Multivariate Analysis of Metabolomic and Nutritional Profiles among Children with Autism Spectrum Disorder
Abstract
:1. Introduction
2. Methods
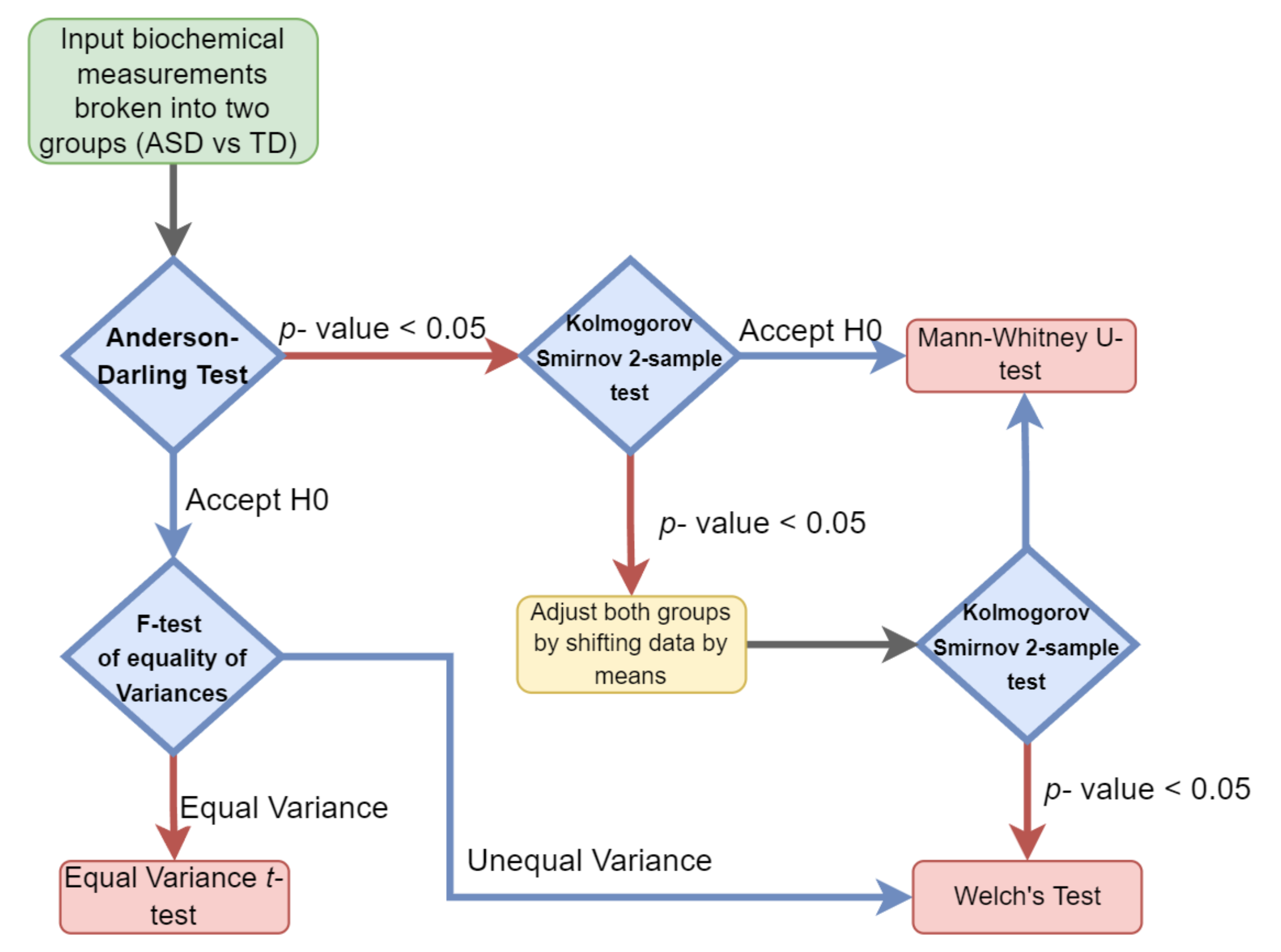
2.1. Univariate Analysis
2.2. Correlation Analysis
2.3. Multivariate Analysis Preprocessing
2.4. Multivariate Analysis
2.5. FDA Application
2.6. SVM Analysis
3. Results
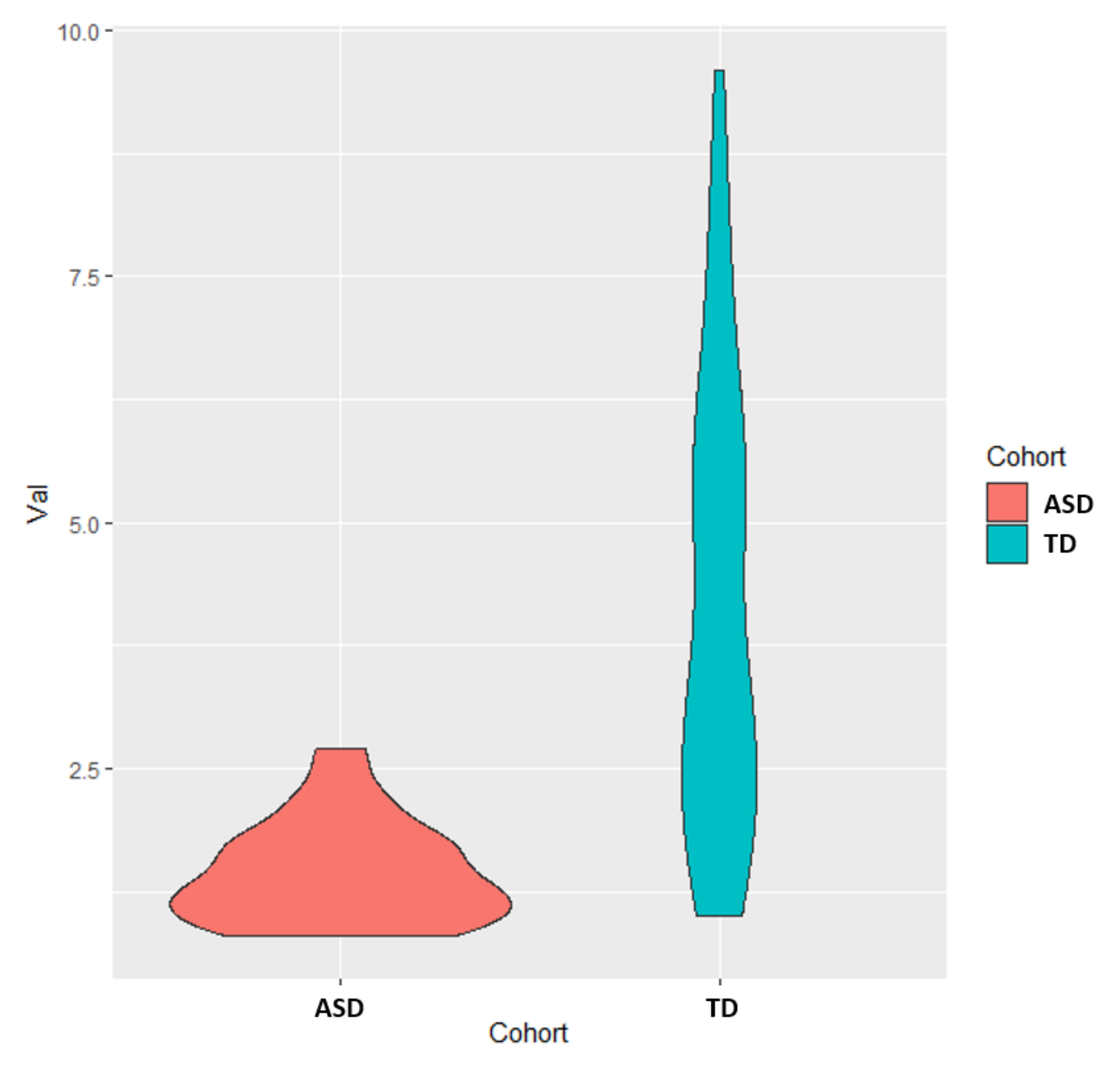
3.1. Univariate Analysis
3.2. Correlation Analysis
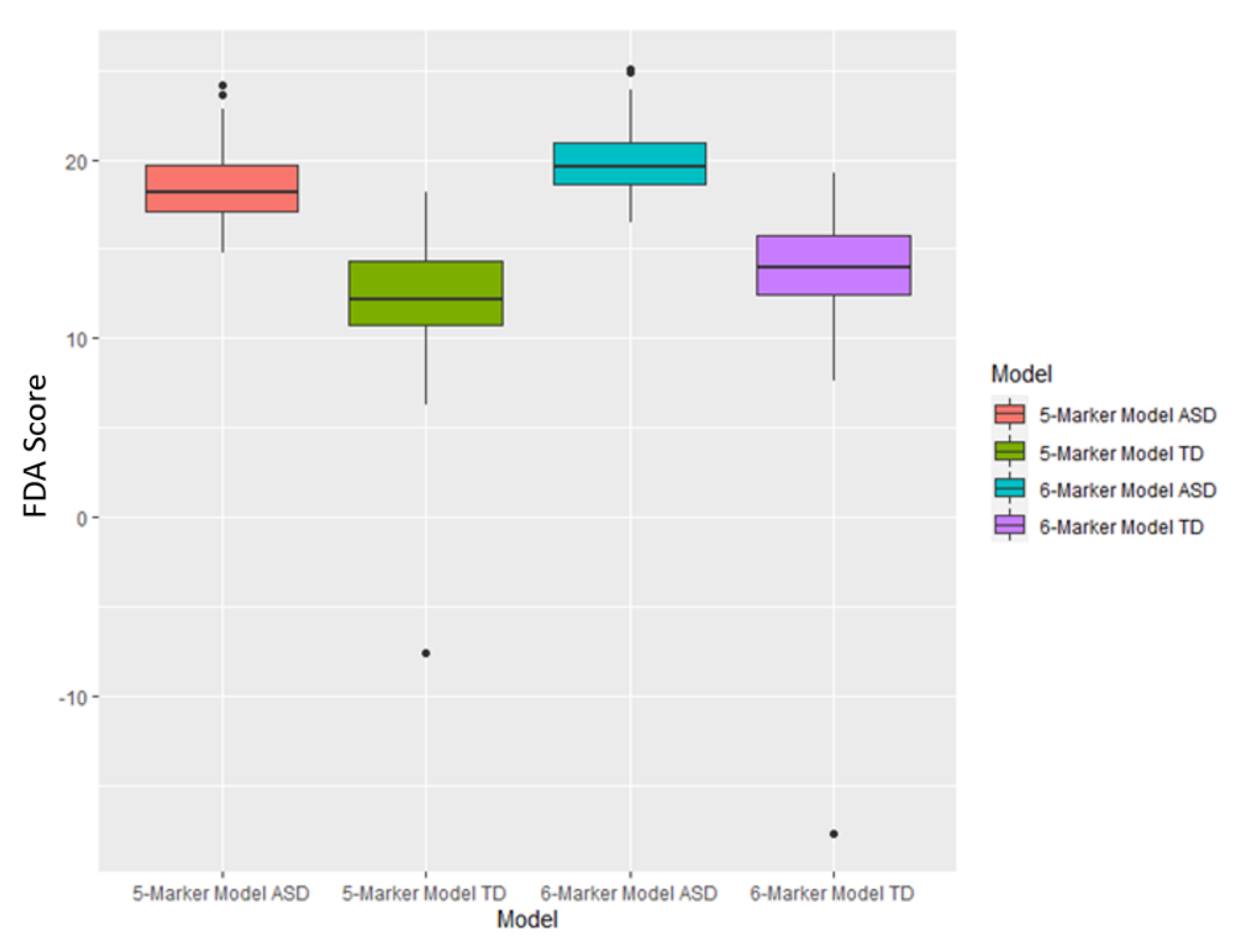
3.3. FDA Models
3.4. SVM Models
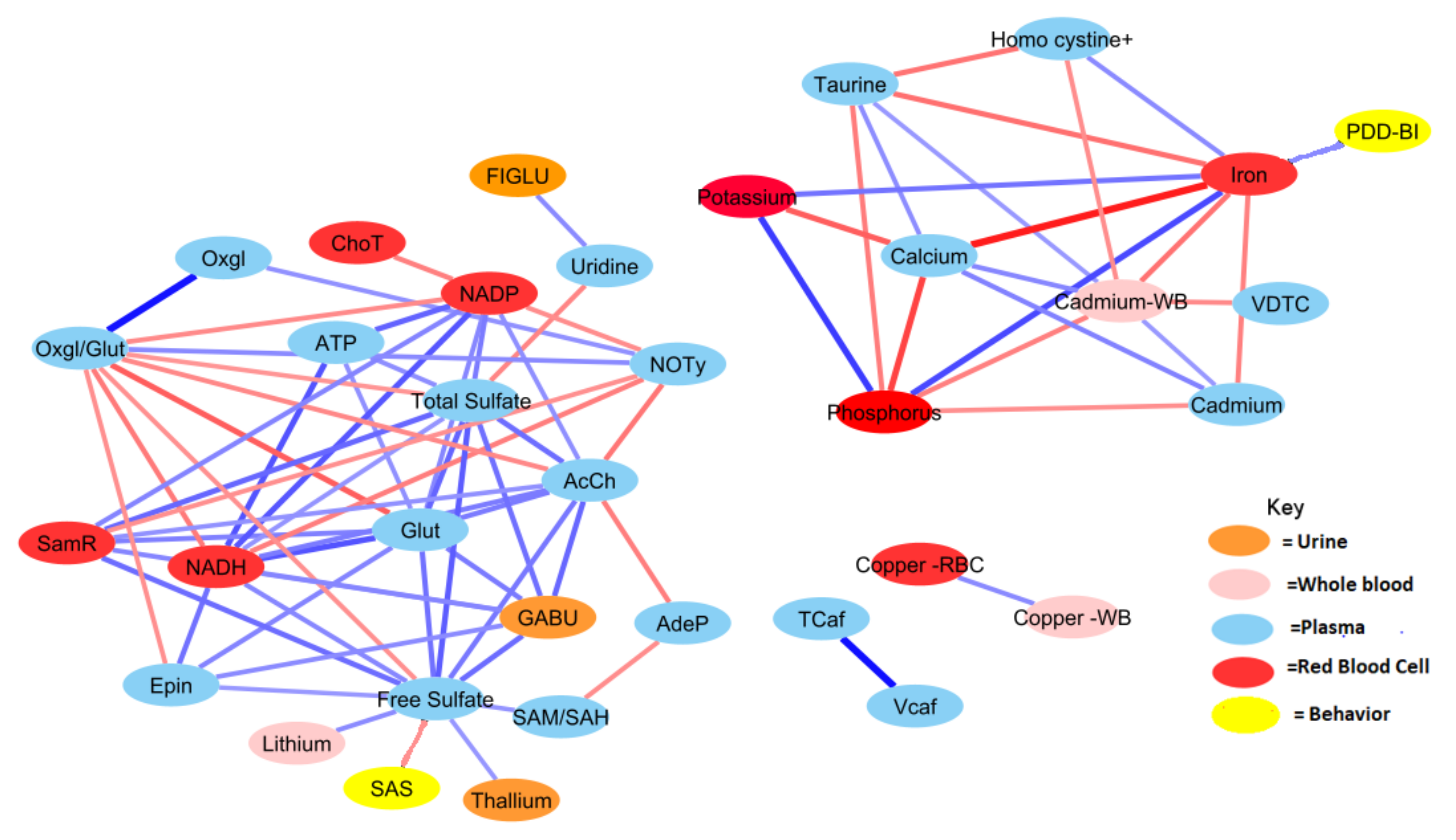
3.5. Metabolite Clusters
4. Discussion
4.1. Univariate Findings
4.2. Correlation Analyses
4.3. Multivariate Analysis for Classifying ASD
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Measurement 1 | Measurement 2 | Pearson Correlation Coefficient |
|---|---|---|
| Cadmium | Calcium | 0.49 |
| Iron (whole blood) | Calcium | −0.89 |
| Taurine | Calcium | 0.45 |
| Cadmium (whole Blood) | Calcium | 0.47 |
| Cadmium | Iron (whole blood) | −0.50 |
| Taurine | Iron (whole blood) | −0.56 |
| Homocysteine + homocystine | Iron (whole blood) | 0.46 |
| Cadmium | Phosphorus | −0.44 |
| Calcium | Phosphorus | −0.74 |
| Iron (whole blood) | Phosphorus | 0.71 |
| Taurine | Phosphorus | −0.51 |
| Cadmium (whole blood) | Phosphorus | −0.50 |
| Homocysteine + homocystine | Taurine | −0.55 |
| Adenosine (Plasma) | Acetylcholine | −0.51 |
| Free sulfate (plasma) | Acetylcholine | 0.53 |
| GABU | Acetylcholine | 0.60 |
| GSSG/GSH ratio | Acetylcholine | −0.47 |
| NADP | Acetylcholine | 0.44 |
| Nitrotyrosine | Acetylcholine | −0.54 |
| SamR | Acetylcholine | 0.43 |
| SAM/SAH | Adenosine (plasma) | −0.45 |
| Glutathione | ATP | 0.44 |
| NADH | ATP | 0.67 |
| NADP | ATP | 0.61 |
| Total sulfate (plasma) | ATP | 0.44 |
| Cadmium | Cadmium (whole blood) | 0.40 |
| Iron (whole blood) | Cadmium (whole blood) | −0.55 |
| Taurine | Cadmium (whole blood) | 0.40 |
| Homocysteine + homocystine | Cadmium (whole blood) | −0.45 |
| Total carotenoids | Cadmium (whole blood) | −0.46 |
| Copper (RBC) | Copper (whole blood) | 0.46 |
| GSSG/GSH ratio | Epinephrine | −0.44 |
| Uridine (plasma) | FIGLU | 0.46 |
| Total Carnitine (carnitine + acetyl-carnitine) | Free carnitine | 0.94 |
| Lithium | Free sulfate (plasma) | 0.45 |
| Thallium | Free sulfate (plasma) | 0.41 |
| Epinephrine | Free sulfate (plasma) | 0.40 |
| GABU | Free sulfate (plasma) | 0.57 |
| GSSG/GSH ratio | Free sulfate (plasma) | −0.43 |
| SAM/SAH | Free sulfate (plasma) | 0.42 |
| SamR | Free sulfate (plasma) | 0.57 |
| Epinephrine | GABU | 0.45 |
| SamR | GABU | 0.47 |
| Acetylcholine | Glutathione | 0.52 |
| Epinephrine | Glutathione | 0.48 |
| Free sulfate (plasma) | Glutathione | 0.56 |
| GABU | Glutathione | 0.52 |
| GSSG/GSH ratio | Glutathione | −0.63 |
| NADH | Glutathione | 0.67 |
| NADP | Glutathione | 0.47 |
| SamR | Glutathione | 0.50 |
| Total Sulfate (plasma) | Glutathione | 0.60 |
| Acetylcholine | NADH | 0.52 |
| Epinephrine | NADH | 0.56 |
| Free sulfate (plasma) | NADH | 0.47 |
| GABU | NADH | 0.41 |
| GSSG/GSH ratio | NADH | −0.52 |
| NADP | NADH | 0.66 |
| Nitrotyrosine | NADH | −0.51 |
| Total Sulfate (plasma) | NADH | 0.42 |
| Choline (total) | NADP | −0.50 |
| GSSG/GSH ratio | NADP | −0.46 |
| Nitrotyrosine | NADP | −0.45 |
| SamR | NADP | 0.48 |
| GSSG/GSH ratio | Nitrotyrosine | 0.46 |
| SamR | Nitrotyrosine | −0.44 |
| GSSG/GSH ratio | Oxidized glutathione | 0.93 |
| Nitrotyrosine | Oxidized glutathione | 0.43 |
| Calcium | Potassium | −0.63 |
| Iron (whole blood) | Potassium | 0.55 |
| Phosphorus | Potassium | 0.77 |
| Acetylcholine | Total sulfate (plasma) | 0.57 |
| Free sulfate (plasma) | Total sulfate (plasma) | 0.64 |
| GABU | Total sulfate (plasma) | 0.56 |
| GSSG/GSH ratio | Total sulfate (plasma) | −0.42 |
| NADP | Total sulfate (plasma) | 0.52 |
| SamR | Total sulfate (plasma) | 0.59 |
| Uridine (plasma) | Total sulfate (plasma) | −0.48 |
| Measurement 1 | Measurement 2 | Pearson Correlation Coefficient |
|---|---|---|
| Glutathione | NADH | 0.48 |
| Glutathione | NADP | 0.49 |
| Glutathione | Epinephrine | 0.41 |
| Glutathione | Norepinephrine | 0.51 |
| Glutathione | Serotonin | 0.45 |
| Glutathione | Acetylcholine | 0.45 |
| Glutathione | Sulfate (total) | 0.55 |
| Glutathione | GABU | 0.49 |
| Glutathione | SAM/SAH | 0.45 |
| ATP | GSSG/GSH ratio | −0.42 |
| ATP | NADH | 0.50 |
| ATP | NADP | 0.67 |
| ATP | Sulfate (free) | 0.58 |
| ATP | AdeP | −0.42 |
| ATP | Uridine | −0.47 |
| ATP | SAM/SAH | 0.46 |
| ATP | Cadmium | −0.53 |
| Figl | NADH | −0.44 |
| Figl | NADP | −0.50 |
| Figl | Norepinephrine | −0.43 |
| Nitrotyrosine | Oxidized glutathione | 0.56 |
| Nitrotyrosine | GSSG/GSH ratio | 0.75 |
| Nitrotyrosine | NADP | −0.51 |
| Nitrotyrosine | Choline (total) | 0.66 |
| Nitrotyrosine | Epinephrine | −0.46 |
| Nitrotyrosine | Norepinephrine | −0.47 |
| Nitrotyrosine | Serotonin | −0.43 |
| Nitrotyrosine | Acetylcholine | −0.72 |
| Nitrotyrosine | Sulfate (total) | −0.64 |
| Nitrotyrosine | Sulfate (free) | −0.51 |
| Nitrotyrosine | AdeP | 0.60 |
| Nitrotyrosine | Uridine | 0.46 |
| Nitrotyrosine | GABU | −0.59 |
| Nitrotyrosine | SamR | −0.54 |
| Nitrotyrosine | SAM/SAH | −0.61 |
| Oxidized glutathione | GSSG/GSH ratio | 0.89 |
| Oxidized glutathione | Choline (total) | 0.46 |
| Oxidized glutathione | Epinephrine | −0.47 |
| Oxidized glutathione | Acetylcholine | −0.51 |
| GSSG/GSH ratio | NADH | −0.41 |
| GSSG/GSH ratio | NADP | −0.50 |
| GSSG/GSH ratio | Choline (total) | 0.57 |
| GSSG/GSH ratio | Epinephrine | −0.57 |
| GSSG/GSH ratio | Norepinephrine | −0.42 |
| GSSG/GSH ratio | Serotonin | −0.48 |
| GSSG/GSH ratio | Acetylcholine | −0.65 |
| GSSG/GSH ratio | Sulfate (total) | −0.58 |
| GSSG/GSH ratio | Sulfate (free) | −0.46 |
| GSSG/GSH ratio | Uridine | 0.43 |
| GSSG/GSH ratio | GABU | −0.56 |
| GSSG/GSH ratio | SAM/SAH | −0.51 |
| NADH | NADP | 0.81 |
| NADH | Choline (total) | −0.45 |
| NADH | Norepinephrine | 0.57 |
| NADH | Serotonin | 0.41 |
| NADH | Acetylcholine | 0.50 |
| NADH | Sulfate (total) | 0.59 |
| NADH | Sulfate (free) | 0.66 |
| NADH | GABU | 0.50 |
| NADH | SAM/SAH | 0.42 |
| NADP | Choline (total) | −0.47 |
| NADP | Epinephrine | 0.46 |
| NADP | Norepinephrine | 0.61 |
| NADP | Serotonin | 0.55 |
| NADP | Acetylcholine | 0.46 |
| NADP | Sulfate (total) | 0.62 |
| NADP | Sulfate (free) | 0.66 |
| NADP | AdeP | −0.41 |
| NADP | Uridine | −0.48 |
| NADP | GABU | 0.60 |
| NADP | SamR | 0.53 |
| NADP | SAM/SAH | 0.50 |
| Choline (total) | Norepinephrine | −0.49 |
| Choline (total) | Acetylcholine | −0.64 |
| Choline (total) | Sulfate (total) | −0.52 |
| Choline (total) | Sulfate (free) | −0.41 |
| Choline (total) | AdeP | 0.49 |
| Choline (total) | SamR | −0.49 |
| Choline (total) | Taurine | −0.53 |
| Free carnitine | Total carnitine (carnitine + acetyl-carnitine) | 0.97 |
| Total carnitine (carnitine + acetyl-carnitine) | Vitamin B5 | −0.42 |
| Total Carnitine (carnitine + acetyl-carnitine) | Serine | 0.48 |
| Epinephrine | Norepinephrine | 0.57 |
| Epinephrine | Serotonin | 0.59 |
| Epinephrine | Acetylcholine | 0.53 |
| Epinephrine | Sulfate (total) | 0.71 |
| Epinephrine | Sulfate (free) | 0.44 |
| Epinephrine | GABU | 0.66 |
| Norepinephrine | Serotonin | 0.59 |
| Norepinephrine | Acetylcholine | 0.49 |
| Norepinephrine | Sulfate (total) | 0.70 |
| Norepinephrine | GABU | 0.74 |
| Norepinephrine | SamR | 0.46 |
| Serotonin | Acetylcholine | 0.43 |
| Serotonin | Sulfate (total) | 0.75 |
| Serotonin | Sulfate (free) | 0.51 |
| Serotonin | GABU | 0.62 |
| Acetylcholine | Sulfate (total) | 0.69 |
| Acetylcholine | Sulfate (free) | 0.54 |
| Acetylcholine | Adenosine | −0.54 |
| Acetylcholine | GABU | 0.61 |
| Acetylcholine | SamR | 0.59 |
| Acetylcholine | SAM/SAH | 0.50 |
| Sulfate (total) | Sulfate (free) | 0.65 |
| Sulfate (total) | GABU | 0.70 |
| Sulfate (total) | SamR | 0.49 |
| Sulfate (total) | SAM/SAH | 0.53 |
| Sulfate (total) | Lithium | 0.45 |
| Sulfate (free) | Adenosine | −0.41 |
| Sulfate (free) | GABU | 0.51 |
| Sulfate (free) | SAM/SAH | 0.49 |
| VDC | Tin | 0.40 |
| Biotin | Cadmium (whole blood) | 0.58 |
| Biotin | Calcium | 0.52 |
| Biotin | Iron (RBC) | −0.46 |
| Biotin | Thallium | −0.46 |
| Biotin | Taurine | 0.46 |
| Biotin | Glutamate | 0.45 |
| Biotin | Vitamin B5 | 0.66 |
| Adenosine | SamR | −0.53 |
| Uridine | SAM/SAH | −0.58 |
| GABU | SamR | 0.44 |
| SamR | SAM/SAH | 0.71 |
| Copper (WB) | Copper (RBC) | 0.70 |
| Cadmium (whole blood) | Calcium | 0.64 |
| Cadmium (whole blood) | Iron (RBC) | −0.56 |
| Calcium | Potassium | −0.47 |
| Calcium | Phosphorus | −0.59 |
| Calcium | Iron (RBC) | −0.76 |
| Potassium | Phosphorus | 0.71 |
| Potassium | Iron (RBC) | 0.50 |
| Potassium | Taurine | −0.40 |
| Phosphorus | Iron (RBC) | 0.65 |
| Iron (RBC) | Taurine | −0.48 |
| Iron (RBC) | Taurine | −0.48 |
| Magnesium | Glutamate | −0.42 |
| Taurine | Homocysteine + homocystine | −0.41 |
| Tryptophan | Vitamin B5 | 0.43 |
References
- Maenner, M.J.; Shaw, K.A.; Bakian, A.V.; Cogswell, M. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States. MMWR Surveill. Summ. 2021, 70 (Suppl. S11), 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vargason, T.; Frye, R.E.; McGuinness, D.L.; Hahn, J. Clustering of Co-occurring Conditions in Autism Spectrum Disorder during Early Childhood: A Retrospective Analysis of Medical Claims Data. Autism Res. 2019, 12, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossignol, D.A.; Genuis, S.J.; Frye, R.E. Environmental Toxicants and Autism Spectrum Disorders: A Systematic Review. Transl. Psychiatry 2014, 4, e360. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, A.; Van de Water, J. The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology 2017, 42, 284–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holingue, C.; Newill, C.; Lee, L.-C.; Pasricha, P.J.; Fallin, M.D. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Review of the Literature on Ascertainment and Prevalence. Autism Res. 2018, 11, 24–36. [Google Scholar] [CrossRef]
- Howsmon, D.P.; Kruger, U.; Melnyk, S.; James, S.J.; Hahn, J. Classification and Adaptive Behavior Prediction of Children with Autism Spectrum Disorder Based upon Multivariate Data Analysis of Markers of Oxidative Stress and DNA Methylation. PLoS Comput. Biol. 2017, 13, e1005385. [Google Scholar] [CrossRef]
- Frye, R.E.; Vassall, S.; Kaur, G.; Lewis, C.; Karim, M.; Rossignol, D. Emerging Biomarkers in Autism Spectrum Disorder: A Systematic Review. Ann. Transl. Med. 2019, 7, 792. [Google Scholar] [CrossRef]
- Vargason, T.; Grivas, G.; Hollowood-Jones, K.L.; Hahn, J. Towards a Multivariate Biomarker-Based Diagnosis of Autism Spectrum Disorder: Review and Discussion of Recent Advancements. Semin. Pediatr. Neurol. 2020, 34, 100803. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-Term Benefit of Microbiota Transfer Therapy on Autism Symptoms and Gut Microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Vargason, T.; Kruger, U.; Roth, E.; Delhey, L.M.; Tippett, M.; Rose, S.; Bennuri, S.C.; Slattery, J.C.; Melnyk, S.; James, S.J.; et al. Comparison of Three Clinical Trial Treatments for Autism Spectrum Disorder Through Multivariate Analysis of Changes in Metabolic Profiles and Adaptive Behavior. Front. Cell. Neurosci. 2018, 12, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejía, J.L.; Hansen, L.H.; Leigh Gibson, E.; Nielsen, D.S.; Costabile, A. A Prebiotic Intervention Study in Children with Autism Spectrum Disorders (ASDs). Microbiome 2018, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- De Sande, M.M.H.; van Buul, V.J.; Brouns, F.J.P.H. Autism and nutrition: The role of the gut–brain axis. Nutr. Res. Rev. 2014, 27, 199–214. [Google Scholar] [CrossRef] [Green Version]
- Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorder: Unique Abnormalities and Targeted Treatments. Semin. Pediatr. Neurol. 2020, 35, 100829. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Niyazov, D.M.; Rossignol, D.A.; Goldenthal, M.; Kahler, S.G.; Frye, R.E. Clinical and Molecular Characteristics of Mitochondrial Dysfunction in Autism Spectrum Disorder. Mol. Diagn. Ther. 2018, 22, 571–593. [Google Scholar] [CrossRef] [Green Version]
- Giulivi, C.; Zhang, Y.-F.; Omanska-Klusek, A.; Ross-Inta, C.; Wong, S.; Hertz-Picciotto, I.; Tassone, F.; Pessah, I.N. Mitochondrial Dysfunction in Autism. JAMA 2010, 304, 2389. [Google Scholar] [CrossRef] [Green Version]
- Frye, R.E.; Rossignol, D.A. Mitochondrial Dysfunction Can Connect the Diverse Medical Symptoms Associated with Autism Spectrum Disorders. Pediatr. Res. 2011, 69, 41R–47R. [Google Scholar] [CrossRef]
- Thorburn, D.R. Mitochondrial Disorders: Prevalence, Myths and Advances. J. Inherit. Metab. Dis. 2004, 27, 349–362. [Google Scholar] [CrossRef]
- Griffiths, K.K.; Levy, R.J. Evidence of Mitochondrial Dysfunction in Autism: Biochemical Links, Genetic-Based Associations, and Non-Energy-Related Mechanisms. Oxid. Med. Cell. Longev. 2017, 2017, 4314025. [Google Scholar] [CrossRef]
- Needham, B.D.; Adame, M.D.; Serena, G.; Rose, D.R.; Preston, G.M.; Conrad, M.C.; Campbell, A.S.; Donabedian, D.H.; Fasano, A.; Ashwood, P.; et al. Plasma and Fecal Metabolite Profiles in Autism Spectrum Disorder. Biol. Psychiatry 2021, 89, 451–462. [Google Scholar] [CrossRef]
- Barone, R.; Alaimo, S.; Messina, M.; Pulvirenti, A.; Bastin, J.; Ferro, A.; Frye, R.E.; Rizzo, R. A Subset of Patients with Autism Spectrum Disorders Show a Distinctive Metabolic Profile by Dried Blood Spot Analyses. Front. Psychiatry 2018, 9, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frye, R.E.; Melnyk, S.; MacFabe, D.F. Unique Acyl-Carnitine Profiles Are Potential Biomarkers for Acquired Mitochondrial Disease in Autism Spectrum Disorder. Transl. Psychiatry 2013, 3, e220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geier, D.A.; Kern, J.K.; Davis, G.; King, P.G.; Adams, J.B.; Young, J.L.; Geier, M.R. A Prospective Double-Blind, Randomized Clinical Trial of Levocarnitine to Treat Autism Spectrum Disorders. Med. Sci. Monit. 2011, 17, PI15–PI23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, S.F.; El-Hamamsy, M.H.; Zaki, O.K.; Badary, O.A. L-Carnitine Supplementation Improves the Behavioral Symptoms in Autistic Children. Res. Autism Spectr. Disord. 2013, 7, 159–166. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Elwell, C.; Johnson, M.H. Mitochondrial Dysfunction in Autism Spectrum Disorders. Autism Open Access 2016, 6, 1000190. [Google Scholar] [CrossRef] [Green Version]
- Citrigno, L.; Muglia, M.; Qualtieri, A.; Spadafora, P.; Cavalcanti, F.; Pioggia, G.; Cerasa, A. The Mitochondrial Dysfunction Hypothesis in Autism Spectrum Disorders: Current Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 5785. [Google Scholar] [CrossRef]
- Gu, F.; Chauhan, V.; Kaur, K.; Brown, W.T.; LaFauci, G.; Wegiel, J.; Chauhan, A. Alterations in Mitochondrial DNA Copy Number and the Activities of Electron Transport Chain Complexes and Pyruvate Dehydrogenase in the Frontal Cortex from Subjects with Autism. Transl. Psychiatry 2013, 3, e299. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, M.R.; Rubin, R.A.; Falcone, T.; Ting, A.H.; Natowicz, M.R. Brain Transcriptional and Epigenetic Associations with Autism. PLoS ONE 2012, 7, e44736. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.; Gutierrez Rios, P.; Kuo, S.-H.; Akman, H.O.; Rosoklija, G.; Tanji, K.; Dwork, A.; Schon, E.A.; DiMauro, S.; Goldman, J.; et al. Mitochondrial Abnormalities in Temporal Lobe of Autistic Brain. Neurobiol. Dis. 2013, 54, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Effect of a Vitamin/Mineral Supplement on Children and Adults with Autism. BMC Pediatr. 2011, 11, 111. [Google Scholar] [CrossRef]
- Quadros, E.V.; Sequeira, J.M.; Brown, W.T.; Mevs, C.; Marchi, E.; Flory, M.; Jenkins, E.C.; Velinov, M.T.; Cohen, I.L. Folate Receptor Autoantibodies Are Prevalent in Children Diagnosed with Autism Spectrum Disorder, Their Normal Siblings and Parents. Autism Res. 2018, 11, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Sequeira, J.M.; Quadros, E.V.; James, S.J.; Rossignol, D.A. Cerebral Folate Receptor Autoantibodies in Autism Spectrum Disorder. Mol. Psychiatry 2013, 18, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Rossignol, D.A.; Scahill, L.; McDougle, C.J.; Huberman, H.; Quadros, E.V. Treatment of Folate Metabolism Abnormalities in Autism Spectrum Disorder. Semin. Pediatr. Neurol. 2020, 35, 100835. [Google Scholar] [CrossRef]
- Alberti, A.; Pirrone, P.; Elia, M.; Waring, R.H.; Romano, C. Sulphation Deficit in “Low-Functioning” Autistic Children: A Pilot Study. Biol. Psychiatry 1999, 46, 420–424. [Google Scholar] [CrossRef]
- Waring, R.H.; Klovrza, L.V. Sulphur Metabolism in Autism. J. Nutr. Environ. Med. 2000, 10, 25–32. [Google Scholar] [CrossRef]
- Pagan, C.; Benabou, M.; Leblond, C.; Cliquet, F.; Mathieu, A.; Lemière, N.; Goubran-Botros, H.; Delorme, R.; Leboyer, M.; Callebert, J.; et al. Decreased Phenol Sulfotransferase Activities Associated with Hyperserotonemia in Autism Spectrum Disorders. Transl. Psychiatry 2021, 11, 23. [Google Scholar] [CrossRef]
- Bowling, F.G.; Heussler, H.S.; McWhinney, A.; Dawson, P.A. Plasma and Urinary Sulfate Determination in a Cohort with Autism. Biochem. Genet. 2013, 51, 147–153. [Google Scholar] [CrossRef]
- Diémé, B.; Mavel, S.; Blasco, H.; Tripi, G.; Bonnet-Brilhault, F.; Malvy, J.; Bocca, C.; Andres, C.R.; Nadal-Desbarats, L.; Emond, P. Metabolomics Study of Urine in Autism Spectrum Disorders Using a Multiplatform Analytical Methodology. J. Proteome Res. 2015, 14, 5273–5282. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhou, J.-M. The Microbiota–Gut–Brain Axis and Its Potential Therapeutic Role in Autism Spectrum Disorder. Neuroscience 2016, 324, 131–139. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef] [Green Version]
- Kushkevych, I.; Cejnar, J.; Treml, J.; Dordević, D.; Kollar, P.; Vítězová, M. Recent Advances in Metabolic Pathways of Sulfate Reduction in Intestinal Bacteria. Cells 2020, 9, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefter, R.; Ciobica, A.; Timofte, D.; Stanciu, C.; Trifan, A. A Descriptive Review on the Prevalence of Gastrointestinal Disturbances and Their Multiple Associations in Autism Spectrum Disorder. Medicina 2019, 56, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, H.K.; Rose, D.; Ashwood, P. The Gut Microbiota and Dysbiosis in Autism Spectrum Disorders. Curr. Neurol. Neurosci. Rep. 2018, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Santocchi, E.; Guiducci, L.; Prosperi, M.; Calderoni, S.; Gaggini, M.; Apicella, F.; Tancredi, R.; Billeci, L.; Mastromarino, P.; Grossi, E.; et al. Effects of Probiotic Supplementation on Gastrointestinal, Sensory and Core Symptoms in Autism Spectrum Disorders: A Randomized Controlled Trial. Front. Psychiatry 2020, 11, 550593. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12 Folate, and the Methionine Remethylation Cycle—Biochemistry, Pathways, and Regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef] [Green Version]
- James, S.J. Autism and Folate-Dependent One-Carbon Metabolism: Serendipity and Critical Branch-Point Decisions in Science. Glob. Adv. Health Med. 2013, 2, 48–51. [Google Scholar] [CrossRef] [Green Version]
- Howsmon, D.P.; Vargason, T.; Rubin, R.A.; Delhey, L.; Tippett, M.; Rose, S.; Bennuri, S.C.; Slattery, J.C.; Melnyk, S.; James, S.J.; et al. Multivariate Techniques Enable a Biochemical Classification of Children with Autism Spectrum Disorder versus Typically-Developing Peers: A Comparison and Validation Study. Bioeng. Transl. Med. 2018, 3, 156–165. [Google Scholar] [CrossRef]
- Ryberg, K.H. Evidence for the Implementation of the Early Start Denver Model for Young Children with Autism Spectrum Disorder. J. Am. Psychiatr. Nurses Assoc. 2015, 21, 327–337. [Google Scholar] [CrossRef]
- Schreibman, L.; Dawson, G.; Stahmer, A.C.; Landa, R.; Rogers, S.J.; McGee, G.G.; Kasari, C.; Ingersoll, B.; Kaiser, A.P.; Bruinsma, Y.; et al. Naturalistic Developmental Behavioral Interventions: Empirically Validated Treatments for Autism Spectrum Disorder. J. Autism Dev. Disord. 2015, 45, 2411–2428. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.Q.; Ding, S.B.; Li, H.B. Blood biomarker levels of methylation capacity in autism spectrum disorder: A systematic review and meta-analysis. Acta Psychiatr. Scand. 2020, 141, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Vargason, T.; Roth, E.; Grivas, G.; Ferina, J.; Frye, R.E.; Hahn, J. Classification of Autism Spectrum Disorder from Blood Metabolites: Robustness to the Presence of Co-Occurring Conditions. Res. Autism Spectr. Disord. 2020, 77, 101644. [Google Scholar] [CrossRef]
- Adams, J.; Howsmon, D.P.; Kruger, U.; Geis, E.; Gehn, E.; Fimbres, V.; Pollard, E.; Mitchell, J.; Ingram, J.; Hellmers, R.; et al. Significant Association of Urinary Toxic Metals and Autism-Related Symptoms—A Nonlinear Statistical Analysis with Cross Validation. PLoS ONE 2017, 12, e0169526. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, J.; Zhang, J.; Liang, C.; Wang, Y.; Chen, B.; Zhao, C.; Wang, J.; Zhang, G.; Zhao, D.; et al. Correlation of Gut Microbiome Between ASD Children and Mothers and Potential Biomarkers for Risk Assessment. Genom. Proteom. Bioinform. 2019, 17, 26–38. [Google Scholar] [CrossRef]
- Qureshi, F.; Adams, J.; Coleman, D.; Quig, D.; Hahn, J. Urinary Essential Elements of Young Children with Autism Spectrum Disorder and Their Mothers. Res. Autism Spectr. Disord. 2020, 72, 101518. [Google Scholar] [CrossRef]
- Ranjan, S.; Nasser, J.A. Nutritional status of individuals with autism spectrum disorders: Do we know enough? Adv. Nutr. 2015, 6, 397–407. [Google Scholar] [CrossRef]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.J.; Bazin, A.P.; Bigega, R.; Carlson, R.S.; Cole, M.G.; Contreras, D.C.; Galvin, M.B.; Gaydorus, S.S.; Holik, S.D.; Jenkins, G.P.; et al. Plasma copper and zinc concentration in individuals with autism correlate with selected symptom severity. Nutr. Metab. Insights 2012, 5, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Noto, A.; Fanos, V.; Barberini, L.; Grapov, D.; Fattuoni, C.; Zaffanello, M.; Casanova, A.; Fenu, G.; De Giacomo, A.; De Angelis, M.; et al. The urinary metabolomics profile of an Italian autistic children population and their unaffected siblings. J. Matern. Fetal Neonatal Med. 2014, 27 (Suppl. S2), 46–52. [Google Scholar] [CrossRef]
- Audhya, T.; Adams, J.B.; Johansen, L. Correlation of Serotonin Levels in CSF, Platelets, Plasma, and Urine. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1496–1501. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.M.; Azevedo, C.; Proença, H.; Vieira, S.M. Missing Data. In Secondary Analysis of Electronic Health Records; Springer: Berlin/Heidelberg, Germany, 2016; pp. 143–162. [Google Scholar] [CrossRef] [Green Version]
- Kontopantelis, E.; White, I.R.; Sperrin, M.; Buchan, I. Outcome-Sensitive Multiple Imputation: A Simulation Study. BMC Med. Res. Methodol. 2017, 17, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, R.A. The Use of Multiple Measurements in Taxonomic Problems. Ann. Eugen. 1936, 7, 179–188. [Google Scholar] [CrossRef]
- Janssens, A.C.J.W.; Aulchenko, Y.S.; Elefante, S.; Borsboom, G.J.J.M.; Steyerberg, E.W.; van Duijn, C.M. Predictive Testing for Complex Diseases Using Multiple Genes: Fact or Fiction? Genet. Med. 2006, 8, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Essa, M.M.; Braidy, N.; Waly, M.I.; Al-Farsi, Y.M.; Al-Sharbati, M.; Subash, S.; Amanat, A.; Al-Shaffaee, M.A.; Guillemin, G.J. Impaired Antioxidant Status and Reduced Energy Metabolism in Autistic Children. Res. Autism Spectr. Disord. 2013, 7, 557–565. [Google Scholar] [CrossRef]
- Aaron, E.; Montgomery, A.; Ren, X.; Guter, S.; Anderson, G.; Carneiro, A.M.D.; Jacob, S.; Mosconi, M.; Pandey, G.N.; Cook, E.; et al. Whole Blood Serotonin Levels and Platelet 5-HT2A Binding in Autism Spectrum Disorder. J. Autism Dev. Disord. 2019, 49, 2417–2425. [Google Scholar] [CrossRef]
- Muller, C.L.; Anacker, A.M.J.; Veenstra-VanderWeele, J. The Serotonin System in Autism Spectrum Disorder: From Biomarker to Animal Models. Neuroscience 2016, 321, 24–41. [Google Scholar] [CrossRef] [Green Version]
- Deutsch, S.I.; Urbano, M.R.; Neumann, S.A.; Burket, J.A.; Katz, E. Cholinergic Abnormalities in Autism. Clin. Neuropharmacol. 2010, 33, 114–120. [Google Scholar] [CrossRef]
- Horder, J.; Petrinovic, M.M.; Mendez, M.A.; Bruns, A.; Takumi, T.; Spooren, W.; Barker, G.J.; Künnecke, B.; Murphy, D.G. Glutamate and GABA in Autism Spectrum Disorder—A Translational Magnetic Resonance Spectroscopy Study in Man and Rodent Models. Transl. Psychiatry 2018, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Hollowood-Jones, K.; Adams, J.B.; Coleman, D.M.; Ramamoorthy, S.; Melnyk, S.; James, S.J.; Woodruff, B.K.; Pollard, E.L.; Snozek, C.L.; Kruger, U.; et al. Altered Metabolism of Mothers of Young Children with Autism Spectrum Disorder: A Case Control Study. BMC Pediatr. 2020, 20, 557. [Google Scholar] [CrossRef]
- Filipek, P.A.; Juranek, J.; Nguyen, M.T.; Cummings, C.; Gargus, J.J. Relative Carnitine Deficiency in Autism. J. Autism Dev. Disord. 2004, 34, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Tanianskii, D.; Jarzebska, N.; Birkenfeld, A.; O’Sullivan, J.; Rodionov, R. Beta-Aminoisobutyric Acid as a Novel Regulator of Carbohydrate and Lipid Metabolism. Nutrients 2019, 11, 524. [Google Scholar] [CrossRef] [Green Version]
- Cheng, N.; Rho, J.M.; Masino, S.A. Metabolic Dysfunction Underlying Autism Spectrum Disorder and Potential Treatment Approaches. Front. Mol. Neurosci. 2017, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosato, A.; Tenori, L.; Cascante, M.; De Atauri Carulla, P.R.; dos Santos, V.A.P.M.; Saccenti, E. From Correlation to Causation: Analysis of Metabolomics Data Using Systems Biology Approaches. Metabolomics 2018, 14, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanley, H.G. Hyperserotonemia and Amine Metabolites in Autistic and Retarded Children. Arch. Gen. Psychiatry 1977, 34, 521. [Google Scholar] [CrossRef]
- Anderson, G.M.; Horne, W.C.; Chatterjee, D.; Cohen, D.J. The Hyperserotonemia of Autism. Ann. N. Y. Acad. Sci. 1990, 600, 331–340. [Google Scholar] [CrossRef]
- Marler, S.; Ferguson, B.J.; Lee, E.B.; Peters, B.; Williams, K.C.; McDonnell, E.; Macklin, E.A.; Levitt, P.; Gillespie, C.H.; Anderson, G.M.; et al. Brief Report: Whole Blood Serotonin Levels and Gastrointestinal Symptoms in Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 1124–1130. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.; Tangen, Ä.; Farde, L.; Bölte, S.; Halldin, C.; Borg, J.; Lundberg, J. Serotonin Transporter Availability in Adults with Autism—A Positron Emission Tomography Study. Mol. Psychiatry 2021, 26, 1647–1658. [Google Scholar] [CrossRef]
- Israelyan, N.; Margolis, K.G. Reprint of: Serotonin as a Link between the Gut-Brain-Microbiome Axis in Autism Spectrum Disorders. Pharmacol. Res. 2019, 140, 115–120. [Google Scholar] [CrossRef]
- Tsujiguchi, H.; Miyagi, S.; Nguyen, T.T.T.; Hara, A.; Ono, Y.; Kambayashi, Y.; Shimizu, Y.; Nakamura, H.; Suzuki, K.; Suzuki, F.; et al. Relationship between Autistic Traits and Nutrient Intake among Japanese Children and Adolescents. Nutrients 2020, 12, 2258. [Google Scholar] [CrossRef]
- Higazi, A.M.; Kamel, H.M.; Abdel-Naeem, E.A.; Abdullah, N.M.; Mahrous, D.M.; Osman, A.M. Expression Analysis of Selected Genes Involved in Tryptophan Metabolic Pathways in Egyptian Children with Autism Spectrum Disorder and Learning Disabilities. Sci. Rep. 2021, 11, 6931. [Google Scholar] [CrossRef] [PubMed]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary Metabolomics of Young Italian Autistic Children Supports Abnormal Tryptophan and Purine Metabolism. Mol. Autism 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, G.; Maes, M. Redox Regulation and the Autistic Spectrum: Role of Tryptophan Catabolites, Immuno-Inflammation, Autoimmunity and the Amygdala. Curr. Neuropharmacol. 2014, 12, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Boccuto, L.; Chen, C.-F.; Pittman, A.R.; Skinner, C.D.; McCartney, H.J.; Jones, K.; Bochner, B.R.; Stevenson, R.E.; Schwartz, C.E. Decreased Tryptophan Metabolism in Patients with Autism Spectrum Disorders. Mol. Autism 2013, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Mussap, M.; Siracusano, M.; Noto, A.; Fattuoni, C.; Riccioni, A.; Rajula, H.S.R.; Fanos, V.; Curatolo, P.; Barberini, L.; Mazzone, L. The Urine Metabolome of Young Autistic Children Correlates with Their Clinical Profile Severity. Metabolites 2020, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Khemakhem, A.M.; Frye, R.E.; El-Ansary, A.; Al-Ayadhi, L.; Bacha, A. Ben. Novel Biomarkers of Metabolic Dysfunction Is Autism Spectrum Disorder: Potential for Biological Diagnostic Markers. Metab. Brain Dis. 2017, 32, 1983–1997. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, J.; Li, Q.; Chen, L.; Wu, L.-J.; Jia, F.-Y.; Hao, Y.; Ke, X.-Y.; Yi, M.-J.; Jin, C.-H.; et al. China Multi-Center Preschool Autism Project (CMPAP): Design and Methodologies to Identify Clinical Symptom Features and Biomarkers of Autism Spectrum Disorders. Front. Psychiatry 2021, 11, 613519. [Google Scholar] [CrossRef]
- Bridgemohan, C.; Cochran, D.M.; Howe, Y.J.; Pawlowski, K.; Zimmerman, A.W.; Anderson, G.M.; Choueiri, R.; Sices, L.; Miller, K.J.; Ultmann, M.; et al. Investigating Potential Biomarkers in Autism Spectrum Disorder. Front. Integr. Neurosci. 2019, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Pierce, K.; Gazestani, V.H.; Bacon, E.; Barnes, C.C.; Cha, D.; Nalabolu, S.; Lopez, L.; Moore, A.; Pence-Stophaeros, S.; Courchesne, E. Evaluation of the Diagnostic Stability of the Early Autism Spectrum Disorder Phenotype in the General Population Starting at 12 Months. JAMA Pediatr. 2019, 173, 578. [Google Scholar] [CrossRef]
- McCarty, P.; Frye, R.E. Early Detection and Diagnosis of Autism Spectrum Disorder: Why Is It So Difficult? Semin. Pediatr. Neurol. 2020, 35, 100831. [Google Scholar] [CrossRef]
- Ning, M.; Daniels, J.; Schwartz, J.; Dunlap, K.; Washington, P.; Kalantarian, H.; Du, M.; Wall, D.P. Identification and Quantification of Gaps in Access to Autism Resources in the United States: An Infodemiological Study. J. Med. Internet Res. 2019, 21, e13094. [Google Scholar] [CrossRef] [PubMed]
- Delaye, J.-B.; Patin, F.; Lagrue, E.; Le Tilly, O.; Bruno, C.; Vuillaume, M.-L.; Raynaud, M.; Benz-De Bretagne, I.; Laumonnier, F.; Vourc’h, P.; et al. Post Hoc Analysis of Plasma Amino Acid Profiles: Towards a Specific Pattern in Autism Spectrum Disorder and Intellectual Disability. Ann. Clin. Biochem. Int. J. Lab. Med. 2018, 55, 543–552. [Google Scholar] [CrossRef]
- Main, P.A.; Angley, M.T.; O’Doherty, C.E.; Thomas, P.; Fenech, M. The Potential Role of the Antioxidant and Detoxification Properties of Glutathione in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Nutr. Metab. 2012, 9, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Shi, X.-J.; Liu, H.; Mao, X.; Gui, L.-N.; Wang, H.; Cheng, Y. Oxidative Stress Marker Aberrations in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis of 87 Studies (N = 9109). Transl. Psychiatry 2021, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Hassan, W.M.; Daghestani, M.; Al-Ayadhi, L.; Ben Bacha, A. Preliminary Evaluation of a Novel Nine-Biomarker Profile for the Prediction of Autism Spectrum Disorder. PLoS ONE 2020, 15, e0227626. [Google Scholar] [CrossRef] [Green Version]
- Osredkar, J.; Gosar, D.; Maček, J.; Kumer, K.; Fabjan, T.; Finderle, P.; Šterpin, S.; Zupan, M.; Jekovec Vrhovšek, M. Urinary Markers of Oxidative Stress in Children with Autism Spectrum Disorder (ASD). Antioxidants 2019, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-J.; Cai, X.-E.; Meng, F.-C.; Song, T.-J.; Wang, X.-X.; Wei, Y.-Z.; Zhai, F.-J.; Long, B.; Wang, J.; You, X.; et al. Comparison of the Metabolic Profiles in the Plasma and Urine Samples Between Autistic and Typically Developing Boys: A Preliminary Study. Front. Psychiatry 2021, 12, 657105. [Google Scholar] [CrossRef]


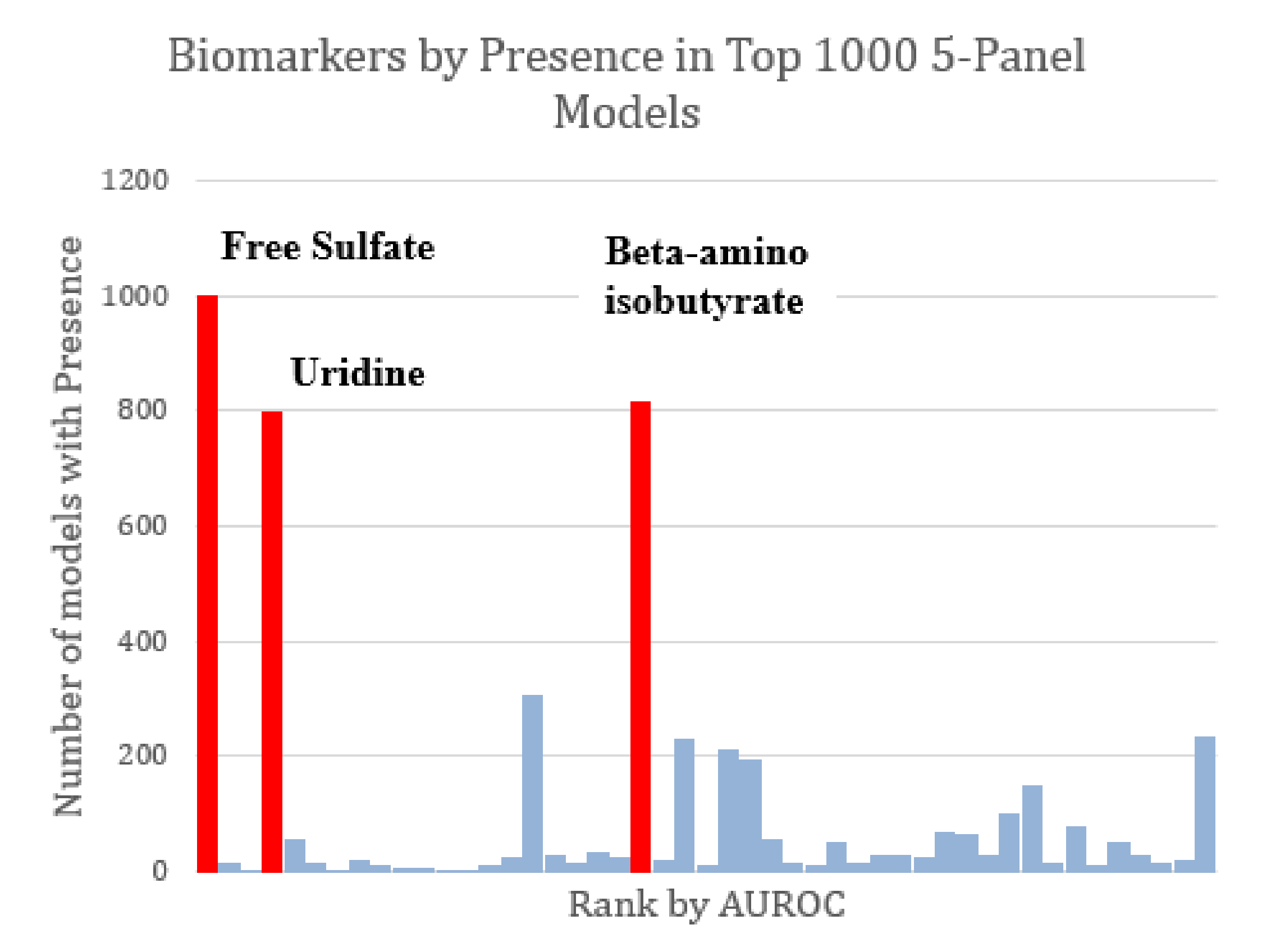
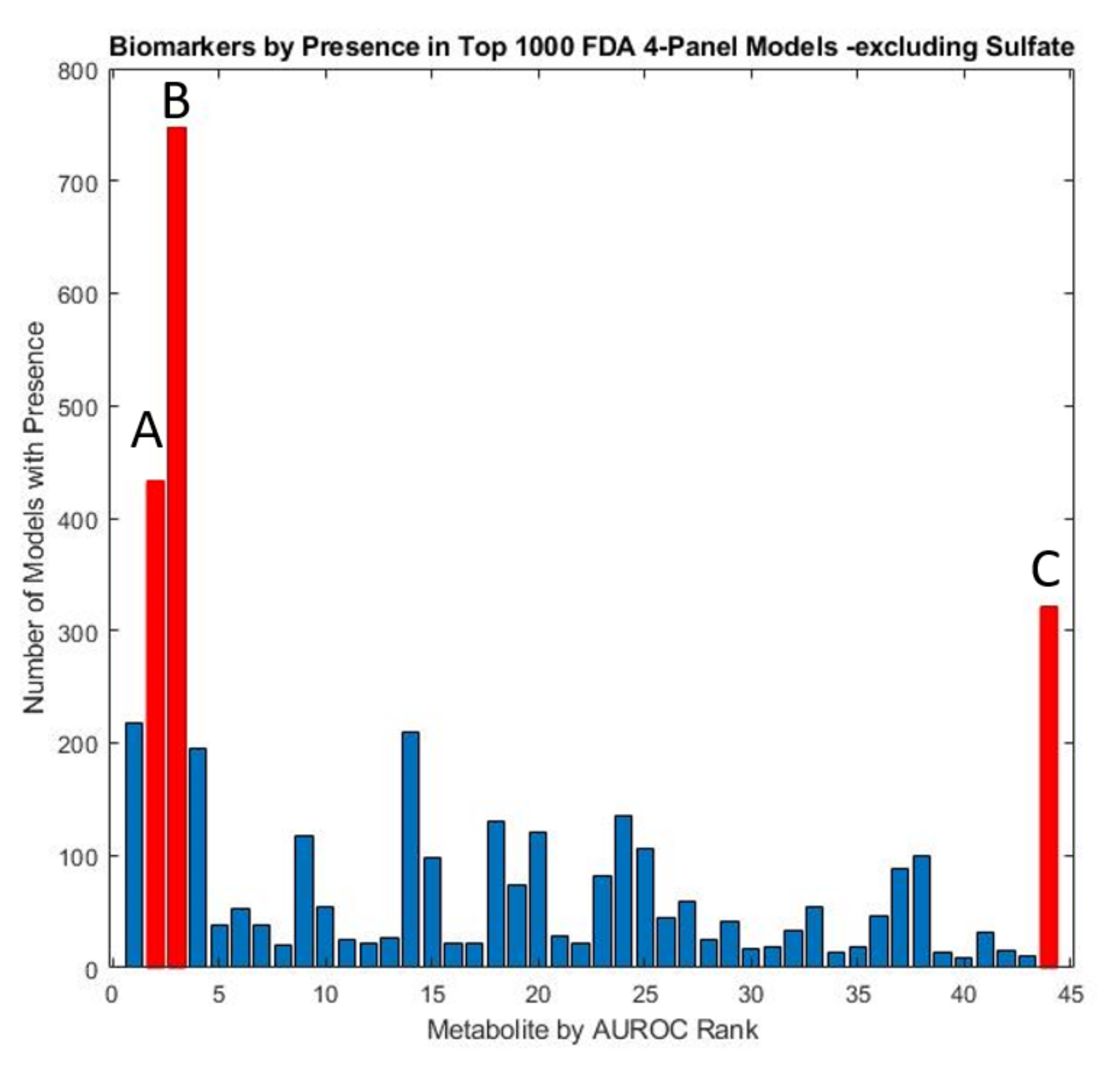

| Name | Source | Mean ASD Value vs. Mean TD Value | p-Value | Test-Type | ASD Correlations | TD Correlations | AUROC |
|---|---|---|---|---|---|---|---|
| Free Sulfate | Plasma | ↓ | 0 | Welch’s test ***** | 12 | 18 | 0.90 |
| Nitrotyrosine | Plasma | ↑ | 0 | Welch’s test ***** | 11 | 19 | 0.87 |
| Total Sulfate | Plasma | ↓ | 0 | Welch’s test ***** | 12 | 19 | 0.85 |
| Uridine (UriP) | Plasma | ↑ | 0 | Welch’s test ***** | 5 | 10 | 0.85 |
| Glutathione (Glut) | Plasma | ↓ | 0 | Welch’s test ***** | 11 | 16 | 0.85 |
| Nicotinamide Adenine Dinucleotide (NAD) + hydrogen (H) (NADH) | RBC | ↓ | 0 | Welch’s test ***** | 12 | 16 | 0.84 |
| Acetylcholine | platelets | ↓ | 0 | Welch’s test ***** | 14 | 16 | 0.81 |
| Nicotinamide adenine dinucleotide phosphate (NADP) | RBC | ↓ | 0 | Welch’s test | 11 | 19 | 0.79 |
| ATP | Plasma | ↓ | 0 | Welch’s test ***** | 5 | 14 | 0.77 |
| S-adenosylmethionine (SAM) | RBC | ↓ | 0 | t-test | 4 | 14 | 0.77 |
| Norepinephrine | platelets | ↓ | 0 | Welch’s test ***** | 0 | 16 | 0.76 |
| Reduced glutathione: oxidised glutathione (GSSG/GSH ratio) | Plasma | ↑ | 0 | Welch’s test ***** | 10 | 18 | 0.75 |
| Total Choline | RBC | ↑ | 0 | t-test | 2 | 16 | 0.75 |
| Serotonin | platelets | ↓ | 0 | Welch’s test ***** | 0 | 13 | 0.75 |
| Tryptophan | Plasma | ↓ | 0 | Welch’s test ***** | 0 | 1 | 0.75 |
| Thallium | Urine | ↑ | 0 | Welch’s test ***** | 1 | 2 | 0.73 |
| Free carnitine | Plasma | ↑ | 0 | t-test | 3 | 5 | 0.71 |
| Oxidized glutathione | Plasma | ↑ | 0 | Welch’s test ***** | 6 | 9 | 0.7 |
| Gamma-aminobutyric acid (GABU) | Urine | ↓ | 0 | Welch’s test ***** | 11 | 16 | 0.7 |
| Total carnitine (carnitine + acetyl-carnitine) | Plasma | ↑ | 0 | t-test | 4 | 4 | 0.69 |
| Beta-amino isobutyrate | Plasma | ↑ | 0 | Welch’s test ***** | 0 | 3 | 0.69 |
| Biotin | Plasma | ↓ | 0 | Welch’s test | 0 | 7 | 0.68 |
| Glutamate | Plasma | ↑ | 0.01 | Welch’s test ***** | 1 | 3 | 0.68 |
| Epinephrine | Platelets | ↓ | 0 | Mann–Whitney | 9 | 15 | 0.67 |
| Total carotenes | Plasma | ↓ | 0.01 | Welch’s test ***** | 4 | 1 | 0.67 |
| Cadmium | WB | ↓ | 0 | Welch’s test ***** | 5 | 1 | 0.67 |
| Iron | RBC | ↑ | 0 | Welch’s test ***** | 7 | 7 | 0.67 |
| Phosphorus | RBC | ↑ | 0 | Welch’s test ***** | 7 | 3 | 0.66 |
| Lithium | WB | ↓ | 0.04 | Welch’s test ***** | 2 | 3 | 0.66 |
| SAM/SAH | Plasma | ↓ | 0.01 | Welch’s test ***** | 6 | 15 | 0.65 |
| Potassium | RBC | ↑ | 0.01 | Welch’s test ***** | 5 | 3 | 0.65 |
| Tin | Urine | ↑ | 0.01 | Mann–Whitney | 1 | 2 | 0.65 |
| Taurine | Plasma | ↓ | 0.01 | Welch’s test ***** | 5 | 7 | 0.65 |
| Vitamin C | Plasma | ↑ | 0.03 | t-test | 1 | 4 | 0.64 |
| Copper | WB | ↑ | 0.02 | t-test | 0 | 1 | 0.64 |
| Formiminoglutamic acid (FIGLU) | Urine | ↑ | 0.03 | t-test | 1 | 3 | 0.63 |
| Copper | RBC | ↑ | 0.03 | t-test | 0 | 1 | 0.63 |
| Magnesium | Plasma | ↓ | 0.02 | Mann–Whitney | 0 | 3 | 0.63 |
| Antimony | Urine | ↑ | 0.03 | Mann–Whitney | 0 | 0 | 0.63 |
| Lead | Urine | ↑ | 0.02 | Mann–Whitney | 1 | 1 | 0.63 |
| Serine | Plasma | ↑ | 0.04 | Welch’s test ***** | 0 | 3 | 0.63 |
| Adenosine | Plasma | ↑ | 0.01 | Welch’s test ***** | 4 | 10 | 0.62 |
| Calcium | RBC | ↓ | 0.02 | Welch’s test ***** | 8 | 6 | 0.61 |
| Vitamin B5 | Plasma | ↓ | 0.02 | Welch’s test | 0 | 8 | 0.61 |
| Cadmium | Urine | ↓ | 0.01 | Mann–Whitney | 6 | 6 | 0.6 |
| Homocysteine + homocystine | Plasma | ↑ | 0.02 | Mann–Whitney | 5 | 1 | 0.6 |
| Number of Markers | Method | Model Constituents | Fitted AUROC | CV AUROC | Sensitivity (TPR) | Specificity (TNR) |
|---|---|---|---|---|---|---|
| 2 | FDA | Free sulfate (plasma) Uridine (plasma) | 0.92 | 0.94 | 0.94 | 0.86 |
| 3 | FDA | Free sulfate (plasma) Uridine (plasma) Beta-amino isobutyrate | 0.95 | 0.96 | 0.92 | 0.89 |
| 4 | FDA | Free sulfate (plasma) Uridine (plasma) Homo cystine Beta-amino isobutyrate | 0.96 | 0.97 | 0.93 | 0.91 |
| 5 | FDA | Free sulfate (plasma) Uridine (plasma) Initial homo cystine Beta-amino isobutyrate Serum magnesium | 0.96 | 0.98 | 0.95 | 0.95 |
| 5 *** | FDA | Free sulfate (plasma) Uridine (plasma) Beta-amino isobutyrate Tryptophan (plasma) Homo cystine (plasma) | 0.96 | 0.97 | 0.93 | 0.89 |
| 5 ‡ | FDA | Glutathione (plasma) Uridine (plasma) Thallium (urine) Glutamate (plasma) Homo cystine (plasma) | 0.94 | 0.95 | 0.98 | 0.75 |
| 5 | SVM | Free sulfate (plasma) Magnesium (Serum) Homo cystine (plasma) Uridine (plasma) Beta-amino isobutyrate | 1.00 | 0.92 | 0.91 | 0.92 |
| 6 | FDA | Free sulfate (plasma) Uridine (plasma) Homo cystine (plasma) Beta-amino isobutyrate Serum magnesium RBC copper | 0.97 | 0.98 | 0.95 | 0.95 |
| Metabolite Pair | Pearson Correlation Coefficient |
|---|---|
| Free sulfate (plasma) | |
| Total sulfate (plasma) | 0.63 |
| GABA | 0.57 |
| SamR | 0.57 |
| Glutathione | 0.56 |
| Acetylcholine | 0.53 |
| NADH | 0.47 |
| Lithium | 0.45 |
| SAM/SAH | 0.42 |
| Thallium (urine) | 0.41 |
| Epinephrine | 0.40 |
| Oxidized glutathione/glutathione | −0.43 |
| Uridine (plasma) | |
| FIGLU | 0.46 |
| Total sulfate (plasma) | −0.48 |
| Homocysteine + homocystine | |
| Iron | 0.46 |
| Cadmium (whole blood) | −0.45 |
| Taurine | −0.55 |
| Beta-amino isobutyrate | |
| **** | |
| Magnesium | |
| **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, F.; Adams, J.B.; Audhya, T.; Hahn, J. Multivariate Analysis of Metabolomic and Nutritional Profiles among Children with Autism Spectrum Disorder. J. Pers. Med. 2022, 12, 923. https://doi.org/10.3390/jpm12060923
Qureshi F, Adams JB, Audhya T, Hahn J. Multivariate Analysis of Metabolomic and Nutritional Profiles among Children with Autism Spectrum Disorder. Journal of Personalized Medicine. 2022; 12(6):923. https://doi.org/10.3390/jpm12060923
Chicago/Turabian StyleQureshi, Fatir, James B. Adams, Tapan Audhya, and Juergen Hahn. 2022. "Multivariate Analysis of Metabolomic and Nutritional Profiles among Children with Autism Spectrum Disorder" Journal of Personalized Medicine 12, no. 6: 923. https://doi.org/10.3390/jpm12060923
APA StyleQureshi, F., Adams, J. B., Audhya, T., & Hahn, J. (2022). Multivariate Analysis of Metabolomic and Nutritional Profiles among Children with Autism Spectrum Disorder. Journal of Personalized Medicine, 12(6), 923. https://doi.org/10.3390/jpm12060923







