.2 | 31.1 | <0.001 |
| Peripheral Artery Disease (%) | 6.4 | 0.8 | 0.6 | 16.1 | 9.1 | <0.001 |
| Cancer (%) | 13.6 | 2.9 | 18.2 | 15.1 | 8.1 | <0.001 |
| Pulmonary disease (%) | 12.6 | 3.1 | 1.5 | 27.8 | 21.0 | <0.001 |
| Smoking (%) | 13.2 | 21.9 | 2.7 | 39.4 | 1.1 | <0.001 |
| Previous major bleeding (%) | 3.5 | 1.4 | 1.9 | 4.5 | 6.1 | <0.001 |
| DOACs (vs. VKAs) (%) | 25.8 | 10.0 | 27.0 | 22.7 | 33.8 | <0.001 |
| Variables not used for cluster analysis | ||||||
| Persistent/permanent AF (%) | 63.3 | 51.4 | 60.7 | 65.7 | 70.8 | <0.001 |
| BMI (kg/m2) | 26.9 ± 4.7 | 28.1 ± 5.5 | 26.7 ± 4.5 | 27.6 ± 4.6 | 25.8 ± 4.6 | <0.001 |
| Obesity (BMI ≥ 30 kg/m2) | 21.1 | 30.1 | 19.9 | 24.1 | 16.6 | <0.001 |
| Creatinine Clearance (mL/min) | 66.8 ± 28.3 | 103.8 ± 33.6 | 67.6 ± 22.8 | 68.6 ± 26.8 | 47.6 ± 17.4 | <0.001 |
| Chronic kidney disease (Creatinine clearance <60 mL/min) (%) | 45.1 | 5.1 | 39.5 | 39.4 | 78.8 | <0.001 |
| Hemoglobin (g/dl) | 13.5 ± 1.8 | 14.5 ± 1.6 | 13.6 ± 1.6 | 13.6 ± 1.8 | 12.7 ± 1.6 | <0.001 |
| Anemia (<12 g/dL for women and <13 g/dL for men) (%) | 24.7 | 11.3 | 19.3 | 30.0 | 34.6 | <0.001 |
| Platelet count (×109/L) | 222.2 ± 68.9 | 223.3 ± 62.0 | 223.0 ± 69.6 | 213.8 ± 70.7 | 229.2 ± 67.7 | <0.001 |
| Thrombocytopenia (<150 × 109/L, %) | 10.7 | 9.0 | 10.3 | 14.7 | 7.9 | <0.001 |
| Hypertension (%) | 80.6 | 59.6 | 78.1 | 86.3 | 88.2 | <0.001 |
| CHA2DS2 VASc score | 3.6 ± 1.5 | 1.5 ± 1.1 | 3.3 ± 1.2 | 3.9 ± 1.4 | 4.7 ± 1.2 | <0.001 |
| HAS-BLED score | 1.3 ± 0.7 | 0.4 ± 0.6 | 1.2 ± 0.6 | 1.5 ± 0.8 | 1.5 ± 0.6 | <0.001 |
| Aspirin (%) | 9.7 | 6.3 | 5.2 | 21.1 | 7.5 | <0.001 |
| Statins (%) | 33.7 | 21.3 | 26.8 | 54.7 | 29.5 | <0.001 |
| Anti-arrhythmic drugs (%) | 25.2 | 32.8 | 26.2 | 25.1 | 20.3 | <0.001 |
| Digoxin (%) | 9.2 | 6.1 | 7.2 | 8.0 | 15.8 | <0.001 |
| Proton pump inhibitors (%) | 45.9 | 32.6 | 37.8 | 58.7 | 53.1 | <0.001 |
| Variables | Hazard Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Cluster 2 (vs. 1) * | 3.306 | 1.204 | 9.077 | 0.020 |
| Cluster 3 (vs. 1) * | 6.702 | 2.433 | 18.461 | <0.001 |
| Cluster 4 (vs. 1) * | 8.927 | 3.238 | 24.605 | <0.001 |
| Persistent/permanent AF | 1.231 | 0.975 | 1.553 | 0.081 |
| Statin | 0.655 | 0.519 | 0.828 | <0.001 |
| Digoxin | 0.963 | 0.692 | 1.339 | 0.822 |
| Proton pump inhibitors | 1.367 | 1.108 | 1.686 | 0.004 |
| Hypertension | 1.009 | 0.747 | 1.363 | 0.953 |
| Obesity | 1.217 | 0.930 | 1.592 | 0.152 |
| Anemia | 1.618 | 1.313 | 1.993 | <0.001 |
| Thrombocytopenia | 1.418 | 1.060 | 1.898 | 0.019 |
| Chronic kidney disease | 2.347 | 1.821 | 3.024 | <0.001 |
| Anti-arrhythmic drugs | 0.713 | 0.552 | 0.922 | 0.010 |
| Aspirin | 0.880 | 0.620 | 1.248 | 0.472 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastori, D.; Antonucci, E.; Milanese, A.; Menichelli, D.; Palareti, G.; Farcomeni, A.; Pignatelli, P.; the START2 Register Investigators. Clinical Phenotypes of Atrial Fibrillation and Mortality Risk—A Cluster Analysis from the Nationwide Italian START Registry. J. Pers. Med. 2022, 12, 785. https://doi.org/10.3390/jpm12050785
Pastori D, Antonucci E, Milanese A, Menichelli D, Palareti G, Farcomeni A, Pignatelli P, the START2 Register Investigators. Clinical Phenotypes of Atrial Fibrillation and Mortality Risk—A Cluster Analysis from the Nationwide Italian START Registry. Journal of Personalized Medicine. 2022; 12(5):785. https://doi.org/10.3390/jpm12050785
Chicago/Turabian StylePastori, Daniele, Emilia Antonucci, Alberto Milanese, Danilo Menichelli, Gualtiero Palareti, Alessio Farcomeni, Pasquale Pignatelli, and the START2 Register Investigators. 2022. "Clinical Phenotypes of Atrial Fibrillation and Mortality Risk—A Cluster Analysis from the Nationwide Italian START Registry" Journal of Personalized Medicine 12, no. 5: 785. https://doi.org/10.3390/jpm12050785
APA StylePastori, D., Antonucci, E., Milanese, A., Menichelli, D., Palareti, G., Farcomeni, A., Pignatelli, P., & the START2 Register Investigators. (2022). Clinical Phenotypes of Atrial Fibrillation and Mortality Risk—A Cluster Analysis from the Nationwide Italian START Registry. Journal of Personalized Medicine, 12(5), 785. https://doi.org/10.3390/jpm12050785







