Glucose Metabolism Derangements and Thyroid Nodules: Does Sex Matter?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Association between GMD and TN According to Sex
3.2. GMD and IR Distributions According to Sex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, M.L.; Schulkin, J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br. J. Nutr. 2008, 99, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 March 2022).
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Unwin, N.; Shaw, J.; Zimmet, P.; Alberti, K.G. Impaired glucose tolerance and impaired fasting glycaemia: The current status on definition and intervention. Diabet. Med. 2002, 19, 708–723. [Google Scholar] [CrossRef]
- Poehlman, E.T.; Tchernof, A. Traversing the menopause: Changes in energy expenditure and body composition. Coron. Artery Dis. 1998, 9, 799–803. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [Green Version]
- Mauvais-Jarvis, F. Estrogen and androgen receptors: Regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol. Metab. 2011, 22, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Dean, D.S.; Gharib, H. Epidemiology of thyroid nodules. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Guth, S.; Theune, U.; Aberle, J.; Galach, A.; Bamberger, C.M. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur. J. Clin. Investig. 2009, 39, 699–706. [Google Scholar] [CrossRef]
- Mortensen, J.D.; Woolner, L.B.; Bennett, W.A. Gross and microscopic findings in clinically normal thyroid glands. J. Clin. Endocrinol. Metab. 1955, 15, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Costante, G.; Crocetti, U.; Schifino, E.; Ludovico, O.; Capula, C.; Nicotera, M.; Arturi, F.; Filetti, S. Slow growth of benign thyroid nodules after menopause: No need for long-term thyroxine suppressive therapy in post-menopausal women. J. Endocrinol. Investig. 2004, 27, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Del Senno, L.; degli Uberti, E.; Hanau, S.; Piva, R.; Rossi, R.; Trasforini, G. In vitro effects of estrogen on tgb and c-myc gene expression in normal and neoplastic human thyroids. Mol. Cell Endocrinol. 1989, 63, 67–74. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, G.G.; Vlantis, A.C.; van Hasselt, C.A. Oestrogen mediates the growth of human thyroid carcinoma cells via an oestrogen receptor-ERK pathway. Cell Prolif. 2007, 40, 921–935. [Google Scholar] [CrossRef]
- Zhang, H.M.; Feng, Q.W.; Niu, Y.X.; Su, Q.; Wang, X. Thyroid Nodules in Type 2 Diabetes Mellitus. Curr. Med. Sci. 2019, 39, 576–581. [Google Scholar] [CrossRef]
- Li, H.; Qian, J. Association of diabetes mellitus with thyroid cancer risk: A meta-analysis of cohort studies. Medicine 2017, 96, e8230. [Google Scholar] [CrossRef]
- Gursoy, A. Rising thyroid cancer incidence in the world might be related to insulin resistance. Med. Hypotheses 2010, 74, 35–36. [Google Scholar] [CrossRef]
- Ayturk, S.; Gursoy, A.; Kut, A.; Anil, C.; Nar, A.; Tutuncu, N.B. Metabolic syndrome and its components are associated with increased thyroid volume and nodule prevalence in a mild-to-moderate iodine-deficient area. Eur. J. Endocrinol. 2009, 161, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Rezzonico, J.; Rezzonico, M.; Pusiol, E.; Pitoia, F.; Niepomniszcze, H. Introducing the thyroid gland as another victim of the insulin resistance syndrome. Thyroid 2008, 18, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, X.; Han, Y.; Teng, W.; Shan, Z. Correlation Between Thyroid Nodules and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 730279. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xu, Y.; Wang, Y.; Li, X.; Lu, C.; Su, J.; Chen, Y.; Ma, Y.; Yin, Y.; Wu, Y.; et al. Gender Disparity in the Relationship between Prevalence of Thyroid Nodules and Metabolic Syndrome Components: The SHDC-CDPC Community-Based Study. Mediat. Inflamm. 2017, 2017, 8481049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Teng, D.; Tong, N.; Wang, G.; Li, Y.; Yu, X.; Shan, Z.; Teng, W. Gender-Specific Associations Between Metabolic Disorders and Thyroid Nodules: A Cross-Sectional Population-Based Study from China. Thyroid. 2022, 32, 571–580. [Google Scholar] [CrossRef]
- Buscemi, S.; Massenti, F.M.; Vasto, S.; Galvano, F.; Buscemi, C.; Corleo, D.; Barile, A.M.; Rosafio, G.; Rini, N.; Giordano, C. Association of obesity and diabetes with thyroid nodules. Endocrine 2018, 60, 339–347. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Yu, X.; Wang, X.; Lin, Z.; Song, B.; Tian, L.; Feng, C.; Shan, Z.; Teng, W. The Relationship and Gender Disparity Between Thyroid Nodules and Metabolic Syndrome Components Based on a Recent Nationwide Cross-Sectional Study and Meta-Analysis. Front. Endocrinol. 2021, 12, 736972. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33, Erratum in Diabetes Care 2021, 44, 2182. [Google Scholar] [CrossRef]
- Rossi, R.; Zatelli, M.C.; Franceschetti, P.; Maestri, I.; Magri, E.; Aguiari, G.; Cavazzini, P.; Degli Uberti, E.C.; del Senno, L. Inhibitory effect of dihydrotestosterone on human thyroid cell growth. J. Endocrinol. 1996, 151, 185–194. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Huang, Y.; Yang, P.; Xu, W. A Mechanism Exploration of Metabolic Syndrome Causing Nodular Thyroid Disease. Int. J. Endocrinol. 2019, 2019, 9376768. [Google Scholar] [CrossRef]
- Răcătăianu, N.; Leach, N.; Bondor, C.I.; Mârza, S.; Moga, D.; Valea, A.; Ghervan, C. Thyroid disorders in obese patients. Does insulin resistance make a difference? Arch. Endocrinol. Metab. 2017, 61, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Istituto Superiore di Sanità. Osservatorio Nazionale Per il Monitoraggio Della Iodoprofilassi in Italia. Available online: https://www.iss.it/osnami-chi-siamo (accessed on 1 March 2022).
- Mayers, R.A.; Soria Montoya, A.; Piscoya Rivera, A.; Silva Caso, W.G. Association between metabolic syndrome and euthyroid nodular goiter: A case-control study. Colomb. Med. 2019, 50, 239–251. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Y.; Fu, S.; Tang, X.; Liu, J.; Zhao, N.; Jing, G.; Niu, Q.; Ma, L.; Teng, W.; et al. The Detection of Thyroid Nodules in Prediabetes Population and Analysis of Related Factors. Risk Manag. Healthc. Policy 2021, 14, 4875–4882. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Brazzale, A.R.; Moro, E.; Vrbíková, J.; Bendlova, B.; Sbrignadello, S.; Tura, A.; Pacini, G. Influence of increasing BMI on insulin sensitivity and secretion in normotolerant men and women of a wide age span. Obesity 2012, 20, 1966–1973. [Google Scholar] [CrossRef] [Green Version]
- Marchese, E.; Rodeghier, C.; Monson, R.S.; McCracken, B.; Shi, T.; Schrock, W.; Martellotto, J.; Oberholzer, J.; Danielson, K.K. Enumerating β-Cells in Whole Human Islets: Sex Differences and Associations with Clinical Outcomes After Islet Transplantation. Diabetes Care 2015, 38, e176–e177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sex | p-Value | Median Age (IQR) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Males n = 74 | Females n = 268 | ||||||
| Median Age (IQR) | TOT n = 342 | 52 (44.5–62.25) | 51 (36–61) | n.s. | – | ||
| THRT | NO | 254 | 55 (74.3%) | 199 (74.3%) | n.s. | 51.5 (37–62) | n.s |
| YES | 88 | 19 (25.7%) | 69 (25.7%) | 51 (40.25–60.5) | |||
| OGTT | NGT | 213 | 34 (45.9%) | 179 (66.8%) | <0.01 | 49 (32–57.5) | <0.01 |
| IGT/T2DM | 129 | 40 (54.1%) | 89 (33.2%) | 56 (48.5–65.5) | |||
| IFG | NO | 247 | 50 (67.6%) | 197 (73.5%) | n.s. | 49 (33–59) | <0.01 |
| YES | 95 | 24 (32.4%) | 71 (26.5%) | 56 (50–63) | |||
| Insulin sensitivity (HOMA index) | IS | 208 | 45 (60.8%) | 163 (60.8%) | n.s. | 51 (39.25–61.75) | n.s. |
| IR | 134 | 29 (39.2%) | 105 (39.2%) | 53 (36–62) | |||
| Median HOMA index (IQR) | 2.06 (1.54–4.25) | 1.98 (1.34–3.25) | n.s. | – | |||
| TN | p-Value | ||||
|---|---|---|---|---|---|
| NO | YES | ||||
| Median Age (IQR) | TOT | 43.5 (25.5–56) | 54 (46–62) | <0.001 | |
| Sex | Males | 74 | 32 (43.2%) | 42 (56.8%) | n.s. |
| Females | 268 | 100 (37.3%) | 168 (62.7%) | ||
| OGTT | NGT | 213 | 85 (39.9%) | 128 (60.1%) | n.s. |
| IGT/T2DM | 129 | 47 (36.4%) | 82 (63.6%) | ||
| OGTT in males | NGT | 34 | 13 (38.2%) | 21 (61.8%) | n.s. |
| IGT/T2DM | 40 | 19 (47.5%) | 21 (52.5%) | ||
| OGTT in females | NGT | 179 | 72 (40.2%) | 107 (59.8%) | n.s. |
| IGT/T2DM | 89 | 28 (31.5%) | 61 (68.5%) | ||
| IFG | NO | 247 | 108 (43.7%) | 139 (56.3%) | 0.002 |
| YES | 95 | 24 (25.3%) | 71 (74.7%) | ||
| HOMA index | IS | 208 | 85 (40.9%) | 123 (59.1%) | n.s. |
| IR | 134 | 47 (35.1%) | 87 (64.9%) | ||
| Outcome Variable | Independent Variable | ULRM | ||
|---|---|---|---|---|
| OR | p-Value | C.I. | ||
| TN | Females | 1.28 | 0.354 | 0.759–2.158 |
| Age | 1.039 | <0.001 | 1.024–1.054 | |
| IGT/T2DM | 1.159 | 0.523 | 0.738–1.82 | |
| IFG | 2.299 | 0.002 | 1.357–3.892 | |
| HOMA index | 1.017 | 0.764 | 0.910–1.137 | |
| Dependent Variable | Independent Variable | MLRM | Predictive Margins | |||
|---|---|---|---|---|---|---|
| OR | p-Value | C.I. | Pr(TN) | C.I. | ||
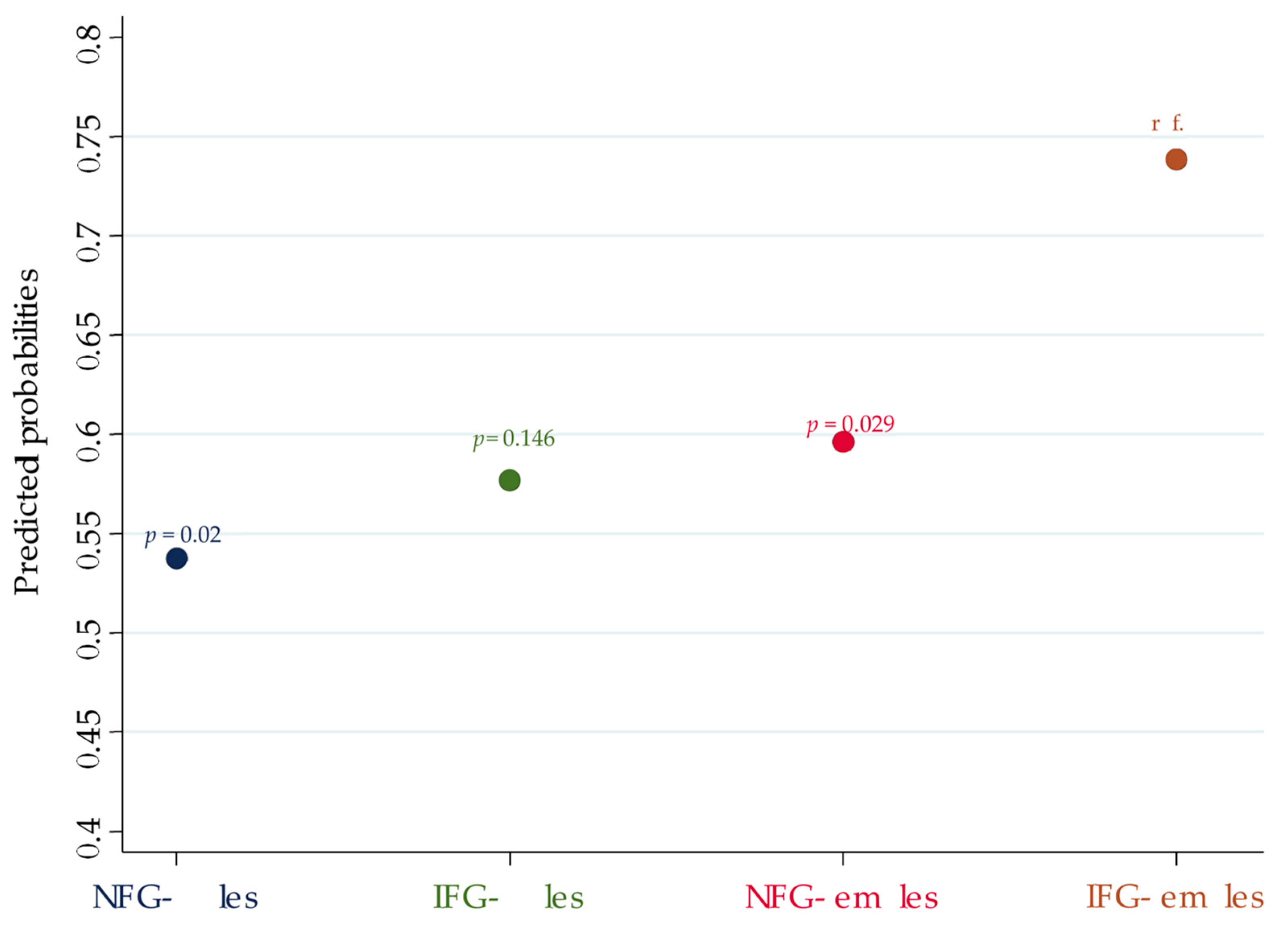
| TN | NFG males | 0.385 | 0.022 | 0.17–0.872 | 0.538 | 0.405–0.67 |
| IFG males | 0.458 | 0.134 | 0.164–1.273 | 0.577 | 0.384–0.769 | |
| NFG females | 0.499 | 0.04 | 0.257–0.968 | 0.596 | 0.529–0.663 | |
| IFG females | reference | // | // | 0.739 | 0.631–0.846 | |
| Age | 1.035 | <0.01 | 1.02–1.051 | // | // | |
| Contrast of Predictive Margins | |||
|---|---|---|---|
| Contrast | p−Value | C.I. | |
| NFG males vs. IFG females | −0.201 | 0.02 | −0.371; −0.031 |
| IFG males vs. IFG females | −0.162 | 0.146 | −0.379; +0.056 |
| NFG females vs. IFG females | −0.142 | 0.029 | −0.271; −0.014 |
| – | Independent Variable | ULRM | MLRM | ||||
|---|---|---|---|---|---|---|---|
| OR or Coefficient * | p-Value | C.I. | OR or Coefficient * | p-Value | C.I. | ||
| IGT/T2DM | Female sex | 0.423 | 0.001 | 0.25–0.71 | 0.462 | 0.008 | 0.261–0.82 |
| Age | 1.043 | <0.001 | 1.027–1.059 | 1.039 | <0.001 | 1.022–1.058 | |
| IFG | 3.886 | <0.001 | 2.37–6.38 | 2.696 | <0.001 | 1.588–4.579 | |
| HOMA index | 1.264 | <0.001 | 1.11–1.438 | 1.239 | 0.003 | 1.075–1.429 | |
| HOMA index | Female sex | −0.761 * | 0.003 | −1.269; −0.253 | −0.605 * | 0.02 | −1.113; −0.097 |
| Age | +0.001 * | 0.849 | −0.012; +0.014 | −0.007 * | 0.290 | −0.021; +0.006 | |
| IGT/T2DM | +0.861 * | <0.001 | +0.434; +1.288 | +0.841 * | <0.001 | +0.390; +1.291 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobbo, A.; Gagliardi, I.; Gobbo, A.; Rossi, R.; Franceschetti, P.; Lupo, S.; Rossi, M.; Bondanelli, M.; Ambrosio, M.R.; Zatelli, M.C. Glucose Metabolism Derangements and Thyroid Nodules: Does Sex Matter? J. Pers. Med. 2022, 12, 903. https://doi.org/10.3390/jpm12060903
Gobbo A, Gagliardi I, Gobbo A, Rossi R, Franceschetti P, Lupo S, Rossi M, Bondanelli M, Ambrosio MR, Zatelli MC. Glucose Metabolism Derangements and Thyroid Nodules: Does Sex Matter? Journal of Personalized Medicine. 2022; 12(6):903. https://doi.org/10.3390/jpm12060903
Chicago/Turabian StyleGobbo, Alberto, Irene Gagliardi, Andrea Gobbo, Roberta Rossi, Paola Franceschetti, Sabrina Lupo, Martina Rossi, Marta Bondanelli, Maria Rosaria Ambrosio, and Maria Chiara Zatelli. 2022. "Glucose Metabolism Derangements and Thyroid Nodules: Does Sex Matter?" Journal of Personalized Medicine 12, no. 6: 903. https://doi.org/10.3390/jpm12060903
APA StyleGobbo, A., Gagliardi, I., Gobbo, A., Rossi, R., Franceschetti, P., Lupo, S., Rossi, M., Bondanelli, M., Ambrosio, M. R., & Zatelli, M. C. (2022). Glucose Metabolism Derangements and Thyroid Nodules: Does Sex Matter? Journal of Personalized Medicine, 12(6), 903. https://doi.org/10.3390/jpm12060903






