Abstract
Autoimmune liver diseases (AILDs) include autoimmune hepatitis, primary biliary cholangitis and primary sclerosing cholangitis. The etiologies of AILD are not well understood but appear to involve a combination of genetic and environmental factors. AILDs commonly affect young individuals and are characterized by a highly variable clinical course. These diseases significantly influence quality of life and can progress toward liver decompensation or the onset of hepatocellular or cholangiocarcinoma; a significant number of patients eventually progress to end-stage liver disease, requiring liver transplantation. In this review, we focus on the sex characteristics and peculiarities of AILD patients and highlight the relevance of a sex-specific analysis in future studies. Understanding the sex differences underlying AILD immune dysregulation may be critical for developing more effective treatments.
1. Introduction
Autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) represent the three major autoimmune liver diseases (AILDs). Furthermore, the spectrum of autoimmune liver disease includes the overlap syndromes (OS) between AIH and PBC or PSC. Overlap syndromes between PBC and PSC have been described in anecdotal cases only. Genetic predisposition, environmental factors and defects in immune regulation underlie the induction and perpetuation of autoimmunity. AIH, PSC and PBC share common pathways of immune-mediated liver injury, involving the hepatic recruitment of CD4+ and CD8+ T cells, which display cytotoxicity against liver or biliary cells, leading to liver fibrosis, cirrhosis and liver failure [1]. An imbalance between effector and regulatory T cells appears to underlie the loss of immune tolerance to self-antigens in many autoimmune diseases with a poorly understood pathway. Several studies have provided evidence of viral or bacterial triggers in AILD etiology, suggesting that autoimmunity may result from immune recognition of microbial peptides that display sequence similarity to autoantigenic peptides, called molecular mimicry [2]. It is also possible that AILD results from the modification of self-antigens by drugs or micro-organisms, making them immunogenic, or from the aberrant exposure of normally sequestered liver antigens to the immune system as a result of liver damage. The observations that particular MHC class II alleles predispose individuals to developing AILD provide a strong argument that antigen presentation to CD4+ T cells is a central event in the pathogenesis [3]. Although the three diseases exhibit similarities in their pathogeneses, they differ in their patterns of liver injury. AIH is characterized by an inflammatory cell infiltrate, mainly composed of cytotoxic T cells and plasma cells, around the portal tracts, which invades and causes progressive destruction of the liver parenchyma, termed interface hepatitis. In contrast, the large intra and extrahepatic bile ducts are targeted in PSC, leading to biliary tree obliteration and resulting in biliary cirrhosis and portal hypertension. In PBC, the small bile ducts are damaged, leading to portal tract destruction and biliary cirrhosis.
To date, available studies have shown a gender role among autoimmune diseases, such as a higher prevalence of AILD in female patients, with the exception of PSC. On the other hand, male sex seems to have a poor prognosis, apart from in AIH, where the mortality is higher in the female than male sex. The aim of this paper is to review the current knowledge regarding the influence of sex on the setting of AILD, underlining the relevance of sex-specific analysis in the prognosis and management of these diseases.
2. Autoimmune Hepatitis
AIH affects the female sex more than the male sex across all ethnicities and ages (children, 60–76%; adults, 71–95%) [3,4,5,6,7,8,9,10,11,12,13,14]. The male-to-female ratio in the population with AIH is considered to have changed over time, indicating a relative increase in the number of male patients. In Japan, the male-to-female ratio was 1:7 in 2004 and 1:4 in 2016 [15]. It should also be stressed that cases with “acute” presentation (transaminases higher than 10 times the upper limit and bilirubin higher than 5 mg/mL) are increasing worldwide. In an Italian multicenter study, among 479 patients diagnosed as AIH, 202 (43%) met the criteria for “acute” onset, and no significant differences were observed between the sexes [16].
Factors responsible for gender differences in immune response are illustrated in Figure 1.
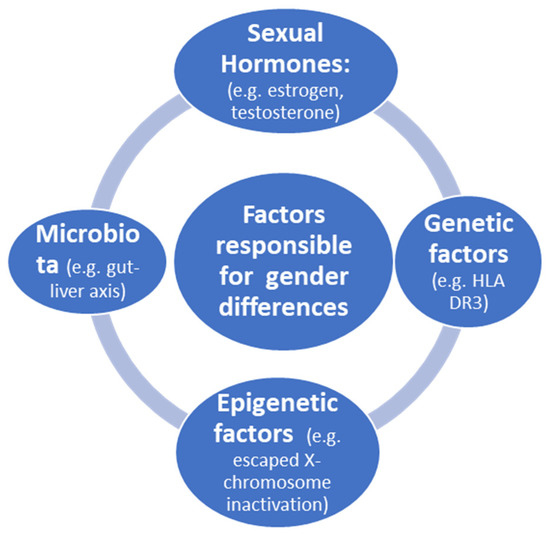
Figure 1.
Factors responsible for gender differences in immune response.
Sexual hormones influence innate immunity. High levels of estrogen reduce the synthesis of interleukin-1β (IL-1β), IL-6 and tumor necrosis factor (TNF) by macrophages and monocytes. On the other hand, low levels of estrogen increase the same cytokines [17]. However, in cases in which cytotoxic T cells target liver cells, estrogen may shift the onset of AIH to the late reproductive phase. This is the case of AIH with onset after menopausal state. Moreover, depending on the reproductive status, before puberty, B-cell-dependent autoimmunity plays a key role in AIH, with the production of autoantibodies [17]. Indeed, estrogen also plays a role in natural killer (NK) cells; low estrogen levels increase the activity of NK cells, as well as that of dendritic cells. Estrogen treatment (compared to pregnancy levels) was found to decrease TNF and IL-12 production in mature mouse dendritic cells [18]. These changes also explain the beneficial effect of pregnancy for AIH. During pregnancy, high levels of estrogen and progesterone exert a tremendous inhibitory influence on most inflammatory pathways, but females might be prone to disease flare thereafter in the vulnerable postpartum phase.
Genetic factors linked to X chromosomes have been extensively studied [19,20,21,22]. During embryonic development, one of the two X chromosomes is randomly inactivated in females. This process is initiated by long non-coding RNA X inactivation and results in a cellular mosaicism, where about one-half of the cells in a given tissue express either the maternal X chromosome or the paternal X chromosome. However, X chromosome inactivation is not complete, with 15 to 23% of genes escaping inactivation, thereby contributing to the emergence of a female-specific heterogeneous population of cells with biallelic expression of some X-linked genes. Although X chromosomes provide clues as to the cause of PBC [19], few studies have been published on AIH.
Epigenetic factors were extensively reviewed by Liu et al. [20]. An important issue is the role of X-chromosome-located microRNAs in immunity, which has been hypothesized to contribute to the enhanced immune response of females [21]. The human X chromosome contains 10% of all microRNAs detected to date. According to recent studies, in several mammalian species, including humans and mice, the X chromosome has a higher density of miRNAs, whereas the Y chromosome has only four miRNA sequences (although not experimentally validated) with two shared by both sex chromosomes [22]. Indeed, miRNAs on the X chromosome may influence sex-specific responses. It has been shown that bone-marrow-derived mesenchymal stem-cell-secreted miR-223-containing exosomes prevent liver injury in an autoimmune hepatitis mouse model by suppressing hepatic NLRP3 and caspase-1 [23]. Moreover, modification of miR-223 further improves its therapeutic efficacy against AIH [23]. In general, miR-223 is involved in the pathogenesis of various liver diseases by influencing immune cell differentiation, neutrophil infiltration, macrophage polarization and inflammasome activation by both metabolic and inflammatory signaling pathways [24]. However, a few studies have been conducted on the epigenetics of AIH utilizing miRNAs, but altogether, these studies suggest the possibility that miRNAs could be used as biomarkers for diagnosis and prognosis of AIH [25].
The microbiome can influence a number of physiological aspects of the host, including the immune response, and several studies have attempted to unravel the role of the microbiome in the pathogenesis of autoimmune diseases [26,27]. Moreover, the sex difference in this setting is currently not sufficiently explored, representing a gap that needs to be filled.
Neoplastic risk for hepatocellular carcinoma (HCC) in AIH is lower than other types of liver disease [28]. HCC develops in patients with AIH and cirrhosis in 1–9% of cases, and the annual incidence in patients with cirrhosis is 1.1–1.9% [29], with the same proportion in females and males [30]. Besides cirrhosis, other risk factors linked to HCC are older age, increased frequency of relapses, concurrent alcohol consumption and a trend for male sex [28].
The long-term outcome of patients with AIH was evaluated in 238 patient (51 men) at a single center from 1971 to 2005 [31], showing increased survival in males; however, the reason was unclear. Moreover, the age at death in women with liver-related causes compared to non-liver causes was significantly lower (39 years. vs. 70.5 years., p = 0.001). Interestingly, HLA a1-B8-DR3 (associated with increased susceptibility to AIH) was more than twice as prevalent in males compared to females. When considering a large cohort of patients including all ages, patients diagnosed under the age of 18 years were found to have a significantly reduced life expectancy [32] and were at high risk of relapses and liver transplantation (LT), with no significant differences observed between the sexes [32].
2.1. Management of AIH
There are no differences in response to therapy according to gender. The goals of treatment include (i) the induction of remission, (ii) the maintenance of remission and (iii) the prevention of progression to cirrhosis and its complications. Standard therapy includes prednisone alone or in combination therapy with azathioprine (AZA). Combination therapy with low-dose prednisolone permits a reduction in steroid-related side effects in the induction phase. The open questions include (i) the outcome of therapy, (ii) the possibility of discontinuing treatment and (iii) alternative or new therapies that could enable better control of the disease. The outcomes of therapy include biochemical remission, complete remission, relapse, treatment failure and stabilization. For patients who have experienced treatment failure or failed to achieved remission, second-line therapies with mycophenolate mofetil (MMF), calcioneurin inhibitors (tacrolimus (TAC)) or cyclosporine have been proposed. In patients with “acute” onset of AIH characterized by jaundice and concomitant coagulopathy a rapid referral to a transplant center may be necessary. This is the case for subjects with acute encephalopathy because it has been shown that this condition is associated with high mortality in cases of corticosteroid therapy [33]. In general, patients with “acute” onset and without hepatic encephalopathy respond well to oral or intravenous steroids (1 mg/kd/day) [34]. Discontinuing treatment is still an important problem. Whereas the British literature in the 1980s recommended indefinite treatment, current AASLD guidelines consider discontinuing treatment a possibility during the course of the disease [4].
2.2. Drug-Induced Liver Injury (DILI) with Autoimmune Hepatitis Characteristics
This is a particular form of DILI mimicking AIH with autoantibodies. The nomenclature was introduced by Czaja in 2011 [35]. Numerous drugs can induce this condition, including nitrofurantoin, hydralazine, methyldopa, interferons and checkpoint inhibitors. A consensus conference on this important issue was held on 1–3 March, 2022 in Parador de Nerja (Spain) under the auspices of EASL. In a recent prospective study from 2004 through 2011, 88 cases of DILI with AIH features were analyzed by the DILI Network [36]. Female sex was preponderant (100% in 42 cases due to nitrofurantoin, 79% in 28 cases due to minocycline, 100% of 11 cases due to methyldopa and 71% of 7 cases due to hydralazine). Overall, 40% of subjects showed spontaneous improvement in liver tests after discontinuation of the implicated drugs [37]. Analysis of liver biopsies revealed that it is very difficult to distinguish between histologic features that favor DILI and those favoring AIH. Recently, DILI with AIH features was reported with the use of immune-activating agents, such as checkpoint inhibitors [38]. Liver changes usually improve with steroid therapy, but some cases are resistant and associated with bile duct injury [39].
3. Primary Biliary Cholangitis
A significant female preponderance is a well-known clinical feature of PBC, whereas differences between sexes in the clinical presentation at PBC diagnosis are not so well-defined. Twelve studies including 51,290 PBC patients worldwide were analyzed to evaluate sex-related differences at PBC diagnosis [40,41,42,43,44,45,46,47,48,49,50]. Seven studies showed that male sex is associated with delayed diagnosis and, consequently, older age at PBC identification [40,43,44,45,47,49,50]. Six studies showed that male patients received PBC diagnosis at more advanced and severe liver disease with cirrhosis and its decompensation events, as well as portal hypertension signs [41,42,44,45,48,51]. Three studies found that male PBC patients presented with worse liver biochemistries [43,44,51]. Finally, six studies showed that fatigue is more associated with female sex at PBC presentation [41,42,44,45,48,51]. Furthermore, Marzioni et al. analyzed data from electronic medical records of patients from 900 general practitioners in Italy, identifying 412 PBC patients and showing that osteoporosis, inflammatory arthritis and other connective tissue diseases were significantly more common in women, whereas inflammatory bowel diseases were significantly more common in men (p < 0.01) [52].
The clinical impact of PBC is highly variable, and one of the most important factors contributing to this variability is the response to primary therapy with ursodeoxycholic acid (UDCA) [53]. Several studies have demonstrated that the efficacy of UDCA therapy strongly determines long-term outcomes [53,54,55]. Moreover, many studies have evaluated the role of sex in the response to UDCA therapy, although results are conflicting because of small sample size, retrospectivity and different criteria used to determine response. Eleven studies including 9748 patients with PBC were analyzed [40,48,55,56,57,58,59,60,61,62,63]. All of these studies, except one [59], evaluated the impact of sex on UDCA therapy response. In particular, four of studies showed that no response to UDCA is more frequent in the male than female sex [40,56,57,58]. However, although these studies presented large sample sizes (7677 PBC patients), they evaluated response to UDCA with four different criteria, and this heterogeneity makes the results not comparable. Particularly, Carbone et al. [40] used the UK-PBC cohort to evaluate UDCA response with Paris I criteria and demonstrated that sex is an independent predictor of therapy failure. Instead, Cheung et al. [56] analyzed the largest cohort of PBC patients utilizing the GLOBE score criteria. Finally, Lammert et al. [57] used the Toronto criteria, and Tian et al. [58] utilized a combination of the Barcelona and Paris I criteria. A similar heterogeneity in the evaluation of UDCA response was observed in six, studies showing that sex has no impact on response to therapy [48,55,60,61,62,63]. In 2016, obeticholic acid (OCA) was approved as a second-line therapy in PBC patients with inadequate response or intolerant to UDCA [64]. Four studies evaluated the response to OCA therapy according to POISE criteria [65,66,67,68]. Only D’Amato et al. [68] considered the role of sex in OCA therapy and showed that sex has no impact on inadequate response to OCA.
There is currently little information available regarding the exact magnitude of HCC risk in PBC patients according to sex. A recent meta-analysis evaluating 18 studies examining the incidence of HCC in PBC patients according to sex showed a pooled HCC incidence rate of 9.82 per 1000 person-years (95% CI 5.92–16.28) in men and 3.82 per 1000 person-years (95% CI 2.85–5.11) in women, with moderate-to-high between-study heterogeneity [69]. Additionally, Trivedi et al. showed that HCC incidence was higher in male UDCA non-responders versus responders (HR 4.44, 95% CI 1.29 to 10.20; p < 0.001) in a cohort of 4565 PBC patients [70]. Moreover, Harada et al. found that the cumulative incidence of HCC was 6.5% in males and 2.0% in females (p < 0.0001) during the 10 years after PBC diagnosis, indicating that male PBC patients had a 3.3-fold higher risk of HCC compared with female PBC patients [71]. Nonetheless, although PBC primarily affects females, the authors postulated that HCC might be more common in male PBC patients because of a lack of estrogen-mediated prevention. In females, the HCC incidence gradually increased according to histological stage, indicating that the terminal stage of PBC, which is a cirrhotic state, may be a risk factor for HCC development in females, whereas males are likely to develop HCC at any stage [71].
The role of sex in the prognosis of PBC patients has been widely evaluated. Seven studies analyzing the presence of ACLD were considered [42,45,48,51,55,56,72]. All of them showed that the presence of ACLD was more frequent in male than in female patients. In particular, Cheung et al. [56] and Marschall et al. [42] demonstrated that male sex had a higher prevalence of portal hypertension and liver decompensation. Moreover, Adejumo et al. [72] showed that male sex had higher risk of jaundice, spontaneous bacterial peritonitis and acute liver failure, whereas female sex had a higher risk of hospitalizations. Furthermore, 11 studies evaluated the role of sex in mortality for PBC patients [42,47,48,50,53,55,56,73,74,75,76]. Eight of these studies demonstrated that males have a higher risk of mortality. In particular, John et al. [55] showed that male sex is a risk factor for death, liver-related mortality and liver decompensation. Lleo et al. [74] demonstrated that male sex was associated to an increased risk of all-cause mortality.
Primary Sclerosing Cholangitis
PSC is considered an immune-mediated disease with atypical features, including prevalence in men, the absence of disease-specific autoantibodies and poor response to immunosuppression. Regarding gender distribution, PSC affects men prevalently; in a large regional population study from Sweden, the mean crude annual incidence of PSC was 1.22 per 100,000 in the total population aged ≥18 years in the period from 1992 to 2005; among men and women, the incidence was 1.8 and 0.7, respectively. The point prevalence of PSC in the same population was 16 (24 among men and 9 among women) per 100,000, and the proportion of men was 71% [77]. In other nations, proportion of men ranged from 51% in New Zealand [78] to 71% in the USA [79] and in Norway [10]. Similarly, in a recent data collection from the National Rare Diseases Registry (RNMR) and the National Mortality Database (NMD) in Italy, 60% of new PSC diagnoses were in male patients, with a male-to-female ratio of 1.5:1 [80]. Mean age at disease onset was 33 years (SD = 17), and mean age at diagnosis was 37 years. There were no statistically significant differences in age at diagnosis, age at onset and diagnostic delay between male and female patients. In other studies, the median age at PSC onset was generally higher in women than men; a large cohort study from Germany published in 2018 evaluated patients with late disease onset (defined as first diagnosis after 50 years), revealing that the proportion of females was significantly higher in the late-onset group compared with the earlier-onset group (50/183 (27%) vs. 15/32 (47%), p = 0.02) [81]. A study population from Sweden was reported to have a time-trend increase in the incidence of large-duct PSC among women but not among men; conversely, the incidence of small-duct PSC increased significantly among men but not among women. Diverging trends were also observed for the incidence of PSC related to IBD, with a significant increase in the incidence of PSC-IBD in women, whereas in men, an increase in PSC without IBD was observed [77].
Patients with PSC are at increased risk of developing several hepatobiliary cancers, mainly cholangiocarcinoma, gallbladder and colon cancer—and hepatocellular carcinoma to a lesser degree. Cholangiocarcinoma (CCA) is a model of malignancies occurring in the inflammatory background. Chronic inflammation of the biliary tree induced by PSC promotes oncogenesis and predisposes to development of CCA through DNA damage, cellular proliferation and oxidative stress [82,83]. The annual risk for CCA in PSC is approximately 2%, with a 10- and 30-year cumulative incidence of 6–11% and 20%, respectively, and an increase of 400-fold when compared with the general population [84,85]. Regarding the sex-specific risk of CCA development, female sex seems to be associated with a lower risk of CCA (HR,0.68; p < 0.001, respectively), as reported in the data from a large international PSC cohort, which included 7121 patients encompassing >30 years of clinical observation. According to multivariate analysis, advancing age at diagnosis is an independent risk factor of CCA development, whereas female sex and having small-duct disease or CD at the time of PSC diagnosis are protective factors against CCA development [86].
Inflammatory bowel disease occurs in 70–80% of patients with PSC, and PSC seems to confer additional risk of developing colorectal cancer (CRC) when compared with the risk in patients with IBD alone [87,88]. CRC can appear in up to 20–30% of PSC-IBD patients, and an annual colonoscopy is recommended [89].
In 2020, a nationwide population-based study from national healthcare registries in England identified incident cases of IBD with and without PSC over ten years; the study showed that patients with PSC-IDB younger than 40 years old had a fourfold higher risk of CRC, whereas there was no difference between groups for patients in which the IBD diagnosis was made in patients older than 60 years. Regarding sex differences, the risk for CRC was significantly lower among women than men (HR 0.46 p < 0.001) [90]. The oncologic additive risk of association between PSC and IBD was confirmed in a Spanish multicenter retrospective cohort study. The risk of CRC was increased four- to fivefold in PSC-IBD patients compared to IBD controls, including patients submitted to annual colonoscopic surveillance [91].
Data related to sex differences in PSC clinical presentation and evolution are scarce. From the Italian registry, including 502 PSC patients in population-based data, the survival rate was 92% at 10 years from diagnosis and 82% at 20 years, considering all causes of deaths. The Kaplan–Meier curves show no significant difference between male and female patients with respect to estimated survival times from diagnosis [80]. The International PSC Study Group published a multicenter outcome study in 2017 to describe the natural history of the disease, including 7121 patients across 17 countries and encompassing ≥30 years of clinical observation from 1980 through 2010. The study registered sex-specific variations in clinical phenotype and correlations with liver disease progression and neoplastic complications. Men comprised the majority of the cohort (66%) and were younger than women (average age of 37 years vs. 40 years). Women more commonly exhibited small-duct PSC phenotype, ulcerative colitis (UC) was less common in women than men (48% vs. 61%, respectively p < 0.001) and small-duct PSC was characterized by a low-risk phenotype in both sexes (adjusted HR for men, 0.23; p < 0.001 and adjusted HR for women, 0.48; p = 0.003). Female sex was an independent protective factor against liver progression; in particular, females maintained a significantly higher transplant-free survival than males matched for age and PSC phenotype. Moreover, the lower prevalence of UC in women may partially account for differences in liver disease progression between the sexes.
4. Overlap Syndromes and Gender
The term overlap syndrome (OS) describes a subtype of clinical syndromes that share common features relating to AIH and PBC or AIH and PSC. Very rarely, an overlap syndrome between PBC and PSC has been described. However, the term remains controversial, and it is not known whether overlaps are situations occurring simultaneously or represent a different development during the natural course of the disease.
PBC-AIH is the most common form of overlap syndromes [92,93]. A systematic review included 17 studies of PBC-AIH comprising a total of 402 patients [94]. Female gender was present in 87–100% of either retrospective or prospective studies.
AIH-PSC overlap syndrome has been described in both children and adults. In children, the syndrome is particularly important, reported in up to 40% of patients with AIH [95]. Adults diagnosed with AIH-PSC overlap are significantly younger at the time of diagnosis than those with classical PSC (24–27 years vs. 39–46 years, respectively) [96,97,98]. The proportion of adult males with AIH-PSC overlap is 69–81%, which is higher than in AIH [99]. However, the proportion of male gender in AIH-PSC undergoing liver transplantation was slightly lower (49%) than the number reported in classical PSC [100].
4.1. Concurrent Autoimmune Disorders in Patients with Autoimmune Liver Diseases: The Effect of Gender
AILDs often coexist with other extrahepatic autoimmune diseases (EHAIDs). Notably, autoimmune thyroid disease and Sjogren’s syndrome (SS) are the most common EHAIDs. The incidence of EHAIDs in patients with AILD is different in AIH, PBC, PSC and OS [101], and there is a lack of data about the effect of gender on the prevalence of EHAIDs in AILD (Table 1).

Table 1.
The effect of gender on the prevalence of EHAIDs in AILD.
An Italian study of 327 AIH patients, showed a significant prevalence of EHAIDs (69% had pure AIH and 31% had EHAIDs). The prevalence was higher in females (72% of pure AIH cases were female; 90% with EHAIDs were female); the most frequent association was with AITDs, which were found in 51% of the 101 patients with EHAIDs [102]. In a Danish cohort study of 2745 patients with AIH (71% women), the prevalence of EHAIDs was higher in women than in men. Among AIH patients, 193 (7%) had two or more EHAIDs, with a maximum five [103]. Multiple logistic regression analysis with regard to PBC showed that only female gender was significantly associated with positivity for EHA conditions [104]. Cumali Efe et al. collected data of 1554 patients with PBC diagnosis between 1994 and 2017 in 20 centers from Europe, USA and Canada. A total of 35 different EHAIDs were diagnosed in 440 (28.3%) patients with PBC; among these, 358 (23%) had one associated EHAID, and AITDs were the most common (11%). Patients with EHAIDs were more commonly female (93% vs. 86%, p < 0.001) [105]. Another Italian study enrolled 361 PBC patients between 1975 and 2012 (22 males, 339 females); 61% of them had EHAIDs, and 39% had pure PBC. In this study, female patients with EHAIDs accounted for 97% of the sample, whereas male patients accounted for 3%. Female patients with pure PBC accounted for 89% of the sample, whereas male patients with pure PBC accounted for 11% [104]. A recent Chinese retrospective study enrolled 505 patients with PBC (65% with pure PBC, 26% with PBC and SS association and 7% with PBC-AIH). Notably, the proportion of female patients was found to be significantly higher in the PBC-AIH (91%) and the PBC-SS (81%) groups, indicating that female gender was significantly associated with positivity for EHA conditions (p < 0.05) [106]. The opposite is true of PSC, where the development of ulcerative colitis was less common in women than men (48% vs. 61%, respectively; p < 0.001) (9). Globally, 50–80% of patients with PSC have concomitant inflammatory bowel disease [107]. Besides overlap of these AILDs, the observation of concurrent diverse autoimmune diseases has been reported frequently in patients with AIH and PBC. Cumali Efe et al. conducted a study of 71 AIH/PBC patients (58 female, 13 male); 31 had at least one EHAID (AITDs were the most common), but no differences in sex were described [108].
4.2. Impact of Gender on Liver Transplantation for Autoimmune Liver Diseases
To date, a significant number of AILD patients have been reported to eventually progress to end-stage liver disease requiring LT. LT in AILD is indicated when liver failure occurs with complications similar to those for end-stage liver disease caused by other etiologies. With regard to PSC, due to the variability of the course of the disease, timing of transplantation is difficult to predict; the risk of development of malignant disorders in the liver/biliary tract, recurrent bacterial infections in the biliary tract and the impact of inflammatory bowel disease (IBD) are all aspects to consider [109]. In particular, both EASL and AASLD guidelines recommend that patients with biliary dysplasia be considered for transplantation to remove malignant development at an early stage before progression to invasive cholangiocarcinoma. A small group of patients diagnosed with cholangiocarcinoma but with very limited disease can also benefit from transplantation. Selection of patients is crucial, and treatment includes neoadjuvant radiochemotherapy according to protocols [110,111]. An unacceptable quality of life because of severe, treatment-resistant pruritus, severe hepatic encephalopathy or recurrent cholangitis may also merit consideration for transplantation. Fatigue in PBC and other cholestatic liver diseases is often severe and disabling. Cross-sectional studies have shown no evidence of improved fatigue after LT, whereas others demonstrated that fatigue can improve after LT, although only significantly in 50% of cases. Whether this improvement is enough to justify organ allocation in patients with fatigue alone, without liver failure, remains an open issue [111,112]. Finally, among the three AILDs, only AIH presents as acute liver failure and hence qualifies patients for high-urgency (HU) liver transplantation [113]. Sex may affect the severity of autoimmune diseases, the disease course and, consequently, the indication to LT [112,114]. Each of the three liver diseases accounts for 2% to 6% of the indications for LT according to the European Liver Transplant Registry [115]. PSC accounts for about 4–5% of European LTs, with a prevalence that seems to be stable over the years [114]. The proportion of LTs for AIH has also remained stable over the time (2%); in contrast, with regard to PBC, a falling transplant rate was registered in Europe, dropping from 8% (from 1988 to 2001) to 4% (from 2000 to 2009). Reasons for this decline may relate to diagnosis in an earlier stage and more effective treatment [112]. With respect to gender differences, men with PBC are older and seem to have a more severe disease course, a higher incidence of HCC and higher overall mortality compared with female individuals [114]. A ELITA (European Liver Transplantation Association) study including LT patients from 1986 to 2016 found that despite a relative decrease, the absolute number of transplantations for PBC is steady on an annual basis. These patients, predominantly female, are slightly older, have higher MELD scores and are more likely to be male compared to 30 years ago. Males were significantly older than females at the time of transplantation, whereas the MELD score did not differ between the sexes [116]. As regards AIH, male patients present at a younger age and show a higher relapse rate compared with females [114]. Comparing overall mortality and need for LT, men with AIH appear to have better survival compared to female patients. Despite this, the proportion of patients who required LT or died because of liver-related illness was not significantly different [31]. Data related to sex differences in PSC are not particularly thorough. Whereas the literature points toward an almost equal sex distribution and no significant differences in age at diagnosis between male and female patients, information regarding sex differences in disease severity is scarce and contradictory. In an American study based on an international online registry established in 2014 among PSC patients or their caretakers, Kuo et al. compared symptoms, disease progression and treatments of PSC and found, in contrast to previous studies, a higher proportion of female individuals (53%) [117]. In a United Network Organ Sharing study assessing outcome after waitlisting for LT in a set of 8272 adults with PSC, young PSC patients were found to be predominantly male individuals (70%) [118].
4.3. Liver Transplantation and Recurrence
In general, LT in this setting has a favorable overall outcome, with current patient and graft survival for all indications in Europe ranging from 64 to 80% at 5 years, depending on the indication (HCC or not), etiology and severity of the disease [51,112,119]. Nevertheless, all three conditions may recur after transplantation and are associated with an increased risk of both acute cellular and chronic ductopenic rejection.
In PSC, recurrent disease (rPSC) affects 10% to 27% of recipients, with an 8.4% graft loss rate due to recurrent disease. An intriguing, well-documented risk factor for rPSC is the link with IBD. Specifically, the absence of inflammation in the intestine, either due to the absence of concurrent IBD or colectomy before or during LT has a protective effect against (rPSC) [112,120], although not all reports concur with this observation.
Males were found to have more graft failure (due to chronic rejection or PSC recurrence) or experience acute rejection, suggesting that age- or sex-related differences can impact outcomes pre- and post-transplantation [118,119,121]. These results were confirmed by a recent study by Berenguer M. et al., which demonstrated that despite no differences in cause of death, post-transplantation (after year 2000) outcome, particularly graft survival, was worse for men than women (10 y graft survival of 56% for men versus 63% for women). Additional factors associated with worse outcomes presented no differences globally in terms of sex and included older recipient and donor age, the presence of CC at LT, the reduced use of grafts and prolonged ischemia time (only for grafts) [114]. Compared to other autoimmune diseases, rPSC is associated with decreased graft survival, and there is no established treatment either before or after LT. Some centers continue to use UDCA for rPSC, as it improves liver biochemistry; nevertheless, it does not improve survival. Given the unmet therapeutic need of PSC patients in general, other modalities to improve bile acid flow and composition are being actively studied.
In autoimmune hepatitis, recurrence affects approximately 25% of liver allografts during the first 5 years after LT and more than 50% after 10 years of follow-up, with 6% graft lost due to recurrence [119,121]. Long-term outcome and prognosis are more favorable in men with AIH compared to women. The reasons for this remain unknown but may reflect either gender alone or the effect of gender on immune responses [31]. Risk factors for recurrence of AIH (rAIH) after OLT have been assessed in several studies, but most remain unvalidated and controversial. Discontinuation of steroid therapy, HLA-DR locus mismatching, elevated IgG before LT and moderate-to-severe inflammation in the explant are reported as significant risk factors for recurrence of AIH, suggesting that recurrence of autoimmune hepatitis may reflect incomplete suppression of disease activity prior to LT [112,120,122]. The treatment of rAIH is empiric and very much depends on the presentation, which can be variable. In asymptomatic disease and with minimal changes in liver biochemistry or histology, minor adjustments with increased immunosuppression may be sufficient to suppress recurrent disease. In more active rAIH, more potent regimens tend to be employed with either an increased dose, recommencement of corticosteroids and/or addition of immunosuppressive agents. Re-transplantation may be required for patients with rAIH who present with liver failure and graft loss [120].
Recurrent PBC is reported in a range from 17% to 42% after LT; however, in contrast to AIH and PSC, graft loss due to recurrent disease is not a major issue in PBC (1.3%) [119]. Sex differences among PBC recurrence are lacking. Several studies have shown that tacrolimus-based immunosuppression is associated with an increased risk of recurrence of PBC, with a reduced time to recurrence compared with ciclosporin. The role of genetic factors has not been investigated thoroughly. The human leukocyte antigen (HLA) profile and HLA donor–recipient mismatch have a controversial association in rPBC [120,122]. Case series have reported that the development of rPBC has little impact on long-term survival or need for re-transplantation. However, this may be related to the small number and short follow-up of patients with rPBC. To date, ursodeoxycholic acid (UDCA) is the only drug accepted for the treatment of patients with PBC and is generally employed after a diagnosis of rPBC has been established. The use of UDCA has been associated with improved liver biochemistry tests in patients with rPBC; however, we lack data documenting a delay in histological progression or improvement in graft and patient survival [120].
4.4. De Novo Autoimmune Hepatitis after Liver Transplantation
De novo AIH is a clinical entity resembling AIH that develops in LT recipients transplanted for other liver disorders. It shows characteristics atypical for AIH, including lymphocytic cholangitis, central perivenulitis and other features consistent with T-cell-mediated rejection. It was originally described in children after LT, predominantly in those with biliary atresia, and was subsequently found in a higher prevalence of LT recipients with PBC. The incidence of de novo AIH is variable because multiple descriptions have been used in case series; however, the disease is rare and does not appear to have an impact on long-term survival. Recipients of female grafts and older donors have a higher prevalence of de novo AIH, suggesting that the risk of AIH may be harbored in the allograft. With regard to immunosuppression, patients maintained on tacrolimus or mycophenolate mofetil have a higher risk of developing de novo AIH, whereas LT recipients treated with ciclosporin have a reduced risk [123].
5. Conclusions
The mechanisms behind the sex differences observed in autoimmune liver diseases, specifically the female predominance in AIH and PBC; the worse disease course in male PBC; male predominance in PSC patients with a lower risk of UC; and cholangiocarcinoma among female patients remain largely unknown. Understanding the effects of sex-related genes and intestinal microbiota underlying AILD immune dysregulation, as well as the role of sex hormones in immune cells, may pave the way for novel treatment strategies for AILD.
In PBC patients, the frequent delay in diagnosis plays an important role among the male sex, leading to more advanced liver disease at PBC presentation; consequently, the risk of ACLD, liver decompensation, HCC development and mortality is higher in male than in female patients, with worse biochemical response rates. Delayed diagnosis could be partially explained by the lower incidence of PBC in male patients, leading clinicians to not consider the disease as a first choice, was well the minor presence of PBC-related symptoms. Despite its rarity, the diagnosis of PBC should be considered in men with elevated cholestatic parameters maintaining a high index of suspicion for PBC to prevent diagnostic delays.
The association between AILDs and the spectrum of EHAIDs seems to be more common in women (Table 1). Further specifically designed studies are needed about the gender impact on EHAID prevalence in patients with AILDs.
In conclusion, gender seems to have an impact on LT in ALD; however, the sex differences with respect to the prevalence, incidence, pathogenesis, risk factors, long-term survival and prognosis in this setting have not been sufficiently explored and require further investigations.
Author Contributions
Writing—original draft preparation, all authors; writing—review and editing, F.I., M.C.; supervision, A.F.; project administration, F.I., M.C.; funding acquisition, P.B. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Department of Surgery, Oncology and Gastroenterology (DiSCOG)-University of Padua, Italy.
Acknowledgments
Membership of the Special Interest Group Gender in Hepatology of the Italian Association for the Study of the Liver (AISF): Patrizia Burra, Maurizia R. Brunetto, Annarosa Floreani, Fabio Marra, Filomena Morisco, Teresa Pollicino, Gloria Taliani, Erica Villa, Alessio Aghemo.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Czaja, A.J.; Manns, M.P. Advances in the diagnosis, pathogenesis, and management of autoimmune hepatitis. Gastroenterology 2010, 139, 58–72.e4. [Google Scholar] [CrossRef] [PubMed]
- Bogdanos, D.P.; Choudhuri, K.; Vergani, D. Molecular mimicry and autoimmune liver disease: Virtuous intentions, malign consequences. Liver 2001, 21, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Coss Adame, E.; Granados, J.; Uribe, M.; Torre, A. Does HLA-DR7 differentiate the overlap syndrome of auto-immune hepatitis-primary biliary cirrhosis (AIH-PBC) from those with auto-immune hepatitis type 1? Ann. Hepatol. 2011, 10, 28–32. [Google Scholar] [PubMed]
- Manns, M.P.; Czaja, A.J.; Gorham, J.D.; Krawitt, E.L.; Mieli-Vergani, G.; Vergani, D.; Vierling, J.M. Diagnosis and management of autoimmune hepatitis. Hepatology 2010, 51, 2193–2219. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, G.V.; Portmann, B.; Reid, F.; Donaldson, P.T.; Doherty, D.G.; McCartney, M.; Mowat, A.P.; Vergani, D.; Milei-Vergani, G. Autoimmune hepatitis in childhood: A 20-year experience. Hepatology 1997, 25, 541–547. [Google Scholar] [CrossRef]
- Radhakrishnan, K.R.; Alkhouri, N.; Worley, S.; Arrigain, S.; Hupertz, V.; Kay, M.; Yerian, L.; Wyllie, R.; Feldstein, A.E. Autoimmune hepatitis in children—Impact of cirrhosis at presentation on natural history and long-term outcome. Dig. Liver Dis. 2010, 42, 724–728. [Google Scholar] [CrossRef]
- Deneau, M.; Jensen, M.K.; Holmen, J.; Williams, M.S.; Book, L.S.; Guthery, S.L. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: Epidemiology and natural history. Hepatology 2013, 58, 1392–1400. [Google Scholar] [CrossRef]
- Jimenez-Rivera, C.; Ling, S.C.; Ahmed, N.; Yap, S.; Aglipay, M.; Borrowman, N.; Graitson, S.; Critch, J.; Rashid, M.; Ng, V.L.; et al. Incidence and characteristics of autoimmune hepatitis. Pediatrics 2015, 136, e1237–e1248. [Google Scholar] [CrossRef] [Green Version]
- Czaja, A.J.; Carpenter, H.A.; Santrach, P.J.; Moore, S.B.; Taswell, H.F.; Homburger, H.A. Evidence against hepatitis viruses as important causes of severe autoimmune hepatitis in the United States. J. Hepatol. 1993, 18, 342–352. [Google Scholar] [CrossRef]
- Boberg, K.M.; Aadland, E.; Jahnsen, J.; Raknerud, N.; Stiris, M.; Bell, H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand. J. Gastroenterol. 1998, 33, 99–103. [Google Scholar] [CrossRef]
- Werner, M.; Prytz, H.; Ohlsson, B.; Almer, S.; Bjornsson, E.; Berquist, A.; Wallerstedt, S.; Sandberg-Gertzén, H.; Hultcrantz, R.; Sangfelt, P.; et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: A nationwide study. Scand. J. Gastroenterol. 2008, 43, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Ngu, J.H.; Bechly, K.; Chapman, B.A.; Burt, M.J.; Barclay, M.L.; Gearry, R.B.; Stedman, C.A.M. Population-based epidemiology study of autoimmune hepatitis: A disease of older women? J. Gastroenterol. Hepatol. 2010, 25, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Gronbaek, L.; Vilstrup, H.; Jepsen, P. Autoimmune hepatitis in Denmark: Incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J. Hepatol. 2014, 60, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Van Gerven, N.M.; Vermer, N.J.; Witte, B.I.; van Erpecum, K.J.; van Buuren, H.R.; Maijers, I.; Visscher, A.P.; Verschuren, E.C.; van Hoek, B.; Coenraad, M.J.; et al. Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Scand. J. Gastroenterol. 2014, 49, 1245–1254. [Google Scholar] [CrossRef]
- Tanaka, A.; Mori, M.; Matsumoto, K.; Ohiza, H.; Takazuma, S.; Takikawa, H. Increase trend in the prevalence and to male-to-female ratio of primary biliary cholangitis, autoimmune hepatitis and primary sclerosing cholangitis in Japan. Hepatol. Res. 2019, 49, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Muratori, P.; Carbone, M.; Stangos, G.; Perini, L.; Lalanne, C.; Ronca, V.; Cazzagon, N.; Bianchi, G.; Lenzi, M.; Floreani, A.; et al. Clinical and prognostic implications of acute onset of autoimmune hepatitis: An Italian multicentre study. Dig. Liver Dis. 2018, 50, 698–702. [Google Scholar] [CrossRef]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.Y.; Buenafe, A.C.; Matejuk, A.; Ito, A.; Zamora, A.; Dwyer, J.; Vandenbark, A.A.; Offner, H. Estrogens inhibition of EAE involves effects on dendritic cell function. J. Neurosci. Res. 2002, 70, 238–248. [Google Scholar] [CrossRef]
- Asselta, R.; Paraboschi, E.M.; Gerussi, A.; Cordell, H.J.; Mells, G.F.; Sandford, R.N.; Jones, D.E.; Nakamura, M.; Ueno, K.; Hitomi, Y. X chromosome contribution to the genetic architecture of primary biliary cholangitis. Gastroenterology 2021, 160, 2483–2495. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Ma, X.; Tang, R. Epigenetics of autoimmune liver diseases: Current progress and future directions. J. Bio-X Res. 2019, 2, 46–55. [Google Scholar] [CrossRef]
- Pinheiro, I.; Dejager, L.; Libert, C. X-chromosome-located microRNAs in immunity: Might they explain male/female differences? Bioassays 2011, 33, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Di Palo, A.; Siniscalchi, C.; Salerno, M.; Russo, A.; Gravholt, C.H.; Potenza, N. What microRNAs could tell us about the X chromosome. Cell Mol. Life Sci. 2020, 77, 4069–4080. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, F.-B.; Chen, D.-Z.; Wu, J.-L.; Hu, E.; Xu, L.M.; Zheng, M.-H.; Li, H.; Huang, Y.; Jin, X.-Y. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol. Immunol. 2018, 93, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Zhang, T.; Lou, G.; Liu, Y. Role of miR-223 in the pathophysiology of liver diseases. Exp. Mol. Med. 2018, 50, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Xing, X.; Xiang, X.; Fan, X.; Men, R.; Ye, T.; Yang, L. MicroRNAs in autoimmune liver diseases: From diagnosis to potential therapeutic targets. Biomed. Pharmacother. 2020, 13, 110558. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Tansel, A.; Katz, L.H.; El-Serag, H.B.; Thrift, A.P.; Parepally, M.; Shakhatreh, M.H.; Kanwal, F. Incidence and determinants of hepatocellular carcinoma in autoimmune hepatitis: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 1207–1217. [Google Scholar] [CrossRef] [Green Version]
- Czaja, A.J. Hepatocellular carcinoma and other malignancies in autoimmune hepatitis. Dig. Dis. Sci. 2013, 58, 1459–1476. [Google Scholar] [CrossRef]
- Yeoman, A.D.; Al-Chalabi, T.; Karani, J.B.; Quaglia, A.; Devlin, J.; Mieli-Vergani, G.; Bomford, A.; O’Grady, J.G.; Harrison, P.M.; Heneghan, M.A. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: Implication for follow-up and screening. Hepatology 2008, 48, 863–870. [Google Scholar] [CrossRef]
- Al-Chalabi, T.; Underhill, J.A.; Portmann, B.C.; McFarlane, I.G.; Heneghan, M.A. Impact of gender on the long-term survival of patients with autoimmune hepatitis. J. Hepatol. 2008, 48, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, M.M.; Metzler, F.; Geiger, E.; Einrich, E.; Hallensleben, M.; Manns, M.P.; Vogel, A. Prediction of short- and long-term outcome in patients with autoimmune hepatitis. Hepatology 2015, 62, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Karkhanis, J.; Verna, E.C.; Chang, M.S.; Stravitz, R.T.; Schilsky, M.; Lee, W.M.; Brown, R.S., Jr.; Acute Liver Failure Study Group. Steroid use in acute liver failure. Hepatology. 2014, 59, 612–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachou, K.; Arvaniti, P.; Azariadis, K.; Lygoura, V.; Gatselis, N.K.; Lyberopoulou, A.; Koukoulis, G.K.; Dalekos, G.N. Prompt initiation of high-dose i.v. corticosteroids seems to prevent progression to liver failure in patients with original acute severe autoimmune hepatitis. Hepatol. Res. 2019, 49, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czaja, A.J. Drug-induced autoimmune-like hepatitis. Dig. Dis. Sci. 2011, 56, 958–976. [Google Scholar] [CrossRef]
- De Boer., Y.S.; Kosinski, A.S.; Urban, T.J.; Zhao, Z.; Long, N.; Chalasani, N.; Kleiner, D.E.; Hoofnagle, J.H. Features of autoimmune hepatitis in patients with drug-induced liver injury. Clin. Gastroenterol. Hepatol. 2017, 15, 103–112.e2. [Google Scholar] [CrossRef] [Green Version]
- Bjornsson, E.S.; Bergmann, O.; Jonasson, J.G.; Grondel, G.; Gudbjornsson, B.; Olafsson, S. Drug-induced autoimmune hepatitis: Response to corticosteroids and lack of relapse after cessation of steroids. Clin. Gastroenterol. Hepatol. 2017, 15, 1635–1636. [Google Scholar] [CrossRef] [Green Version]
- Myers, G. Immune-related adverse events of immune checkpoint inhibitors: A brief review. Curr. Oncol. 2018, 25, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Doherty, G.J.; Duckworth, A.M.; Davies, S.E.; Mells, G.F.; Brais, R.; Harden, S.V.; Parkinson, C.A.; Corrie, P.G. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO Open 2017, 2, e000268. [Google Scholar] [CrossRef] [Green Version]
- Carbone, M.; Mells, G.F.; Pells, G.; Dawwas, M.F.; Newton, J.L.; Heneghan, M.A.; Neuberger, J.M.; Day, D.B.; Ducker, S.J.; UK PBC Consortium; et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology 2013, 144, 560–569.e7. [Google Scholar] [CrossRef]
- Ali, A.H.; Sinakos, E.; Silveira, M.G.; Jorgensen, R.A.; Angulo, P.; Lindor, K.D. Varices in early histological stage primary biliary cirrhosis. J. Clin. Gastroenterol. 2011, 45, e66–e71. [Google Scholar] [CrossRef] [PubMed]
- Marschall, H.U.; Henriksson, I.; Lindberg, S.; Söderdahl, F.; Thuresson, M.; Wahlin, S.; Ludvigsson, J.F. Incidence, prevalence, and outcome of primary biliary cholangitis in a nationwide Swedish population-based cohort. Sci. Rep. 2019, 9, 11525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drazilova, S.; Babinska, I.; Gazda, J.; Halanova, M.; Janicko, M.; Kucinsky, B.; Safcak, D.; Martinkova, D.; Tarbajova, L.; Cekanova, A.; et al. Epidemiology and clinical course of primary biliary cholangitis in Eastern Slovakia. Int. J. Public Health 2020, 65, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Duan, W.; Li, M.; Li, S.; Lv, T.; Tian, Q.; Wang, Q.; Wu, X.; Zhao, X.; Wang, X.; et al. Prognosis of 732 ursodeoxycholic acid-treated patients with primary biliary cholangitis: A single center follow-up study from China. J. Gastroenterol. Hepatol. 2019, 34, 1236–1241. [Google Scholar] [CrossRef]
- Abdulkarim, M.; Zenouzi, R.; Sebode, M.; Schulz, L.; Quaas, A.; Lohse, A.W.; Schramm, C.; Weiler-Normann, C. Sex differences in clinical presentation and prognosis in patients with primary biliary cholangitis. Scand. J. Gastroenterol. 2019, 54, 1391–1396. [Google Scholar] [CrossRef]
- Yagi, M.; Tanaka, A.; Abe, M.; Namisaki, T.; Yoshiji, H.; Takahashi, A.; Ohira, H.; Komori, A.; Yamagiwa, S.; Kikuchi, K.; et al. Symptoms and health-related quality of life in Japanese patients with primary biliary cholangitis. Sci. Rep. 2018, 8, 12542. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.S.; Seto, W.K.; Fung, J.; Lai, C.L.; Yuen, M.F. Epidemiology and Natural History of Primary Biliary Cholangitis in the Chinese: A Territory-Based Study in Hong Kong between 2000 and 2015. Clin. Transl. Gastroenterol. 2017, 8, e116. [Google Scholar] [CrossRef] [Green Version]
- Gatselis, N.K.; Zachou, K.; Lygoura, V.; Azariadis, K.; Arvaniti, P.; Spyrou, E.; Papadamou, G.; Koukoulis, G.K.; Dalekos, G.N.; Rigopoulou, E.I. Geoepidemiology, clinical manifestations and outcome of primary biliary cholangitis in Greece. Eur. J. Intern. Med. 2017, 42, 81–88. [Google Scholar] [CrossRef]
- Kim, K.A.; Ki, M.; Choi, H.Y.; Kim, B.H.; Jang, E.S.; Jeong, S.H. Population-based epidemiology of primary biliary cirrhosis in South Korea. Aliment. Pharmacol. Ther. 2016, 43, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Boonstra, K.; Bokelaar, R.; Stadhouders, P.H.; Tuynman, H.A.; Poen, A.C.; van Nieuwkerk, K.M.; Witteman, E.M.; Hamann, D.; Witteman, B.J.; Beuers, U.; et al. Increased cancer risk in a large population-based cohort of patients with primary biliary cirrhosis: Follow-up for up to 36 years. Hepatol. Int. 2014, 8, 266–274. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Golabi, P.; Epstein, R.S.; Strauss, M.E.; Nader, F.; Racila, A. Factors Associated with Potential Progressive Course of Primary Biliary Cholangitis: Data from Real-world US Database. J. Clin. Gastroenterol. 2019, 53, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Marzioni, M.; Bassanelli, C.; Ripellino, C.; Urbinati, D.; Alvaro, D. Epidemiology of primary biliary cholangitis in Italy: Evidence from a real-world database. Dig. Liver Dis. 2019, 51, 724–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammers, W.J.; Hirschfield, G.M.; Corpechot, C.; Nevens, F.; Lindor, K.D.; Janssen, H.L.; Floreani, A.; Ponsioen, C.Y.; Mayo, M.J.; Invernizzi, P.; et al. Development and Validation of a Scoring System to Predict Outcomes of Patients with Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology 2015, 149, 1804–1812.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Shi, T.Y.; Shi, X.H.; Wang, L.; Yang, Y.J.; Liu, B.; Gao, L.X.; Shuai, Z.W.; Kong, F.; Chen, H.; et al. Early biochemical response to ursodeoxycholic acid and long-term prognosis of primary biliary cirrhosis: Results of a 14-year cohort study. Hepatology 2013, 58, 264–272. [Google Scholar] [CrossRef]
- John, B.V.; Aitcheson, G.; Schwartz, K.B.; Khakoo, N.S.; Dahman, B.; Deng, Y.; Goldberg, D.; Martin, P.; Taddei, T.H.; Levy, C.; et al. Male Sex Is Associated with Higher Rates of Liver-Related Mortality in Primary Biliary Cholangitis and Cirrhosis. Hepatology 2021, 74, 879–891. [Google Scholar] [CrossRef]
- Cheung, A.C.; Lammers, W.J.; Murillo Perez, C.F.; van Buuren, H.R.; Gulamhusein, A.; Trivedi, P.J.; Lazaridis, K.N.; Ponsioen, C.Y.; Floreani, A.; Hirschfield, G.M.; et al. Effects of Age and Sex of Response to Ursodeoxycholic Acid and Transplant-free Survival in Patients with Primary Biliary Cholangitis. Clin. Gastroenterol. Hepatol. 2019, 17, 2076–2084.e2. [Google Scholar] [CrossRef]
- Lammert, C.; Juran, B.D.; Schlicht, E.; Chan, L.L.; Atkinson, E.J.; de Andrade, M.; Lazaridis, K.N. Biochemical response to ursodeoxycholic acid predicts survival in a North American cohort of primary biliary cirrhosis patients. J. Gastroenterol. 2014, 49, 1414–1420. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Liu, Y.; Sun, K.; Zhou, X.; Ma, S.; Zhang, M.; Zhou, X.; Wang, L.; Han, Y. A nomogram based on pretreatment clinical parameters for the prediction of inadequate biochemical response in primary biliary cholangitis. J. Clin. Lab. Anal. 2020, 34, e23501. [Google Scholar] [CrossRef]
- Melchor-Mendoza, Y.K.; Martínez-Benítez, B.; Mina-Hawat, A.; Rodríguez-Leal, G.; Duque, X.; Moran-Villota, S. Ursodeoxycholic Acid Therapy in Patients with Primary Biliary Cholangitis with Limited Liver Transplantation Availability. Ann. Hepatol. 2017, 16, 430–435. [Google Scholar] [CrossRef]
- Delgado, J.S.; Vodonos, A.; Delgado, B.; Jotkowitz, A.; Rosenthal, A.; Fich, A.; Novack, V. Primary biliary cirrhosis in Southern Israel: A 20 year follow up study. Eur. J. Intern. Med. 2012, 23, e193–e198, Erratum in Lancet 2015, 386, 1536. [Google Scholar] [CrossRef]
- Chen, J.; Xue, D.; Gao, F.; Tao, L.; Li, Y.; Zhang, Q.; Wang, R.; Sun, L.; Yang, X.; Liu, Y.; et al. Influence factors and a predictive scoring model for measuring the biochemical response of primary biliary cholangitis to ursodeoxycholic acid treatment. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Madir, A.; Božin, T.; Mikolašević, I.; Milić, S.; Štimac, D.; Mijić, M.; Filipec Kanižaj, T.; Biloglav, Z.; Lucijanić, M.; Lucijanić, I.; et al. Epidemiological and clinical features of primary biliary cholangitis in two Croatian regions: A retrospective study. Croat. Med. J. 2019, 60, 494–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortez-Pinto, H.; Liberal, R.; Lopes, S.; Machado, M.V.; Carvalho, J.; Dias, T.; Santos, A.; Agostinho, C.; Figueiredo, P.; Loureiro, R.; et al. Predictors for incomplete response to ursodeoxycholic acid in primary biliary cholangitis. Data from a national registry of liver disease. United Eur. Gastroenterol. J. 2021, 9, 699–706. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Clinical Practice Guidelines: The Diagnosis and Management of Patients with Primary Biliary Cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.B.; Ismail, M.; Kanagalingam, G.; Mason, A.L.; Swain, M.G.; Vincent, C.; Yoshida, E.M.; Tsien, C.; Flemming, J.A.; Janssen, H.L.A.; et al. Real-World Effectiveness of Obeticholic Acid in Patients with Primary Biliary Cholangitis. Hepatol. Commun. 2020, 4, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Garcia Buey, L.; Molina, E.; Casado, M.; Conde, I.; Berenguer, M.; Jorquera, F.; Simón, M.A.; Olveira, A.; Hernández-Guerra, M.; et al. Effectiveness and safety of obeticholic acid in a Southern European multicentre cohort of patients with primary biliary cholangitis and suboptimal response to ursodeoxycholic acid. Aliment Pharmacol. Ther. 2021, 53, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.H.; Hirschfield, G.M.; Floreani, A.; Mayo, M.J.; Parés, A.; Liberman, A.; Malecha, E.S.; Pencek, R.; MacConell, L.; Hansen, B.E. Obeticholic acid is associated with improvements in AST-to-platelet ratio index and GLOBE score in patients with primary biliary cholangitis. JHEP Rep. 2020, 3, 100191. [Google Scholar] [CrossRef]
- D’Amato, D.; De Vincentis, A.; Malinverno, F.; Viganò, M.; Alvaro, D.; Pompili, M.; Picciotto, A.; Palitti, V.P.; Russello, M.; Storato, S.; et al. Real-world experience with obeticholic acid in patients with primary biliary cholangitis. JHEP Rep. 2021, 3, 100248. [Google Scholar] [CrossRef]
- Natarajan, Y.; Tansel, A.; Patel, P.; Emologu, K.; Shukla, R.; Qureshi, Z.; El-Serag, H.B.; Thrift, A.P.; Kanwal, F. Incidence of Hepatocellular Carcinoma in Primary Biliary Cholangitis: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2021, 66, 2439–2451. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Lammers, W.J.; van Buuren, H.R.; Parés, A.; Floreani, A.; Janssen, H.L.; Invernizzi, P.; Battezzati, P.M.; Ponsioen, C.Y.; Corpechot, C.; et al. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: A multicentre international study. Gut 2016, 65, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Harada, K.; Hirohara, J.; Ueno, Y.; Nakano, T.; Kakuda, Y.; Tsubouchi, H.; Ichida, T.; Nakanuma, Y. Incidence of and risk factors for hepatocellular carcinoma in primary biliary cirrhosis: National data from Japan. Hepatology 2013, 57, 1942–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adejumo, A.C.; Akhtar, D.H.; Dennis, B.B.; Cholankeril, G.; Alayo, Q.; Ogundipe, O.A.; Kim, D.; Ahmed, A. Gender and Racial Differences in Hospitalizations for Primary Biliary Cholangitis in the USA. Dig. Dis. Sci. 2021, 66, 1461–1476. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, T.; Shen, Y.; Xi, X.; Yang, L. Underestimated Male Prevalence of Primary Biliary Cholangitis in China: Results of a 16-yr cohort study involving 769 patients. Sci. Rep. 2017, 7, 6560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lleo, A.; Jepsen, P.; Morenghi, E.; Carbone, M.; Moroni, L.; Battezzati, P.M.; Podda, M.; Mackay, I.R.; Gershwin, M.E.; Invernizzi, P. Evolving Trends in Female to Male Incidence and Male Mortality of Primary Biliary Cholangitis. Sci. Rep. 2016, 6, 25906. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, Y.; Haller, I.V.; Romanelli, R.J.; VanWormer, J.J.; Rodriguez, C.V.; Anderson, H.; Boscarino, J.A.; Schmidt, M.A.; Daida, Y.G.; et al. Increasing Prevalence of Primary Biliary Cholangitis and Reduced Mortality with Treatment. Clin. Gastroenterol. Hepatol. 2018, 16, 1342–1350.e1. [Google Scholar] [CrossRef] [Green Version]
- Sayiner, M.; Golabi, P.; Stepanova, M.; Younossi, I.; Nader, F.; Racila, A.; Younossi, Z.M. Primary Biliary Cholangitis in Medicare Population: The Impact on Mortality and Resource Use. Hepatology 2019, 69, 237–244. [Google Scholar] [CrossRef]
- Lindkvist, B.; Benito de Valle, M.; Gullberg, B.; Bjornsson, E. Incidence and Prevalence of Primary SclerosingCholangitis in a Defined Adult Population in Sweden. Hepatology 2010, 52, 571–577. [Google Scholar] [CrossRef]
- Lamba, M.; Hieng Ngu, J.; Stedman, C.A.M. Trends in Incidence of Autoimmune Liver Diseases and Increasing Incidence of Autoimmune Hepatitis. Clin. Gastroenterol. Hepatol. 2020, 19, 573–579.e1. [Google Scholar] [CrossRef]
- Bakhshi, Z.; Hilscher, M.B.; Gores, G.J.; Harmsen, W.S.; Viehman, J.K.; LaRusso, N.F.; Gossard, A.A.; Lazaridis, K.N.; Lindor, K.D.; Eaton, J.E. An update on primary sclerosing cholangitis epidemiology, outcomes and quantification of alkaline phosphatase variability in a population-based cohort. J. Gastroenterol. 2020, 55, 523–532. [Google Scholar] [CrossRef]
- Carbone, M.; Kodra, Y.; Rocchetti, A.; Manno, V.; Minelli, G.; Gerussi, A.; Ronca, V.; Malinverno, F.; Cristoferi, L.; Floreani, A.; et al. Primary Sclerosing Cholangitis: Burden of Disease and Mortality Using Data from the National Rare Diseases Registry in Italy. Int. J. Environ. Res. Public Health 2020, 17, 3095. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Crothers, H.; Mytton, J.; Bosch, S.; Iqbal, T.; Ferguson, J.; Hirschfield, G.M. Effects of Primary Sclerosing Cholangitis on Risks of Cancer and Death in People with Inflammatory Bowel Disease, Based on Sex, Race, and Age. Gastroenterology 2020, 159, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Gores, G.J. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013, 145, 1215–1229. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, M.; La Russo, N.F.; Burgart, L.J.; Gores, G.J. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000, 60, 184–190. [Google Scholar] [PubMed]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, D.; Ekbom, A.; Ihre, T. Survival and risk of cholangiocarcinoma in patients with primary sclerosing cholangitis. A population-based study. Scand. J. Gastroenterol. 1997, 32, 1042–1045. [Google Scholar] [CrossRef]
- Weismüller, T.J.; Trivedi, P.J.; Bergquist, A.; Imam, M.; Lenzen, H.; Ponsioen, C.Y.; Holm, K.; Gotthardt, D.; Färkkilä, M.A.; Marschall, H.U.; et al. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate with Course of Primary Sclerosing Cholangitis. Gastroenterology 2017, 152, 1975–1984.e8. [Google Scholar] [CrossRef] [Green Version]
- Soetikno, R.M.; Lin, O.S.; Heidenreich, P.A.; Young, H.S.; Blackstone, M.O. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: A meta-analysis. Gastrointest. Endosc. 2002, 56, 48–54. [Google Scholar] [CrossRef]
- Zheng, H.H.; Jiang, X.L. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: A meta-analysis of 16 observational studies. Eur. J. Gastroenterol. Hepatol. 2016, 28, 383–390. [Google Scholar] [CrossRef]
- Karlsen, T.H. Primary sclerosing cholangitis: 50 years of a gut-liver relationship and still no love? Gut 2016, 65, 1579–1581. [Google Scholar] [CrossRef]
- Rupp, C.; Rössler, A.; Zhou, T.; Rauber, C.; Friedrich, K.; Wannhoff, A.; Weiss, K.H.; Sauer, P.; Schirmacher, P.; Süsal, C.; et al. Impact of age at diagnosis on disease progression in patients with primary sclerosing cholangitis. United Eur. Gastroenterol. J. 2018, 6, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Guerra, I.; Bujanda, L.; Castro, J.; Merino, O.; Tosca, J.; Camps, B.; Gutiérrez, A.; Gordillo Ábalos, J.; de Castro, L.; Iborra, M.; et al. Clinical Characteristics, Associated Malignancies and Management of Primary Sclerosing Cholangitis in Inflammatory Bowel Disease Patients: A Multicentre Retrospective Cohort Study. J. Crohns Colitis 2019, 13, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.J.; Ali, A.H.; Lindor, K.D. Primary biliary cirrhosis. Lancet 2015, 386, 1565–1575. [Google Scholar] [CrossRef]
- Floreani, A.; Franceschet, I.; Cazzagon, N. Primary biliary cirrhosis: Overlaps with other autoimmune disorders. Semin. Liver Dis. 2014, 34, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.L.; Danford, C.J.; Patwardhan, V.; Bonder, A. Treatment of overlap syndromes in autoimmune liver disease: A systematic review and meta-analysis. J. Clin. Med. 2020, 9, 1449. [Google Scholar] [CrossRef]
- Gregorio, G.V.; Portmann, B.; Karani, J.; Harrison, P.; Donaldson, P.T.; Vergani, D.; Mieli-Vergani, G. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: A 16-year prospective study. Hepatology 2001, 33, 544–553. [Google Scholar] [CrossRef]
- Abdalian, R.; Dhar, P.; Jhaveri, K.; Haider, M.; Guindi, M.; Heathcote, E.J. Prevalence of sclerosing cholangitis in adults with autoimmune hepatitis: Evaluating the role of routine magnetic resonance imaging. Hepatology 2008, 47, 949–957. [Google Scholar] [CrossRef]
- Al-Chalabi, T.; Portmann, B.C.; Bernal, W.; McFarlane, I.G.; Heneghan, M.A. Autoimmune hepatitis overlap syndromes: An evaluation of treatment response, long-term outcome and survival. Aliment Pharmacol. Ther. 2008, 28, 209–220. [Google Scholar] [CrossRef]
- Gheorghe, L.; Iacob, S.; Gheorghe, C.; Iacob, R.; Simionov, I.; Vadan, R.; Becheanu, G.; Parvulescu, I.; Toader, C. Frequency and predictive factors for overlap syndrome between autoimmune hepatitis and primary cholestatic liver disease. Eur. J. Gastroenterol. Hepatol. 2004, 16, 585–592. [Google Scholar] [CrossRef]
- Lüth, S.; Kanzler, S.; Frenzel, C.; Kasper, H.U.; Dienes, H.P.; Schramm, C.; Galle, P.R.; Herkel, J.; Lohse, A.W. Characteristics and long-term prognosis of the autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. J. Clin. Gastroenterol. 2009, 43, 75–80. [Google Scholar] [CrossRef]
- Chayanupatkul, M.; Fiel, M.I.; Schiano, T.D. The clinical characteristics, pre- and post-liver transplantation outcomes in patients having autoimmune overlap syndromes. Clin. Transplant. 2020, 34, e13841. [Google Scholar] [CrossRef]
- Guo, L.; Zhou, L.; Zhang, N.; Deng, B.; Wang, B. Extrahepatic Autoimmune Diseases in Patients with Autoimmune Liver Diseases: A Phenomenon Neglected by Gastroenterologists. Gastroenterol. Res. Pract. 2017, 2017, 2376231. [Google Scholar] [CrossRef]
- Muratori, P.; Fabbri, A.; Lalanne, C.; Lenzi, M.; Muratori, L. Autoimmune liver disease and concomitant extrahepatic autoimmune disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Grønbaek, L.; Vilstrup, H.; Pedersen, L.; Jepsen, P. Extrahepatic autoimmune diseases in patients with autoimmune hepatitis and their relatives: A Danish nationwide cohort study. Liver Int. 2019, 39, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Floreani, A.; Franceschet, I.; Cazzagon, N.; Spinazzè, A.; Buja, A.; Furlan, P.; Baldo, V.; Gershwin, M.E. Extrahepatic autoimmune conditions associated with primary biliary cirrhosis. Clin. Rev. Allergy Immunol. 2015, 48, 192–197. [Google Scholar] [CrossRef]
- Efe, C.; Torgutalp, M.; Henriksson, I.; Alalkim, F.; Lytvyak, E.; Trivedi, H.; Eren, F.; Fischer, J.; Chayanupatkul, M.; Coppo, C.; et al. Extrahepatic autoimmune diseases in primary biliary cholangitis: Prevalence and significance for clinical presentation and disease outcome. J. Gastroenterol. Hepatol. 2021, 36, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, K.; Liu, C.; Duan, F.; Cheng, J.; Yang, S. Clinical Characteristics and Prognosis of Concomitant Primary Biliary Cholangitis and Autoimmune Diseases: A Retrospective Study. Can. J. Gastroenterol. Hepatol. 2021, 2021, 5557814. [Google Scholar] [CrossRef] [PubMed]
- Núñez, F.P.; Quera, P.R.; Gomollón, F. Primary sclerosing cholangitis and inflammatory bowel disease: Intestine-liver interrelation. Gastroenterol. Hepatol. 2019, 42, 316–325, (In English and Spanish). [Google Scholar] [CrossRef]
- Efe, C.; Wahlin, S.; Ozaslan, E.; Berlot, A.H.; Purnak, T.; Muratori, L.; Quarneti, C.; Yüksel, O.; Thiéfin, G.; Muratori, P. Autoimmune hepatitis/primary biliary cirrhosis overlap syndrome and associated extrahepatic autoimmune diseases. Eur. J. Gastroenterol. Hepatol. 2012, 24, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Neuberger, J. Liver transplantation in PBC and PSC: Indications and disease recurrence. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 446–454. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. [Google Scholar] [CrossRef]
- Chapman, R.; Fevery, J.; Kalloo, A.; Nagorney, D.M.; Boberg, K.M.; Shneider, B.; Gores, G.J.; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010, 51, 660–678. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Neuberger, J.M. Autoimmune liver disease, autoimmunity and liver transplantation. J. Hepatol. 2014, 60, 210–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for the Study of the Liver. EASL Clinical Practical Guidelines on the Management of Acute (Fulminant) Liver Failure. J. Hepatol. 2017, 66, 1047–1081. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, M.; Di Maira, T.; Baumann, U.; Mirza, D.F.; Heneghan, M.A.; Klempnauer, J.L.; Bennet, W.; Ericzon, B.G.; Line, P.D.; Lodge, P.A.; et al. Characteristics, Trends, and Outcomes of Liver Transplantation for Primary Sclerosing Cholangitis in Female Versus Male Patients: An Analysis from the European Liver Transplant Registry. Transplantation 2021, 105, 2255–2262. [Google Scholar] [CrossRef]
- Adam, R.; Karam, V.; Delvart, V.; O’Grady, J.; Mirza, D.; Klempnauer, J.; Castaing, D.; Neuhaus, P.; Jamieson, N.; Salizzoni, M.; et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J. Hepatol. 2012, 57, 675–688. [Google Scholar] [CrossRef] [Green Version]
- Harms, M.H.; Janssen, Q.P.; Adam, R.; Duvoux, C.; Mirza, D.; Hidalgo, E.; Watson, C.; Wigmore, S.J.; Pinzani, M.; Isoniemi, H.; et al. Trends in liver transplantation for primary biliary cholangitis in Europe over the past three decades. Aliment Pharmacol. Ther. 2019, 49, 285–295. [Google Scholar] [CrossRef]
- Kuo, A.; Gomel, R.; Safer, R.; Lindor, K.D.; Everson, G.T.; Bowlus, C.L. Characteristics and Outcomes Reported by Patients with Primary Sclerosing Cholangitis through an Online Registry. Clin. Gastroenterol. Hepatol. 2019, 17, 1372–1378. [Google Scholar] [CrossRef] [Green Version]
- Henson, J.B.; Patel, Y.A.; Wilder, J.M.; Zheng, J.; Chow, S.C.; King, L.Y.; Muir, A.J. Differences in Phenotypes and Liver Transplantation Outcomes by Age Group in Patients with Primary Sclerosing Cholangitis. Dig. Dis. Sci. 2017, 62, 3200–3209. [Google Scholar] [CrossRef]
- Nevens, F. PBC-transplantation and disease recurrence. Best Pract. Res. Clin. Gastroenterol. 2018, 34–35, 107–111. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Bhanji, R.A.; Wasilenko, S.; Mason, A.L. Systematic review: Recurrent autoimmune liver diseases after liver transplantation. Aliment Pharmacol. Ther. 2017, 45, 485–500. [Google Scholar] [CrossRef]
- Duclos-Vallee, J.C.; Sebagh, M. Recurrence of autoimmune disease, primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis after liver transplantation. Liver Transpl. 2009, 15 (Suppl. 2), S25–S34. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Mells, G.F.; Alexander, G.J.; Westbrook, R.H.; Heneghan, M.A.; Sandford, R.N.; Neuberger, J.M. Calcineurin inhibitors and the IL12A locus influence risk of recurrent primary biliary cirrhosis after liver transplantation. Am. J. Transplant. 2013, 13, 1110–1111. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Vargas-Vorackova, F.; Ma, M.; Bain, V.G.; Burak, K.; Kumar, T.; Mason, A.L. Incidence and risk factors associated with de novo autoimmune hepatitis after liver transplantation. Liver Int. 2012, 32, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).