Improvement of Ocular Surface Disease by Lateral Tarsoconjunctival Flap in Thyroid-Associated Orbitopathy Patients with Lid Retraction
Abstract
:1. Introduction
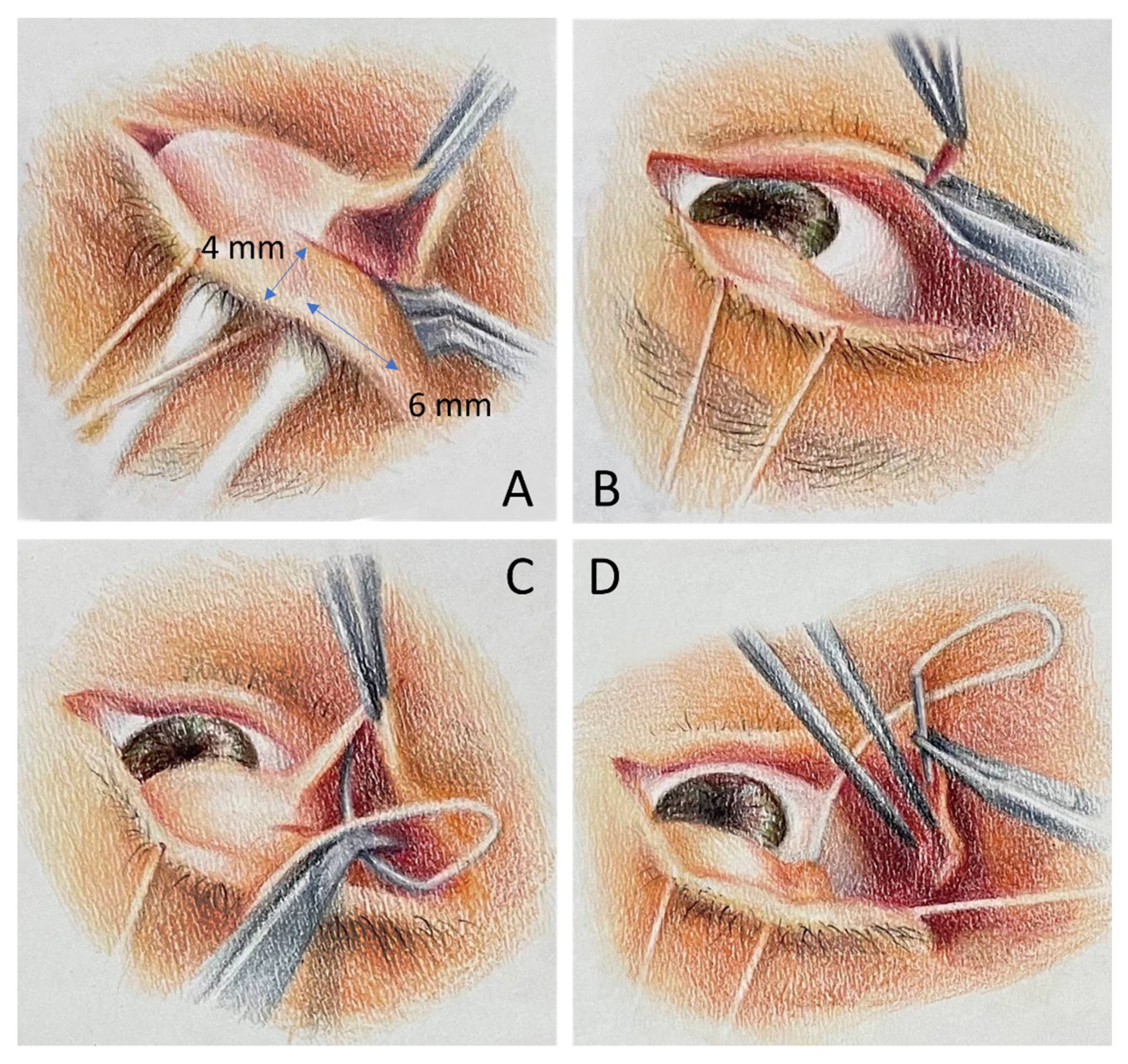
2. Materials and Methods
- (1)
- To enforce horizontal force, we performed the tarsal strip technique in some patients who presented with horizontal laxity at the time of surgery.
- (2)
- To improve medial support of the lower lid, we added a collagen graft at the pretarsal plane in patients who presented with residual medial lower lid retraction despite LTF during the surgery or inferior scleral show more than 3 mm in the preoperative evaluation.
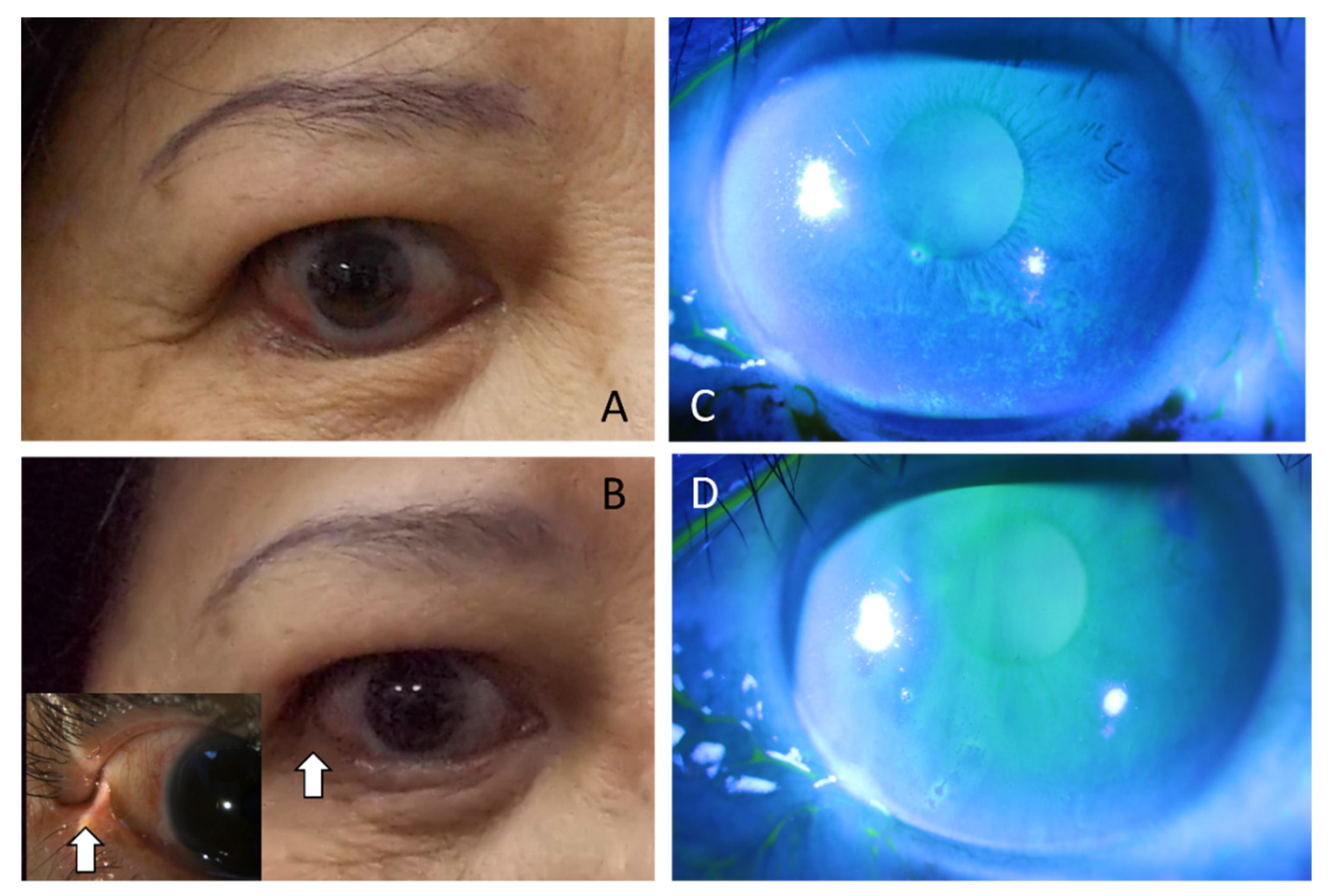
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salvi, M.; Campi, I. Medical Treatment of Graves’ Orbitopathy. Horm. Metab. Res. 2015, 47, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Weetman, A.P. Thyroid-Associated Eye Disease: Pathophysiology. Lancet 1991, 338, 25–28. [Google Scholar] [CrossRef]
- Baldeschi, L.; Wakelkamp, I.M.M.J.; Lindeboom, R.; Prummel, M.F.; Wiersinga, W.M. Early versus Late Orbital Decompression in Graves’ Orbitopathy. A Retrospective Study in 125 Patients. Ophthalmology 2006, 113, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Neag, E.J.; Smith, T.J. 2021 Update on Thyroid-Associated Ophthalmopathy. J. Endocrinol. Investig. 2021, 45, 235–259. [Google Scholar] [CrossRef]
- Sun, R.; Zhou, H.F.; Fan, X.Q. Ocular Surface Changes in Graves’ Ophthalmopathy. Int. J. Ophthalmol. 2021, 14, 616–621. [Google Scholar] [CrossRef]
- Selter, J.H.; Gire, A.I.; Sikder, S. The Relationship between Graves’ Ophthalmopathy and Dry Eye Syndrome. Clin. Ophthalmol. 2015, 9, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Moura Brasil, M.V.O.; Moura Brasil, O.F.; Vieira, R.P.; Vaisman, M.; Brasil Do Amaral Filho, O.M. Tear Film Analysis and Its Relation with Palpebral Fissure Height and Exophthalmos in Graves’ Ophthalmopathy. Arq. Bras. Oftalmol. 2005, 68, 615–618. [Google Scholar] [CrossRef]
- Achtsidis, V.; Tentolouris, N.; Theodoropoulou, S.; Panagiotidis, D.; Vaikoussis, E.; Saldana, M.; Gouws, P.; Theodossiadis, P.G. Dry Eye in Graves’ Ophthalmopathy: Correlation with Corneal Hypoesthesia. Eur. J. Ophthalmol. 2013, 23, 473–479. [Google Scholar] [CrossRef]
- Tirakunwichcha, S.; Lerdchanapornchai, V.; Reinprayoon, U.; Saonanon, P.; Snabboon, T. Prevalence of Dry Eye Disease in Autoimmune Thyroid Disease and the Association of Dry Eye with Clinical Signs of Thyroid Associated Ophthalmopathy: Observational, Noncomparative, Cross-Sectional Study. Asian Biomed. 2016, 10, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Vaidya, A.; Kakizaki, H. Changes in Eyelid Pressure and Dry Eye Status after Orbital Decompression in Thyroid Eye Disease. J. Clin. Med. 2021, 10, 3687. [Google Scholar] [CrossRef]
- Conger, J.R.; Grob, S.R.; Limongi, R.M.; Tao, J.P. Lateral Tarsoconjunctival Flap Suspension Treatment of Post Blepharoplasty Lower Eyelid Retraction. Ophthalmic Plast. Reconstr. Surg. 2020, 36, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Kazim, M.; Gold, K.G. A review of surgical techniques to correct upper eyelid retraction associated with thyroid eye disease. Curr. Opin. Ophthalmol. 2011, 22, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, G.O.; Fazil, K.; Yilmaz, B.S.; Ozturker, C.; Günaydın, Z.K.; Taskapili, M.; Kaynak, P. An algorithm for Botulinum toxin A injection for upper eyelid retraction associated with thyroid eye disease: Long-term results. Orbit 2020, 40, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Salour, H.; Bagheri, B.; Aletaha, M.; Babsharif, B.; Kleshadi, M.; Abrishami, M.; Bagheri, A. Transcutaneous Dysport Injection for Treatment of Upper Eyelid Retraction Associated with Thyroid Eye Disease. Orbit 2010, 29, 114–118. [Google Scholar] [CrossRef]
- Rajabi, M.T.; Jafari, H.; Mazloumi, M.; Tabatabaie, S.Z.; Rajabi, M.B.; Hasanlou, N.; Abtahi, S.M.; Goldberg, R.A. Lower Lid Retraction in Thyroid Orbitopathy: Lamellar Shortening or Proptosis? Int. Ophthalmol. 2013, 34, 801–804. [Google Scholar] [CrossRef]
- Mourits, M.P.; Koornneef, L. Lid Lengthening by Sclera Interposition for Eyelid Retraction in Graves’ Ophthalmopathy. Br. J. Ophthalmol. 1991, 75, 344–347. [Google Scholar] [CrossRef] [Green Version]
- Kersten, R.C.; Kulwin, D.R.; Levartovsky, S.; Tiradellis, H.; Tse, D.T. Management of Lower-Lid Retraction With Hard-Palate Mucosa Grafting. Arch. Ophthalmol. 1990, 108, 1339–1343. [Google Scholar] [CrossRef]
- Olver, J.M.; Rose, G.E.; Khaw, P.T.; Collin, J.R.O. Correction of Lower Eyelid Retraction in Thyroid Eye Disease: A Randomised Controlled Trial of Retractor Tenotomy with Adjuvant Antimetabolite versus Scleral Graft. Br. J. Ophthalmol. 1998, 82, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Lang, S.; Wei, Y.H. Correction of Lower Lid Retraction Using TarSys Bioengineered Grafts for Graves Ophthalmopathy. Am. J. Ophthalmol. 2013, 156, 387–392.e1. [Google Scholar] [CrossRef]
- Lemp, M.A. Report of the National Eye Institute/Industry Workshop on Clinical Trials in Dry Eyes. CLAO J. 1995, 21, 221–232. [Google Scholar]
- Ujhelyi, B.; Gogolak, P.; Erdei, A.; Nagy, V.; Balazs, E.; Rajnavolgyi, E.; Berta, A.; Nagy, E.V. Graves’ Orbitopathy Results in Profound Changes in Tear Composition: A Study of Plasminogen Activator Inhibitor-1 and Seven Cytokines. Thyroid 2012, 22, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Iskeleli, G.; Karakoc, Y.; Abdula, A. Tear Film Osmolarity in Patients with Thyroid Ophthalmopathy. Jpn. J. Ophthalmol. 2008, 52, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Bartley, G.B.; Gorman, C.A. Diagnostic Criteria for Graves’ Ophthalmopathy. Am. J. Ophthalmol. 1995, 119, 792–795. [Google Scholar] [CrossRef]
- Cruz, A.A.V.; Ribeiro, S.F.T.; Garcia, D.M.; Akaishi, P.M.; Pinto, C.T. Graves Upper Eyelid Retraction. Surv. Ophthalmol. 2013, 58, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Graves’ Eye Disease: Orbital Compliance and Other Physical Measurements. Trans. Am. Ophthalmol. Soc. 1984, 82, 492. Available online: https://pubmed.ncbi.nlm.nih.gov/6398935/ (accessed on 4 March 2022).
- Day, R.M. Ocular Manifestations of Thyroid Disease: Current Concepts. Arch. Ophthalmol. 1960, 64, 324–341. [Google Scholar] [CrossRef]
- Kim, K.H.; Baek, J.S.; Lee, S.; Lee, J.H.; Choi, H.S.; Kim, S.J.; Jang, J.W. Causes and Surgical Outcomes of Lower Eyelid Retraction. Korean J. Ophthalmol. 2017, 31, 290. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.P.; Vemuri, S.; Patel, A.D.; Compton, C.; Nunery, W.R. Lateral Tarsoconjunctival Onlay Flap Lower Eyelid Suspension in Facial Nerve Paresis. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 342–345. [Google Scholar] [CrossRef]
- Wei, Y.H.; Chen, W.L.; Hu, F.R.; Liao, S.L. In Vivo Confocal Microscopy of Bulbar Conjunctiva in Patients with Graves’ Ophthalmopathy. J. Med. Assoc. 2015, 114, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.Q.; Cheng, J.W.; Cai, J.P.; Le, Q.H.; Ma, X.Y.; Gao, L.D.; Wei, R.L. Observation of Corneal Langerhans Cells by In Vivo Confocal Microscopy in Thyroid-Associated Ophthalmopathy. Curr. Eye Res. 2016, 41, 927–932. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kwak, A.Y.; Lee, S.Y.; Yoon, J.S.; Jang, S.Y. Meibomian Gland Dysfunction in Graves’ Orbitopathy. Can. J. Ophthalmol. 2015, 50, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Xu, N.; Song, Y.; Wang, P.; Yang, H. Inflammatory Cytokine Profiles in the Tears of Thyroid-Associated Ophthalmopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.Y.; Xiao, J.; Olafsson, J.; Chen, X.; Lagali, N.S.; Ræder, S.; Utheim, Ø.A.; Dartt, D.A.; Utheim, T.P. Meibomian Gland Morphology Is a Sensitive Early Indicator of Meibomian Gland Dysfunction. Am. J. Ophthalmol. 2019, 200, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckstein, A.K.; Finkenrath, A.; Heiligenhaus, A.; Renzing-Köhler, K.; Esser, J.; Krüger, C.; Quadbeck, B.; Steuhl, K.P.; Gieseler, R.K. Dry Eye Syndrome in Thyroid-Associated Ophthalmopathy: Lacrimal Expression of TSH Receptor Suggests Involvement of TSHR-Specific Autoantibodies. Acta Ophthalmol. Scand. 2004, 82, 291–297. [Google Scholar] [CrossRef]
- Kishazi, E.; Dor, M.; Eperon, S.; Oberic, A.; Turck, N.; Hamedani, M. Differential Profiling of Lacrimal Cytokines in Patients Suffering from Thyroid-Associated Orbitopathy. Sci. Rep. 2018, 8, 10792. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Sadeghi, P.B.; Akpek, E.K. Occult Thyroid Eye Disease in Patients Presenting with Dry Eye Symptoms. Am. J. Ophthalmol. 2009, 147, 919–923. [Google Scholar] [CrossRef]
- Chen, Q. The Expression of Interleukin-15 and Interleukin-17 in Tears and Orbital Tissues of Graves Ophthalmopathy Patients. J. Cell. Biochem. 2019, 120, 6299–6303. [Google Scholar] [CrossRef]
- McMonnies, C.W. Blink Efficiency: A Neglected Area of Ocular Surface Disease Management? Investig. Ophthalmol. Vis. Sci. 2011, 52, 4484. [Google Scholar] [CrossRef]
- Foulks, G.N.; Bron, A.J. Meibomian Gland Dysfunction: A Clinical Scheme for Description, Diagnosis, Classification, and Grading. Ocul. Surf. 2003, 1, 107–126. [Google Scholar] [CrossRef]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Anatomy, Physiology, and Pathophysiology of the Meibomian Gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Kakizaki, H. Meibomian Gland Dysfunction in Cranial Nerve VII Palsy. Ophthalmic Plast. Reconstr. Surg. 2015, 31, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.H.; Patel, A.; Lu, D.W.; Tao, J. Lateral Tarsoconjunctival Onlay Flap in Multi-Vector Lower Eyelid Retraction. J. Med. Sci. 2016, 36, 171. [Google Scholar] [CrossRef]
- Chang, H.S.; Lee, D.; Taban, M.; Douglas, R.S.; Goldberg, R.A. “En-Glove” Lysis of Lower Eyelid Retractors with AlloDerm and Dermis-Fat Grafts in Lower Eyelid Retraction Surgery. Ophthalmic Plast. Reconstr. Surg. 2011, 27, 137–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dailey, R.A.; Marx, D.P.; Ahn, E.S. Porcine Dermal Collagen in Lower Eyelid Retraction Repair. Ophthalmic Plast. Reconstr. Surg. 2015, 31, 233–241. [Google Scholar] [CrossRef] [PubMed]

| No. of Subjects (N) (%) | 42 (100%) |
|---|---|
| Male (N) (%) | 5 (11.9%) |
| Female (N) (%) | 37 (88.1%) |
| Age at operation (years) (mean) (SD) | 46.4 (12.3) |
| Age at operation (years) (min) (max) | (30.2) (57.6) |
| Underlying hyperthyroidism (N) (%) | 39 (92.9%) |
| History of prior ocular surgery | 33 (78.6%) |
| Clinical Activity Score at operation (mean) (SD) | 2.1 (0.6) |
| Follow-up period (month) (mean) (SD) | 10.6 (4.2) |
| Follow-up period (month)(min) (max) | (6.1) (20.4) |
| Baseline | 1 Month | 3 Months | p-Value | |
|---|---|---|---|---|
| OSDI (mean)(SD) | 48.7 (10.8) | 36.5 (9.8) | 24.3 (8.2) | <0.01 * |
| TBUT (sec)(mean)(SD) | 3.9 (0.6) | 4.6 (0.9) | 6.8 (1.3) | <0.01 * |
| Schirmer’s I test (mm)(mean)(SD) | 5.4 (3.4) | 11.3 (3.7) | 14.3 (3.9) | <0.01 * |
| Corneal staining score (mean)(SD) | 4.6 (2.5) | 4.0 (2.6) | 3.7 (2.1) | <0.01 * |
| Conjunctival staining score (mean)(SD) | 5.2 (1.6) | 4.7 (2.3) | 3.0 (2.8) | <0.01 * |
| Gland dropout (mean)(SD) | 4.8 (0.5) | 4.1 (0.3) | 3.4 (0.5) | <0.01 * |
| Control | Baseline | 1 Month | 3 Months | |
|---|---|---|---|---|
| IL-6 (mean)(SD)(pg/mL) | 1.2 (0.3) | 39.6 (9.63) | 6.3 (2.1) | 1.9 (0.3) |
| IL-8 (mean)(SD) (pg/mL) | 8.0 (2.2) | 344.9 (57.2) | 59.4 (12.7) | 25.6 (8.3) |
| IL-18 (mean)(SD) (pg/mL) | 0.7 (0.1) | 8.3 (2.3) | 3.0 (0.3) | 2.2 (0.4) |
| MCP-1 (mean)(SD) (pg/mL) | 100.1 (13.8) | 339.3 (68.8) | 279.3 (53.5) | 230.4 (48.7) |
| Tear osmolarity (SD) (mOsm/L) | 306.2 (16.4) | 321.4 (19.6) | 316.2 (14.3) | 308.6 (18.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-K.; Hsieh, M.-W.; Chang, H.-C.; Chen, Y.-H.; Chien, K.-H. Improvement of Ocular Surface Disease by Lateral Tarsoconjunctival Flap in Thyroid-Associated Orbitopathy Patients with Lid Retraction. J. Pers. Med. 2022, 12, 802. https://doi.org/10.3390/jpm12050802
Hsu C-K, Hsieh M-W, Chang H-C, Chen Y-H, Chien K-H. Improvement of Ocular Surface Disease by Lateral Tarsoconjunctival Flap in Thyroid-Associated Orbitopathy Patients with Lid Retraction. Journal of Personalized Medicine. 2022; 12(5):802. https://doi.org/10.3390/jpm12050802
Chicago/Turabian StyleHsu, Chih-Kang, Meng-Wei Hsieh, Hsu-Chieh Chang, Yi-Hao Chen, and Ke-Hung Chien. 2022. "Improvement of Ocular Surface Disease by Lateral Tarsoconjunctival Flap in Thyroid-Associated Orbitopathy Patients with Lid Retraction" Journal of Personalized Medicine 12, no. 5: 802. https://doi.org/10.3390/jpm12050802






