Three-Dimensional Evaluation of Isodose Radiation Volumes in Cases of Severe Mandibular Osteoradionecrosis for the Prediction of Recurrence after Segmental Resection
Abstract
:1. Introduction
2. Materials and Method
2.1. Patients
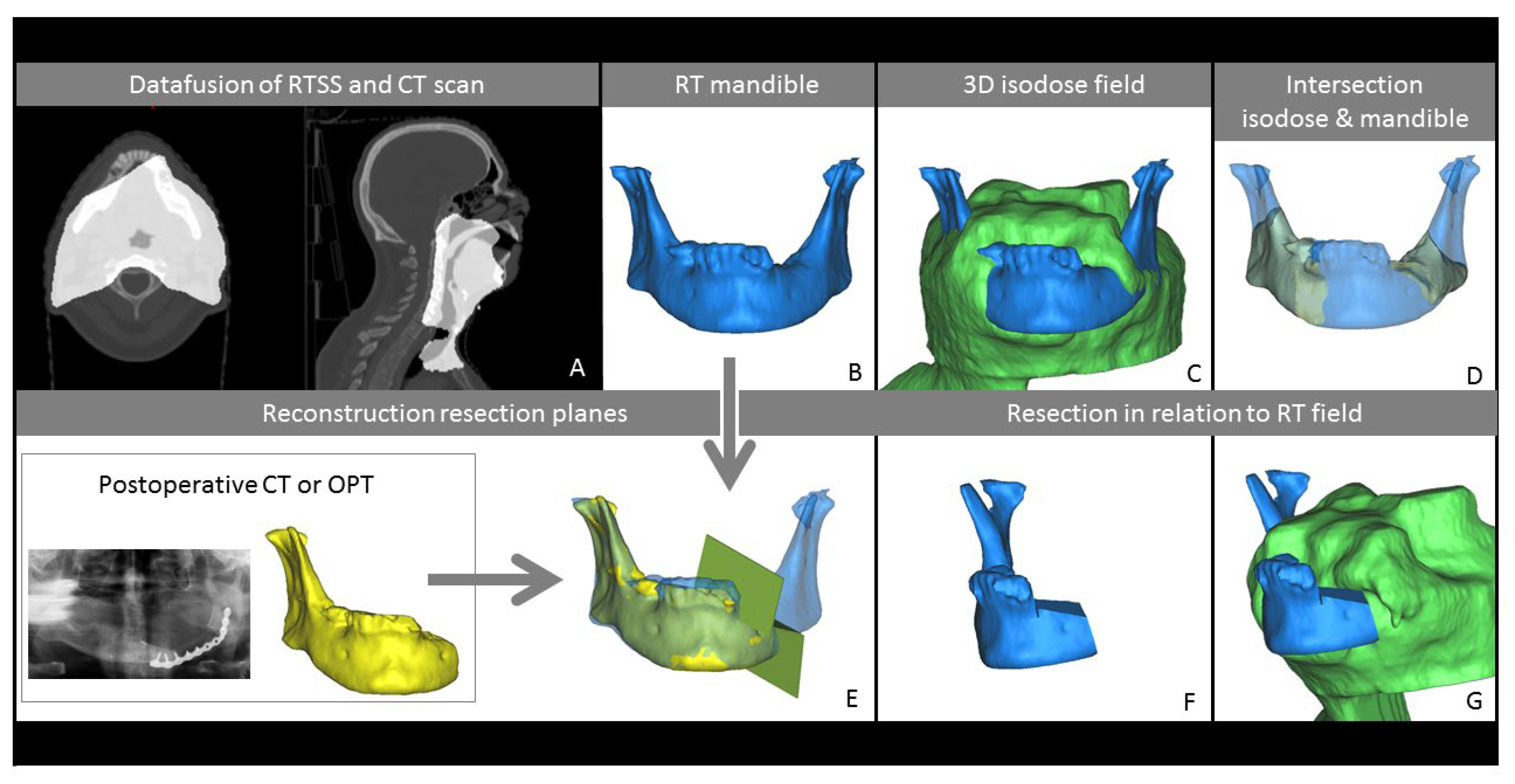
2.2. Processing of Imaging Data
2.3. Measurements
3. Results
3.1. Patient Characteristics
3.2. Recurrent Cases
3.3. Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| GTV | Gross tumour volume |
| DVH | Dose volume histogram |
| IMRT | Intensity modulated radiotherapy |
| MRONJ | Medication-related osteonecrosis of the jaw |
| ORN | Osteoradionecrosis |
| PTV | Planning target volume |
| RT | Radiotherapy |
| Vm | Mandible volume |
| VSP | Virtual surgical planning |
References
- Reuther, T.; Schuster, T.; Mende, U.; Kübler, A. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients—A report of a thirty year retrospective review. Int. J. Oral Maxillofac. Surg. 2003, 32, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Mallya, S.M.; Tetradis, S. Imaging of Radiation- and Medication-Related Osteonecrosis. Radiol. Clin. N. Am. 2018, 56, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Suárez, C.; Genden, E.M.; De Bree, R.; Strojan, P.; Langendijk, J.A.; Mäkitie, A.A.; Smee, R.; Eisbruch, A.; Lee, A.W.; et al. Parameters Associated With Mandibular Osteoradionecrosis. Am. J. Clin. Oncol. Cancer Clin. Trials 2018, 41, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.J.; Koom, W.S.; Lee, C.G.; Kim, Y.B.; Yoo, S.W.; Keum, K.C.; Kim, G.E.; Choi, E.C.; Cha, I. Risk Factors and Dose–Effect Relationship for Mandibular Osteoradionecrosis in Oral and Oropharyngeal Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Firmino, R.; Meira, H.; Vasconcelos, B.; Noronha, V.; Santos, V. Osteoradionecrosis prevalence and associated factors: A ten years retrospective study. Med. Oral Patol. Oral y Cir. Bucal 2018, 23, e633–e638. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.; Hofstede, T.M.; Sturgis, E.M.; Garden, A.S.; Lindberg, M.E.; Wei, Q.; Tucker, S.L.; Dong, L. Osteoradionecrosis and Radiation Dose to the Mandible in Patients With Oropharyngeal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 85, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Studer, G.; Studer, S.P.; Zwahlen, R.A.; Huguenin, P.; Grätz, K.W.; Lütolf, U.M.; Glanzmann, C. Osteoradionecrosis of the mandible: Minimized risk profile following intensity-modulated radiation therapy (IMRT). Strahlenther. Onkol. 2006, 182, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.G.; Herson, J.; Daly, T.E.; Zimmerman, S. Radiation necrosis of the mandible: A 10 year study. Part I. Factors influencing the onset of necrosis. Int. J. Radiat. Oncol. Biol. Phys. 1980, 6, 543–548. [Google Scholar] [CrossRef]
- Emami, B. Tolerance of Normal Tissue to Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Aarup-Kristensen, S.; Hansen, C.R.; Forner, L.; Brink, C.; Eriksen, J.G.; Johansen, J. Osteoradionecrosis of the mandible after radiotherapy for head and neck cancer: Risk factors and dose-volume correlations. Acta Oncol. 2019, 58, 1373–1377. [Google Scholar] [CrossRef]
- Manzano, B.R.; Santaella, N.G.; Oliveira, M.A.; Rubira, C.M.F.; de Santos, P.S. Retrospective study of osteoradi-onecrosis in the jaws of patients with head and neck cancer. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studer, G.; Grätz, K.W.; Glanzmann, C. Osteoradionecrosis of the Mandibula in Patients Treated with Different Fractionations. Strahlenther. Onkol. 2004, 180, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Glanzmann, C.; Grätz, K. Radionecrosis of the mandibula: A retrospective analysis of the incidence and risk factors. Radiother. Oncol. 1995, 36, 94–100. [Google Scholar] [CrossRef]
- Marx, R.E. A New Concept of Its Pathophysiology. Growth 1983, 41, 283–288. [Google Scholar]
- Wanifuchi, S.; Akashi, M.; Ejima, Y.; Shinomiya, H.; Minamikawa, T.; Furudoi, S.; Otsuki, N.; Sasaki, R.; Nibu, K.-I.; Komori, T. Cause and occurrence timing of osteoradionecrosis of the jaw: A retrospective study focusing on prophylactic tooth extraction. Oral Maxillofac. Surg. 2016, 20, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Grisar, K.; Schol, M.; Schoenaers, J.; Dormaar, T.; Coropciuc, R.; Poorten, V.V.; Politis, C. Osteoradionecrosis and medication-related osteonecrosis of the jaw: Similarities and differences. Int. J. Oral Maxillofac. Surg. 2016, 45, 1592–1599. [Google Scholar] [CrossRef]
- Fortunato, L.; Amato, M.; Simeone, M.; Bennardo, F.; Barone, S.; Giudice, A. Numb chin syndrome: A reflection of malignancy or a harbinger of MRONJ? A multicenter experience. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 389–394. [Google Scholar] [CrossRef]
- Nadella, K.R.; Kodali, R.M.; Guttikonda, L.K.; Jonnalagadda, A. Osteoradionecrosis of the Jaws: Clinico-Therapeutic Management: A Literature Review and Update. J. Maxillofac. Oral Surg. 2015, 14, 891–901. [Google Scholar] [CrossRef] [Green Version]
- Studer, G.; Bredell, M.; Studer, S.; Huber, G.; Glanzmann, C. Risikoprofil für Osteoradionekrosen des Kiefers in der IMRT-Ära. Strahlenther. Onkol. 2016, 192, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Ben-David, M.A.; Diamante, M.; Radawski, J.D.; Vineberg, K.A.; Stroup, C.; Murdoch-Kinch, C.-A.; Zwetchkenbaum, S.R.; Eisbruch, A. Lack of Osteoradionecrosis of the Mandible After Intensity-Modulated Radiotherapy for Head and Neck Cancer: Likely Contributions of Both Dental Care and Improved Dose Distributions. Int. J. Radiat. Oncol. 2007, 68, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Anderson Head and Neck Cancer Symptom Working Group. Dose-volume correlates of mandibular osteoradionecrosis in Oropharynx cancer patients receiv-ing intensity-modulated radiotherapy: Results from a case-matched comparison. Radiother. Oncol. 2017, 124, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Dore, F.; Filippi, L.; Biasotto, M.; Chiandussi, S.; Cavalli, F.; di Lenarda, R. Bone scintigraphy and SPECT/CT of bisphos-phonate-induced osteonecrosis of the jaw. J. Nucl. Med. 2009, 50, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, A.S.; He, R.; Ding, Y.; Wang, J.; Fahim, J.; Elgohari, B.; Elhalawani, H.; Kim, A.D.; Ahmed, H.; Garcia, J.A.; et al. Quantitative Dynamic Contrast-Enhanced MRI Identifies Radiation-Induced Vascular Damage in Patients With Advanced Osteoradionecrosis: Results of a Prospective Study. Int. J. Radiat. Oncol. 2020, 108, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Zaghi, S.; Miller, M.; Blackwell, K.; Palla, B.; Lai, C.; Nabili, V. Analysis of surgical margins in cases of mandibular oste-oradionecrosis that progress despite extensive mandible resection and free tissue transfer. Am. J. Otolaryngol. 2012, 33, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Alam, D.S.; Nuara, M.; Christian, J. Analysis of Outcomes of Vascularized Flap Reconstruction in Patients with Advanced Mandibular Osteoradionecrosis. Otolaryngol. Neck Surg. 2009, 141, 196–201. [Google Scholar] [CrossRef]
- Curi, M.M.; dos Santos, M.O.; Feher, O.; Faria, J.C.M.; Rodrigues, M.L.; Kowalski, L.P. Management of Extensive Osteoradionecrosis of the Mandible With Radical Resection and Immediate Microvascular Reconstruction. J. Oral Maxillofac. Surg. 2007, 65, 434–438. [Google Scholar] [CrossRef]
- Marx, R.E. A new concept in the treatment of osteoradionecrosis. J. Oral Maxillofac. Surg. 1983, 41, 351–357. [Google Scholar] [CrossRef]
- Kraeima, J.; Steenbakkers, R.J.H.M.; Spijkervet, F.K.L.; Roodenburg, J.L.N.; Witjes, M.J.H. Secondary surgical man-agement of osteoradionecrosis using three-dimensional isodose curve visualization: A report of three cases. Int. J. Oral Maxillofac. Surg. 2018, 47, 214–219. [Google Scholar] [CrossRef]
- Kraeima, J.; Schepers, R.H.; van Ooijen, P.M.A.; Steenbakkers, R.J.H.M.; Roodenburg, J.L.N.; Witjes, M.J.H. Integration of oncologic margins in three-dimensional virtual planning for head and neck surgery, including a validation of the software pathway. J. Cranio Maxillofac. Surg. 2015, 43, 1374–1379. [Google Scholar] [CrossRef]


| Value | % | ||
|---|---|---|---|
| Age | |||
| Median (range) | 60 | (43–76) | |
| sex | |||
| male | 21 | 64% | |
| Female | 12 | 36% | |
| Smoking status | |||
| Never | 6 | 23% | |
| Former | 11 | 42% | |
| Current | 9 | 35% | |
| unknown | 7 | ||
| Smoking pack-year | |||
| Mean (SD) | 31 | (23) | |
| Alcohol history | |||
| occasional | 5 | 19% | |
| Former | 11 | 41% | |
| Current | 13 | 48% | |
| unknown | 6 | ||
| Tumour location | |||
| Base of tonque | 11 | 48% | |
| Tonsil | 8 | 35% | |
| Other | 4 | 17% | |
| unknown | 10 | ||
| T stage | |||
| T1 | 1 | 3% | |
| T2 | 12 | 38% | |
| T3 | 6 | 19% | |
| T4 | 13 | 41% | |
| unknown | 1 | ||
| N stage | |||
| N0 | 6 | 19% | |
| N1 | 5 | 16% | |
| N2 | 21 | 66% | |
| unknown | 1 | ||
| Primary treatment | |||
| RT | 3 | 9% | |
| Surgery + RT | 7 | 21% | |
| RCT | 20 | 61% | |
| Surgery + RCT | 3 | 9% | |
| Time RT-ORN | |||
| Months (range) | 28 | (1–76) | |
| Reconstruction method | |||
| Fibula (unknown) | 12 | ||
| 21 | (21) | ||
| Follow-up initial ORN | |||
| Months (range) | 69 | (19–142) | |
| Dental status | |||
| Edentulous | 11 | 33% | |
| dental extractions | 16 | 59% | |
| unknown | 6 | ||
| HBO therapy | 18 | 55% | |
| Radiation dose | |||
| Median (range) | 70 Gy | (56–72) | |
| Radiation fractions | |||
| Median (range) | 33 | (28–45) | |
| RT = radiotherapy | |||
| RCT = radiotherapy + chemotherapy | |||
| Vm | V56 | V-PTV | Vm56 | Vm-PTV | Vm56R | Vm-PTV-R | VmR | Vm56/Vm | Vm-PTV/Vm | VmR/Vm | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 33) | Mean (mL) | 62.7 | 772.8 | 221.5 | 27.7 | 9.2 | 9.6 | 3.3 | 39.4 | 42% | 15% | 35% |
| min | 26.4 | 33.8 | 0.0 | 4.0 | 0.0 | 0.0 | 0.0 | 23.9 | 9% | 0% | 7% | |
| max | 95.0 | 1760.9 | 623.2 | 77.5 | 38.6 | 53.1 | 31.4 | 36.0 | 83% | 51% | 78% | |
| Non-recurrent (n = 28) | Mean (mL) | 65.4 | 843.5 | 238.6 | 30.7 | 9.8 | 10.9 | 3.8 | 40.3 | 45% | 15% | 37% |
| min | 26.4 | 183.6 | 0.0 | 4.5 | 0.0 | 0.0 | 0.0 | 23.9 | 10% | 0% | 7% | |
| max | 95.0 | 1760.9 | 623.2 | 77.5 | 38.6 | 53.1 | 31.4 | 36.0 | 83% | 51% | 78% | |
| Recurrent (n = 5) | Mean (mL) | 47.8 | 376.9 | 125.7 | 10.9 | 5.9 | 2.2 | 0.0 | 34.8 | 23% | 13% | 26% |
| min | 32.2 | 33.8 | 33.8 | 4.0 | 0.0 | 0.7 | 0.0 | 29.1 | 9% | 0% | 10% | |
| max | 58.4 | 763.0 | 212.1 | 19.6 | 19.6 | 4.8 | 0.0 | 35.1 | 44% | 44% | 52% | |
| >70 Gy Non-recurrent (n = 20) | Mean (mL) | 67.8 | 950.7 | 235.6 | 29.9 | 6.7 | 7.7 | 1.5 | 43.7 | 43% | 10% | 34% |
| min | 26.4 | 183.6 | 0.0 | 6.7 | 0.0 | 0.0 | 0.0 | 23.9 | 12% | 0% | 7% | |
| max | 95.0 | 1760.9 | 604.8 | 77.5 | 33.7 | 32.5 | 10.2 | 42.2 | 83% | 44% | 63% | |
| >70 Gy Recurrent (n = 2) | Mean (mL) | 57.3 | 751.3 | 196.7 | 12.2 | 1.9 | 0.8 | 0.0 | 41.0 | 21% | 3% | 29% |
| min | 56.3 | 739.7 | 181.3 | 10.9 | 0.0 | 0.7 | 0.0 | 42.6 | 19% | 0% | 23% | |
| max | 58.4 | 763.0 | 212.1 | 13.4 | 3.9 | 0.8 | 0.0 | 39.4 | 24% | 7% | 34% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glas, H.H.; Kraeima, J.; Tribius, S.; Leusink, F.K.J.; Rendenbach, C.; Heiland, M.; Stromberger, C.; Rashad, A.; Fuller, C.D.; Mohamed, A.S.R.; et al. Three-Dimensional Evaluation of Isodose Radiation Volumes in Cases of Severe Mandibular Osteoradionecrosis for the Prediction of Recurrence after Segmental Resection. J. Pers. Med. 2022, 12, 834. https://doi.org/10.3390/jpm12050834
Glas HH, Kraeima J, Tribius S, Leusink FKJ, Rendenbach C, Heiland M, Stromberger C, Rashad A, Fuller CD, Mohamed ASR, et al. Three-Dimensional Evaluation of Isodose Radiation Volumes in Cases of Severe Mandibular Osteoradionecrosis for the Prediction of Recurrence after Segmental Resection. Journal of Personalized Medicine. 2022; 12(5):834. https://doi.org/10.3390/jpm12050834
Chicago/Turabian StyleGlas, Haye H., Joep Kraeima, Silke Tribius, Frank K. J. Leusink, Carsten Rendenbach, Max Heiland, Carmen Stromberger, Ashkan Rashad, Clifton D. Fuller, Abdallah S. R. Mohamed, and et al. 2022. "Three-Dimensional Evaluation of Isodose Radiation Volumes in Cases of Severe Mandibular Osteoradionecrosis for the Prediction of Recurrence after Segmental Resection" Journal of Personalized Medicine 12, no. 5: 834. https://doi.org/10.3390/jpm12050834
APA StyleGlas, H. H., Kraeima, J., Tribius, S., Leusink, F. K. J., Rendenbach, C., Heiland, M., Stromberger, C., Rashad, A., Fuller, C. D., Mohamed, A. S. R., Lai, S. Y., & Witjes, M. J. H. (2022). Three-Dimensional Evaluation of Isodose Radiation Volumes in Cases of Severe Mandibular Osteoradionecrosis for the Prediction of Recurrence after Segmental Resection. Journal of Personalized Medicine, 12(5), 834. https://doi.org/10.3390/jpm12050834












