B-Type Natriuretic Peptide at Admission Is a Predictor of All-Cause Mortality at One Year after the First Acute Episode of New-Onset Heart Failure with Preserved Ejection Fraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
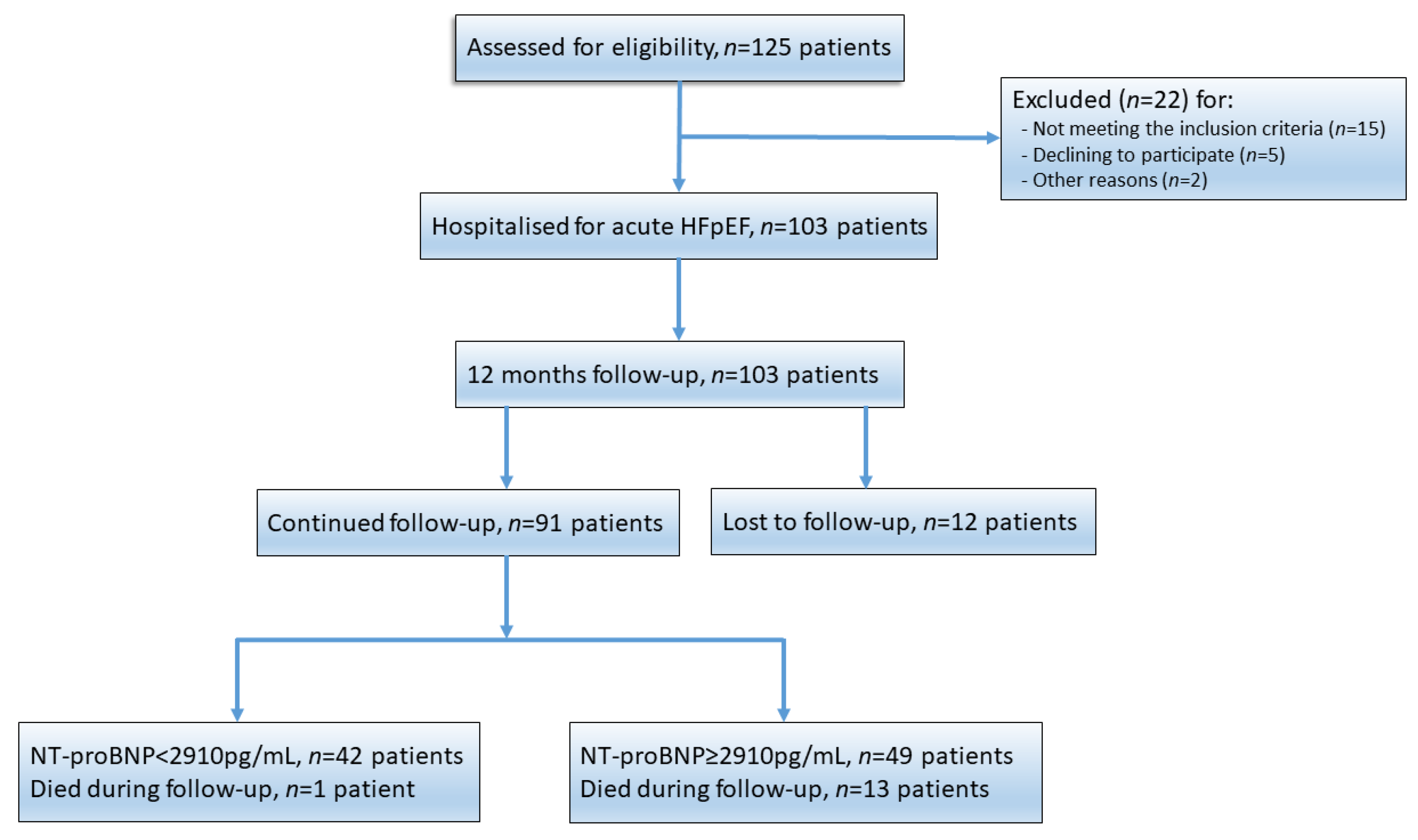
3.1. Patient Population
3.2. Baseline Characteristics
3.3. NT-proBNP—Independent Predictor for Mortality at 12 Months
3.4. NT-proBNP Threshold for 12-Month Mortality
3.5. All-Cause Rehospitalisation and Mortality
3.6. Clinical and Echocardiographic Outcomes
3.7. Predictors of an High-Set NT-proBNP (≥2910 pg/mL)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Parissis, J.; Karavidas, A.; Karvounis, C.; Triposkiadis, F.; Filippatos, G.; Lekakis, J.; Barbetseas, J.; Giannadaki, M.; Kakouros, S.; et al. In-Hospital Management of Acute Heart Failure: Practical Recommendations and Future Perspectives. Int. J. Cardiol. 2015, 201, 231–236. [Google Scholar] [CrossRef]
- Withaar, C.; Lam, C.S.P.; Schiattarella, G.G.; de Boer, R.A.; Meems, L.M.G. Heart Failure with Preserved Ejection Fraction in Humans and Mice: Embracing Clinical Complexity in Mouse Models. Eur. Heart J. 2021, 42, 4420–4430. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, S.; Antón-Ladislao, A.; Quiros, R.; Lara, A.; Rilo, I.; Morillas, M.; Murga, N.; Gallardo, M.S.; Lafuente, I.; Aguirre, U.; et al. Short-Term Mortality Risk Score for de Novo Acute Heart Failure (ESSIC-FEHF). Eur. J. Intern. Med. 2020, 77, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Lee, G.Y.; Choi, J.-O.; Jeon, E.-S.; Lee, H.-Y.; Cho, H.-J.; Lee, S.E.; Kim, M.-S.; Kim, J.-J.; Hwang, K.-K.; et al. Outcomes of de Novo and Acute Decompensated Heart Failure Patients according to Ejection Fraction. Heart 2017, 104, 525–532. [Google Scholar] [CrossRef]
- Franco, J.; Formiga, F.; Chivite, D.; Manzano, L.; Carrera, M.; Arévalo-Lorido, J.C.; Epelde, F.; Cerqueiro, J.M.; Serrado, A.; Pérez-Barquero, M.M. New onset heart failure--Clinical characteristics and short-term mortality. A RICA (Spanish registry of acute heart failure) study. Eur. J. Intern. Med. 2015, 26, 357–362. [Google Scholar] [CrossRef]
- García Sarasola, A.; Alquézar Arbé, A.; Gil, V.; Martín-Sánchez, F.J.; Jacob, J.; Llorens, P.; Rizzi, M.; Fuenzalida, C.; Calderón, S.; Miró, Ò. NOVICA: Characteristics and Outcomes of Patients Who Have a First Episode of Heart Failure (de Novo). Rev. Clínica Española (Engl. Ed.) 2019, 219, 469–476. [Google Scholar] [CrossRef]
- Nadar, S.K.; Shaikh, M.M. Biomarkers in Routine Heart Failure Clinical Care. Card. Fail. Rev. 2019, 5, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Santaguida, P.L.; Don-Wauchope, A.C.; Oremus, M.; McKelvie, R.; Ali, U.; Hill, S.A.; Balion, C.; Booth, R.A.; Brown, J.A.; Bustamam, A.; et al. BNP and NT-ProBNP as Prognostic Markers in Persons with Acute Decompensated Heart Failure: A Systematic Review. Heart Fail. Rev. 2014, 19, 453–470. [Google Scholar] [CrossRef]
- Salah, K.; Stienen, S.; Pinto, Y.M.; Eurlings, L.W.; Metra, M.; Bayes-Genis, A.; Verdiani, V.; Tijssen, J.G.P.; Kok, W.E. Prognosis and NT-ProBNP in Heart Failure Patients with Preserved versus Reduced Ejection Fraction. Heart 2019, 105, 1182–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheorghiade, M.; Follath, F.; Ponikowski, P.; Barsuk, J.H.; Blair, J.E.A.; Cleland, J.G.; Dickstein, K.; Drazner, M.H.; Fonarow, G.C.; Jaarsma, T.; et al. Assessing and Grading Congestion in Acute Heart Failure: A Scientific Statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and Endorsed by the European Society of Intensive Care Medicine. Eur. J. Heart Fail. 2010, 12, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.B.; Smith, A.; Apple, F.S. Optimum Blood Collection Intervals for B-Type Natriuretic Peptide Testing in Patients with Heart Failure. Am. J. Cardiol. 2004, 93, 1562–1563. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, C.; Sievert, K.; Meucci, F.; Sievert, H. A Novel Implantable Left Atrial Sensor for Pressure Monitoring in Heart Failure. EuroIntervention 2020, 16, 432–433. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, D.; Restivo, A.; Canonico, F.; Rodolico, D.; Mattia, G.; Francesco, B.; Vergallo, R.; Trani, C.; Aspromonte, N.; Crea, F. Experience of Remote Cardiac Care during the COVID-19 Pandemic: The V-LAP TM Device in Advanced Heart Failure. Eur. J. Heart Fail. 2020, 22, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, H.; Dobrev, D.; Heijman, J. Angiotensin Receptor-Neprilysin Inhibitor (ARNI) and Cardiac Arrhythmias. Int. J. Mol. Sci. 2021, 22, 8994. [Google Scholar] [CrossRef]
- McKee, P.A.; Castelli, W.P.; McNamara, P.M.; Kannel, W.B. The Natural History of Congestive Heart Failure: The Framingham Study. N. Engl. J. Med. 1971, 285, 1441–1446. [Google Scholar] [CrossRef]
- Pieske, B.; Butler, J.; Filippatos, G.; Lam, C.; Maggioni, A.P.; Ponikowski, P.; Shah, S.; Solomon, S.; Kraigher-Krainer, E.; Samano, E.T.; et al. Rationale and Design of the SOluble Guanylate Cyclase StimulatoR in HeArT FailurE Studies (SOCRATES). Eur. J. Heart Fail. 2014, 16, 1026–1038. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Rienstra, M.; Tay, W.T.; Liu, L.C.Y.; Hummel, Y.M.; van der Meer, P.; de Boer, R.A.; Van Gelder, I.C.; van Veldhuisen, D.J.; Voors, A.A.; et al. Atrial Fibrillation in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2017, 5, 92–98. [Google Scholar] [CrossRef]
- Human Experimentation: Code of Ethics of W.M.A. Br. Med. J. 1964, 2, 177. [CrossRef] [Green Version]
- Youden, W.J. Index for Rating Diagnostic Tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NICE Guideline: Chronic Heart Failure in Adults: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng106 (accessed on 24 January 2022).
- Shchekochikhin, D.; Nikiforova, T.; Shilova, A.; Nesterov, A.; Baturina, O.; Gognieva, D.; Kozlovskaya, N.; Syrkin, A.; Kopylov, P. Evaluation of Discriminative Capacity of Two Formulas of CKD-EPI to Predict Complications after the First Episode of Heart Failure with Preserved Ejection Fraction. Int. J. Nephrol. Renov. Dis. 2019, 12, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Manzano-Fernández, S.; Januzzi, J.L.; Boronat-García, M.; Pastor, P.; Albaladejo-Otón, M.D.; Garrido, I.P.; Bayes-Genis, A.; Valdés, M.; Pascual-Figal, D.A. Impact of Kidney Dysfunction on Plasma and Urinary N-Terminal Pro-B-Type Natriuretic Peptide in Patients with Acute Heart Failure. Congest. Heart Fail. 2010, 16, 214–220. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Taylor, J.; Freemantle, N.; Goode, K.M.; Rigby, A.S.; Tendera, M. Relationship between Plasma Concentrations of N-Terminal pro Brain Natriuretic Peptide and the Characteristics and Outcome of Patients with a Clinical Diagnosis of Diastolic Heart Failure: A Report from the PEP-CHF Study. Eur. J. Heart Fail. 2012, 14, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Bosseau, C.; Galli, E.; Donal, E. Prognostic Value of BNP in Heart Failure with Preserved or Reduced Ejection Fraction. Heart 2015, 101, 1855–1856. [Google Scholar] [CrossRef]
- Brouwers, F.P.; de Boer, R.A.; van der Harst, P.; Voors, A.A.; Gansevoort, R.T.; Bakker, S.J.; Hillege, H.L.; van Veldhuisen, D.J.; van Gilst, W.H. Incidence and Epidemiology of New Onset Heart Failure with Preserved vs. Reduced Ejection Fraction in a Community-Based Cohort: 11-Year Follow-up of PREVEND. Eur. Heart J. 2013, 34, 1424–1431. [Google Scholar] [CrossRef]
- Spinar, J.; Spinarova, L.; Malek, F.; Ludka, O.; Krejci, J.; Ostadal, P.; Vondrakova, D.; Labr, K.; Spinarova, M.; Pavkova Goldbergova, M.; et al. Prognostic Value of NT-ProBNP Added to Clinical Parameters to Predict Two-Year Prognosis of Chronic Heart Failure Patients with Mid-Range and Reduced Ejection Fraction—A Report from FAR NHL Prospective Registry. PLoS ONE 2019, 14, e0214363. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Jiang, D.; Wen, X.; Cheng, X.; Sun, M.; He, B.; You, L.; Lei, P.; Tan, X.; Qin, S.; et al. Prognostic Value of NT-ProBNP in Patients with Severe COVID-19. Respir. Res. 2020, 21, 83. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zeng, Z.; Cheng, J.; Hu, G.; Li, Y.; Wei, L.; Zhou, Y.; Ran, P. Prognostic Role of NT-ProBNP for In-Hospital and 1-Year Mortality in Patients with Acute Exacerbations of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akpınar, E.E.; Hoşgün, D.; Akpınar, S.; Ateş, C.; Baha, A.; Gülensoy, E.S.; Ogan, N. Do N-Terminal Pro-Brain Natriuretic Peptide Levels Determine the Prognosis of Community Acquired Pneumonia? J. Bras. Pneumol. 2019, 45, e20180417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergaro, G.; Gentile, F.; Meems, L.; Aimo, A.; Januzzi, J.L., Jr.; Richards, A.M.; Lam, C.; Latini, R.; Staszewsky, L.; Anand, I.S.; et al. NT-proBNP for Risk Prediction in Heart Failure: Identification of Optimal Cutoffs Across Body Mass Index Categories. JACC. Heart Fail. 2021, 9, 653–663. [Google Scholar] [CrossRef]
- Taylor, C.J.; Roalfe, A.K.; Iles, R.; Hobbs, F.D.R. The Potential Role of NT-ProBNP in Screening for and Predicting Prognosis in Heart Failure: A Survival Analysis. BMJ Open 2014, 4, e004675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Ni, G.; Wu, Q.; Zhou, Y.; Yao, W.; Zhang, H.; Li, X. Prognostic Value of N-Terminal Pro-B-Type Natriuretic Peptide and Glomerular Filtration Rate in Patients with Acute Heart Failure. Front. Cardiovasc. Med. 2020, 7, 123. [Google Scholar] [CrossRef]
- Zamfirescu, M.-B.; Ghilencea, L.N.; Popescu, M.-R.; Bejan, G.C.; Ghiordanescu, I.M.; Popescu, A.-C.; Myerson, S.G.; Dorobanțu, M. A Practical Risk Score for Prediction of Early Readmission after a First Episode of Acute Heart Failure with Preserved Ejection Fraction. Diagnostics 2021, 11, 198. [Google Scholar] [CrossRef]
- Duchnowski, P.; Hryniewiecki, T.; Kuśmierczyk, M.; Szymanski, P. N-Terminal of the Prohormone Brain Natriuretic Peptide Is a Predictor of Hemodynamic Instability in Valve Disease. Biomark. Med. 2019, 13, 353–358. [Google Scholar] [CrossRef]
- Duchnowski, P.; Hryniewiecki, T.; Koźma, M.; Mariusz, K.; Piotr, S. High-Sensitivity Troponin T Is a Prognostic Marker of Hemodynamic Instability in Patients Undergoing Valve Surgery. Biomark. Med. 2018, 12, 1303–1309. [Google Scholar] [CrossRef]
- López Castro, J.; Almazán Ortega, R.; Pérez De Juan Romero, M.; González Juanatey, J.R. Factores pronósticos de mortalidad de la insuficiencia cardíaca en una cohorte del noroeste de España. Estudio EPICOUR [Mortality prognosis factors in heart failure in a cohort of North-West Spain. EPICOUR study]. Rev. Clin. Esp. 2019, 210, 438–447. [Google Scholar] [CrossRef]
- Mouriño López, V.M.; Cid Conde, L.; Alves Pérez, M.T.; López Castro, J. Long-Term Survival of a Cohort of Patients with Heart Failure: Perspective from the Real World. Eur. Geriatr. Med. 2017, 8, 304–309. [Google Scholar] [CrossRef]




| Characteristics | Value |
|---|---|
| Number of subjects | 91 |
| Length of in-hospital stay, days, median (IQR) | 7.5 (5) |
| Age at diagnosis, yo, mean ± SD (95% CI) | 73 ± 10.6 (70.8–75.3) |
| Female gender, n (%) | 62 (68.1%) |
| Cardiovascular risk factors | |
| Hypertension, n (%) | 91 (100%) |
| Diabetes mellitus, n (%) | 51 (56%) |
| Tobacco smoking (current or former), n (%) | 25 (27.5%) |
| Hypercholesterolemia, n (%) | 75 (82.4%) |
| BMI (kg/m2), mean ± SD (95% CI) | 32.1 ± 6.3 (30.8–33.5) |
| Previous medical history | |
| CAD, n (%) | 21 (23.1%) |
| MI, n (%) | 13 (14.3%) |
| Stroke, n (%) | 18 (19.8%) |
| Atrial fibrillation, n (%) | 63 (69.2%) |
| Lung disease, n (%) | 44 (48.4%) |
| Sleep apnoea, n (%) | 12 (13.2%) |
| Assessment at admission | |
| Non-Invasive ventilation, n (%) | 20 (22%) |
| Mechanical ventilation, n (%) | 6 (6.6%) |
| Peripheral oedema, n (%) | 53 (58.2%) |
| SpO2 (%), median (IQR) | 89 (6) |
| HR (beats/min), median (IQR) | 96 (55) |
| SBP (mm Hg), mean ± SD (95% CI) | 185.4 ± 34.8 (178.2–192.7) |
| DBP (mm Hg), mean ± SD (95% CI) | 99.2 ± 18.1 (95.5–103) |
| Serum sodium (mmol/L), median (IQR) | 140 (5) |
| eGFR (mL/min/1.73 m2), mean ± SD (95% CI) | 66.5 ± 28.8 (60.5–72.5) |
| Hb (g/dL), mean ± SD (95% CI) | 12 ± 2.0 (11.6–12.4) |
| NT-proBNP (ng/L), median (IQR) | 3074 (5241) |
| Medication at discharge | |
| ACEI, n (%) | 46 (50.5%) |
| ARB/ARNI, n (%) | 36 (39.6%) |
| Amlodipine, n (%) | 51 (56%) |
| Antiarhythmics, n (%) | 6 (6.6%) |
| Anticoagulants, n (%) | 59 (64.8%) |
| Beta-blockers, n (%) | 77 (84.6%) |
| Digoxin, n (%) | 19 (20.9%) |
| Loop diuretics, n (%) | 79 (86.8%) |
| MRA, n (%) | 42 (46.2%) |
| Nitrates, n (%) | 11 (12.1%) |
| Statines, n (%) | 66 (72.5%) |
| Characteristics | NT-proBNP < 2910 ng/mL | NT-proBNP ≥ 2910 ng/mL | p-Value |
|---|---|---|---|
| Number (%) | 42 (46.2%) | 49 (53.8%) | |
| Age (yr) mean ± SD (95% CI) | 70.4 ± 10.2 (67.2–73.6) | 75.3 ± 10.5 (72.3–78.3) | 0.026 |
| Male gender, n (%) | 17 (40.5%) | 12 (24.5%) | 0.10 |
| Smoking status, n (%) | 15 (35.7%) | 10 (20.4%) | 0.10 |
| Medical history | |||
| Diabetes mellitus, n (%) | 25 (59.5%) | 26 (53.1%) | 0.53 |
| Hypercholesterolemia, n (%) | 40 (95.2%) | 35 (71.4%) | 0.003 |
| Atrial Fibrillation, n (%) | 23 (54.8%) | 40 (81.6%) | 0.006 |
| Coronary artery disease, n (%) | 13 (31%) | 8 (16.3%) | 0.099 |
| Lung disease, n (%) | 22 (52.4%) | 22 (44.9%) | 0.47 |
| Sleep apnea, n (%) | 5 (11.9%) | 7 (14.3%) | 0.73 |
| Number of comorbidities, median (IQR) | 6 (2) | 6 (3) | 0.46 |
| At admission | |||
| Pulmonary edema, n (%) | 15 (36.6%) | 15 (31.3%) | 0.59 |
| Peripheral edema, n (%) | 22 (52.4%) | 31 (63.3%) | 0.29 |
| BMI (kg/m2), mean ± SD (95% CI) | 32.7 ± 5.12 (31.1–34.3) | 31.6 ± 7.1 (29.6–33.7) | 0.43 |
| SpO2 (%), median (IQR) | 89 (7) | 89 (7) | 0.91 |
| Heart rate (beats/min), median (IQR) | 93.5 (45) | 96 (58) | 0.45 |
| SBP (mm Hg), mean ± SD (95% CI) | 189.5 ± 35.8 (178.4–200.7) | 181.9 ± 33.9 (172.2–191.7) | 0.3 |
| DBP (mm Hg), mean ± SD (95% CI) | 102.1 ± 19 (96.2–108.1) | 96.7 ± 17 (91.8–101.6) | 0.17 |
| eGFR (mL/min/1.73 m2),mean ± SD (95% CI) | 76.4 ± 29.9 (67.1–85.8) | 58 ± 25.1 (50.8–65.2) | 0.002 |
| Haemoglobin (g/dL), mean ± SD (95% CI) | 12.4 ± 1.5 (11.9–12.9) | 11.6 ± 2.3 (10.5–12.3) | 0.064 |
| NT-proBNP (ng/L), median (IQR) | 1529.5 (1698) | 6700 (5298) | 0.001 |
| Echocardiography at admission | |||
| Pulmonary edema, n (%) | 15 (36.6%) | 15 (31.3%) | 0.59 |
| Peripheral edema, n (%) | 22 (52.4%) | 31 (63.3%) | 0.29 |
| LVEF (%), mean ± SD (95% CI) | 55 (7) | 55 (10) | 0.51 |
| LVEDD (mm), mean ± SD (95% CI) | 48.5 ± 5.2 (46.9–50.1) | 46.9 ± 5.7 (45.3–48.6) | 0.17 |
| LV mass (g/m2), median (IQR) | 125 (36) | 122 (34.5) | 0.63 |
| LVOT VTI (cm), median (IQR) | 18.85 (6) | 18 (7) | 0.5 |
| TAPSE (mm), mean ± SD (95% CI) | 20.9 ± 3.8 (19.7–22.1) | 19 ± 3.7 (17.9–20) | 0.017 |
| TAPSE < 17 mm, n (%) | 6 (14.3%) | 15 (30.6%) | 0.065 |
| Systolic PAP (mm Hg),mean ± SD (95% CI) | 41.1 ± 15.7 (36.2–46) | 40.5 ± 14.4 (36.4–44.6) | 0.83 |
| Systolic PAP > 35, n (%) | 26 (61.9%) | 33 (67.3%) | 0.58 |
| IVC diameter > 21 mm, n (%) | 18 (42.9%) | 22 (44.9%) | 0.84 |
| IVC collapse < 50%, n (%) | 11 (26.2%) | 24 (49%) | 0.026 |
| LAVi (mL/m2), mean ± SD (95% CI) | 47 ± 10.6 (43.7–50.3) | 55.7 ± 12 (52.2–59.1) | 0.001 |
| LAVi > 34 mL/m2, n (%) | 39 (92.9%) | 48 (98%) | 0.23 |
| E/e’ratio | 14.9 ± 4.9 (13.3–16.4) | 14.2 ± 4.4 (12.9–15.5) | 0.506 |
| Stroke volume, median (IQR) | 65.5 (26) | 60 (33) | 0.053 |
| Right Atrium area > 18 cm2 | 24 (57.1) | 39 (79.6) | 0.021 |
| In-hospital stay (days), median (IQR) | 6 (4) | 9 (6) | 0.08 |
| Rehospitalisation at 12 months, n (%) | 18 (42.9%) | 26 (53.1%) | 0.33 |
| Median time to readmission (days) | 75 (105) | 30 (45) | 0.008 |
| All-cause mortality at 12 months, n (%) | 1 (2.4%) | 13 (26.5%) | 0.001 |
| Time to death (days), median (IQR) | 360 | 90 (252) | 0.002 |
| Medication at discharge | |||
| ACEI, n (%) | 19 (45.2%) | 27 (55.1%) | 0.253 |
| Amlodipine, n (%) | 26 (61.9%) | 25 (51%) | 0.409 |
| ARB/ARNI, n (%) | 21 (50%) | 15 (30.6%) | 0.084 |
| Anticoagulants, n (%) | 25 (59.5%) | 34 (69.4%) | 0.204 |
| Antiarhythmics, n (%) | 4 (9.5%) | 2 (4%) | 0.325 |
| Beta-blockers, n (%) | 34 (80.9%) | 43 (87.8%) | 0.149 |
| Digoxin, n (%) | 8 (19%) | 11 (22.4%) | 0.619 |
| Loop diuretics, n (%) | 34 (80.9%) | 45 (91.8%) | 0.028 |
| MRA, n (%) | 23 (54.8%) | 24 (49%) | 0.729 |
| Nitrates, n (%) | 4 (9.5%) | 7 (14.3%) | 0.445 |
| Statines, n (%) | 36 (85.7%) | 30 (61.2%) | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghilencea, L.-N.; Bejan, G.-C.; Zamfirescu, M.-B.; Stănescu, A.M.A.; Matei, L.-L.; Manea, L.-M.; Kilic, I.D.; Bălănescu, S.-M.; Popescu, A.-C.; Myerson, S.G. B-Type Natriuretic Peptide at Admission Is a Predictor of All-Cause Mortality at One Year after the First Acute Episode of New-Onset Heart Failure with Preserved Ejection Fraction. J. Pers. Med. 2022, 12, 890. https://doi.org/10.3390/jpm12060890
Ghilencea L-N, Bejan G-C, Zamfirescu M-B, Stănescu AMA, Matei L-L, Manea L-M, Kilic ID, Bălănescu S-M, Popescu A-C, Myerson SG. B-Type Natriuretic Peptide at Admission Is a Predictor of All-Cause Mortality at One Year after the First Acute Episode of New-Onset Heart Failure with Preserved Ejection Fraction. Journal of Personalized Medicine. 2022; 12(6):890. https://doi.org/10.3390/jpm12060890
Chicago/Turabian StyleGhilencea, Liviu-Nicolae, Gabriel-Cristian Bejan, Marilena-Brîndusa Zamfirescu, Ana Maria Alexandra Stănescu, Lavinia-Lucia Matei, Laura-Maria Manea, Ismail Dogu Kilic, Serban-Mihai Bălănescu, Andreea-Catarina Popescu, and Saul Gareth Myerson. 2022. "B-Type Natriuretic Peptide at Admission Is a Predictor of All-Cause Mortality at One Year after the First Acute Episode of New-Onset Heart Failure with Preserved Ejection Fraction" Journal of Personalized Medicine 12, no. 6: 890. https://doi.org/10.3390/jpm12060890









