Prognostic Significance of Perineural Invasion in Patients with Stage II/III Gastric Cancer Undergoing Radical Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Information
2.2. Clinical Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- La Vecchia, C.; Negri, E.; D’Avanzo, B.; Franceschi, S. Electric refrigerator use and gastric cancer risk. Br. J. Cancer 1990, 62, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, S.; Gan, Y.; Song, X.; Chen, Y.; Liao, N.; Chen, S.; Lv, C. Association between refrigerator use and the risk of gastric cancer: A systematic review and meta-analysis of observational studies. PLoS ONE 2018, 13, e0203120. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.J.; Kook, M.-C.; Kim, Y.-I.; Cho, S.; Lee, J.Y.; Kim, C.G.; Park, B.; Nam, B.-H. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N. Engl. J. Med. 2018, 378, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, P.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Chen, Y.; Zhou, Q.; Wang, H.; Zhou, W.; Ding, Y.; Lu, J.; Wu, G.; Xu, N.; Teng, L. Predicting peritoneal dissemination of gastric cancer in the era of precision medicine: Molecular characterization and biomarkers. Cancers 2020, 12, 2236. [Google Scholar] [CrossRef]
- Deng, J.; You, Q.; Gao, Y.; Yu, Q.; Zhao, P.; Zheng, Y.; Fang, W.; Xu, N.; Teng, L. Prognostic value of perineural invasion in gastric cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e88907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebig, C.; Ayala, G.; Wilks, J.; Verstovsek, G.; Liu, H.; Agarwal, N.; Berger, D.H.; Albo, D. Perineural invasion is an independent predictor of outcome in colorectal cancer. J. Clin. Oncol. 2009, 27, 5131–5137. [Google Scholar] [CrossRef] [Green Version]
- Schorn, S.; Demir, I.E.; Haller, B.; Scheufele, F.; Reyes, C.M.; Tieftrunk, E.; Sargut, M.; Goess, R.; Friess, H.; Ceyhan, G.O. The influence of neural Invasion on survival and tumor recurrence in pancreatic ductal adenocarcinoma—A systematic review and meta-analysis. Surg. Oncol. 2017, 26, 105–115. [Google Scholar] [CrossRef]
- Chen, S.H.; Zhang, B.Y.; Zhou, B.; Zhu, C.Z.; Sun, L.Q.; Feng, Y.J. Perineural invasion of cancer: A complex crosstalk between cells and molecules in the perineural niche. Am. J. Cancer Res. 2019, 9, 1–21. [Google Scholar]
- Knijn, N.; Mogk, S.C.; Teerenstra, S.; Simmer, F.; Nagtegaal, I.D. Perineural invasion is a strong prognostic factor in colorectal cancer: A systematic review. Am. J. Surg. Pathol. 2016, 40, 103–112. [Google Scholar] [CrossRef]
- Liebl, F.; Demir, I.E.; Mayer, K.; Schuster, T.; D’Haese, J.G.; Becker, K.; Langer, R.; Bergmann, F.; Wang, K.; Rosenberg, R.; et al. The impact of neural invasion severity in gastrointestinal malignancies: A clinicopathological study. Ann. Surg. 2014, 260, 900–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobin, L.H. TNM Classification of Malignant Tumours, 7th ed.; Wiley-Blackwell: Oxford, UK, 2009. [Google Scholar]
- Zhao, B.; Lv, W.; Mei, D.; Luo, R.; Bao, S.; Huang, B.; Lin, J. Perineural invasion as a predictive factor for survival outcome in gastric cancer patients: A systematic review and meta-analysis. J. Clin. Pathol. 2020, 73, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased recursive partitioning: A conditional inference framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]
- Aurello, P.; Berardi, G.; Tierno, S.M.; Rampioni Vinciguerra, G.L.; Socciarelli, F.; Laracca, G.G.; Giulitti, D.; Pilozzi, E.; Ramacciato, G. Influence of perineural invasion in predicting overall survival and disease-free survival in patients with locally advanced gastric cancer. Am. J. Surg. 2017, 213, 748–753. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Lin, J.; Chen, L.Z.; Chen, Y.; Wang, X.J.; Guo, Z.Q.; Yu, J.M. Perineural invasion and postoperative complications are independent predictors of early recurrence and survival following curative resection of gastric cancer. Cancer Manag. Res. 2020, 12, 7601–7610. [Google Scholar] [CrossRef]
- Jiang, N.; Deng, J.-Y.; Liu, Y.; Ke, B.; Liu, H.-G.; Liang, H. Incorporation of perineural invasion of gastric carcinoma into the 7th edition tumor-node-metastasis staging system. Tumour Biol. 2014, 35, 9429–9436. [Google Scholar] [CrossRef] [PubMed]
- De Franco, L.; Marrelli, D.; Voglino, C.; Vindigni, C.; Ferrara, F.; Di Mare, G.; Iudici, L.; Marini, M.; Roviello, F. Prognostic value of perineural invasion in resected gastric cancer patients according to Lauren histotype. Pathol. Oncol. Res. 2018, 24, 393–400. [Google Scholar] [CrossRef]
- Chou, H.-H.; Kuo, C.-J.; Hsu, J.-T.; Chen, T.-H.; Lin, C.-J.; Tseng, J.-H.; Yeh, T.-S.; Hwang, T.-L.; Jan, Y.-Y. Clinicopathologic study of node-negative advanced gastric cancer and analysis of factors predicting its recurrence and prognosis. Am. J. Surg. 2013, 205, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Zhu, W.; Zhao, X.; Li, M.; Shu, Y.; Wang, D.; Li, X. Perineural invasion and postoperative adjuvant chemotherapy efficacy in patients with gastric cancer. Front. Oncol. 2020, 10, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.Z.; Zhang, W.H.; Chen, X.L.; Liu, K.; Yang, K.; Zhou, Z.G.; Hu, J.K. Upper lesser curvature skeletonization in radical distal gastrectomy. J. Surg. Res. 2015, 193, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lu, M.; Li, J.; Yang, Z.; Feng, Q.; Zhou, M.; Zhang, Z.; Shen, L. The patterns and timing of recurrence after curative resection for gastric cancer in China. World J. Surg. Oncol. 2016, 14, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Chen, S.; Chen, M. Schwann cells in the tumor microenvironment: Need more attention. J. Oncol. 2022, 2022, 1058667. [Google Scholar] [CrossRef] [PubMed]
- Martyn, G.V.; Shurin, G.V.; Keskinov, A.A.; Bunimovich, Y.L.; Shurin, M.R. Schwann cells shape the neuro-immune environs and control cancer progression. Cancer Immunol. Immunother. 2019, 68, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Fan, Q.; Wang, Y.; Cui, Y.; Wang, Z.; Yang, L.; Sun, X.; Wang, Y. Schwann cell-derived CCL2 promotes the perineural invasion of cervical cancer. Front. Oncol. 2020, 10, 19. [Google Scholar] [CrossRef]
- Demir, I.E.; Kujundzic, K.; Pfitzinger, P.L.; Saricaoglu, Ö.C.; Teller, S.; Kehl, T.; Reyes, C.M.; Ertl, L.S.; Miao, Z.; Schall, T.J.; et al. Early pancreatic cancer lesions suppress pain through CXCL12-mediated chemoattraction of Schwann cells. Proc. Natl. Acad. Sci. USA 2017, 114, E85–E94. [Google Scholar] [CrossRef] [Green Version]
- Deborde, S.; Omelchenko, T.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.F.; Chernichenko, N.; Lee, S.Y.; Barajas, F.; Chen, C.H.; et al. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Investig. 2016, 126, 1538–1554. [Google Scholar] [CrossRef] [Green Version]
| Parameters | Total | Perineural Invasion | p Value | |
|---|---|---|---|---|
| Negative | Positive | |||
| Number | 1913 | 820 (42.9) | 1093 (57.1) | |
| Age (year), mean ± SD | 63.68 ± 13.48 | 65.01 ± 12.75 | 62.68 ± 13.92 | <0.001 |
| ≤65 | 935 | 374 (45.6) | 561 (51.3) | 0.013 |
| >65 | 978 | 446 (54.4) | 532 (48.7) | |
| Sex | 0.040 | |||
| Male | 1186 | 530 (64.6) | 656 (60.0) | |
| Female | 727 | 290 (35.4) | 437 (40.0) | |
| Tumor size (cm), mean ± SD | 5.06 ± 2.84 | 4.82 ± 2.63 | 5.23 ± 2.98 | 0.002 |
| Location | 0.014 | |||
| Upper | 382 | 144 (17.6) | 238 (21.8) | |
| Middle | 344 | 155 (18.9) | 189 (17.3) | |
| Lower | 1119 | 501 (61.1) | 618 (56.5) | |
| Entire | 56 | 15 (1.8) | 41 (3.8) | |
| Other | 12 | 5 (0.6) | 7 (0.6) | |
| Number of LNs retrieved, mean ± SD | 36.50 ± 16.78 | 35.36 ± 16.38 | 37.35 ± 17.03 | 0.010 |
| Type of gastrectomy | <0.001 | |||
| Total | 608 | 220 (26.8) | 388 (35.5) | |
| Partial | 1305 | 600 (73.2) | 705 (64.5) | |
| T status | <0.001 | |||
| T1 | 64 | 63 (7.7) | 1 (0.1) | |
| T2 | 164 | 108 (13.2) | 56 (5.1) | |
| T3 | 382 | 165 (20.1) | 217 (19.9) | |
| T4a | 1161 | 435 (53.0) | 726 (66.4) | |
| T4b | 142 | 49 (6.0) | 93 (8.5) | |
| N status | <0.001 | |||
| N0 | 332 | 195 (23.8) | 137 (12.5) | |
| N1 | 308 | 175 (21.3) | 133 (12.2) | |
| N2 | 459 | 219 (26.7) | 240 (22.0) | |
| N3a | 494 | 160 (19.5) | 334 (30.6) | |
| N3b | 320 | 71 (8.7) | 249 (22.8) | |
| Stage (AJCC eighth edition) | <0.001 | |||
| IIA | 203 | 152 (18.5) | 51 (4.7) | |
| IIB | 357 | 214 (26.1) | 143 (13.1) | |
| IIIA | 548 | 241 (29.4) | 307 (28.1) | |
| IIIB | 454 | 135 (16.5) | 319 (29.2) | |
| IIIC | 351 | 78 (9.5) | 273 (25.0) | |
| Histological type | <0.001 | |||
| Differentiated | 710 | 397 (48.4) | 313 (28.6) | |
| Undifferentiated | 1203 | 423 (51.6) | 780 (71.4) | |
| Lymphatic invasion | 1263 | 417 (50.9) | 846 (77.4) | <0.001 |
| Vascular invasion | 336 | 66 (8.0) | 270 (24.7) | <0.001 |
| Surgical complication | 260 | 103 (12.6) | 157 (14.4) | 0.255 |
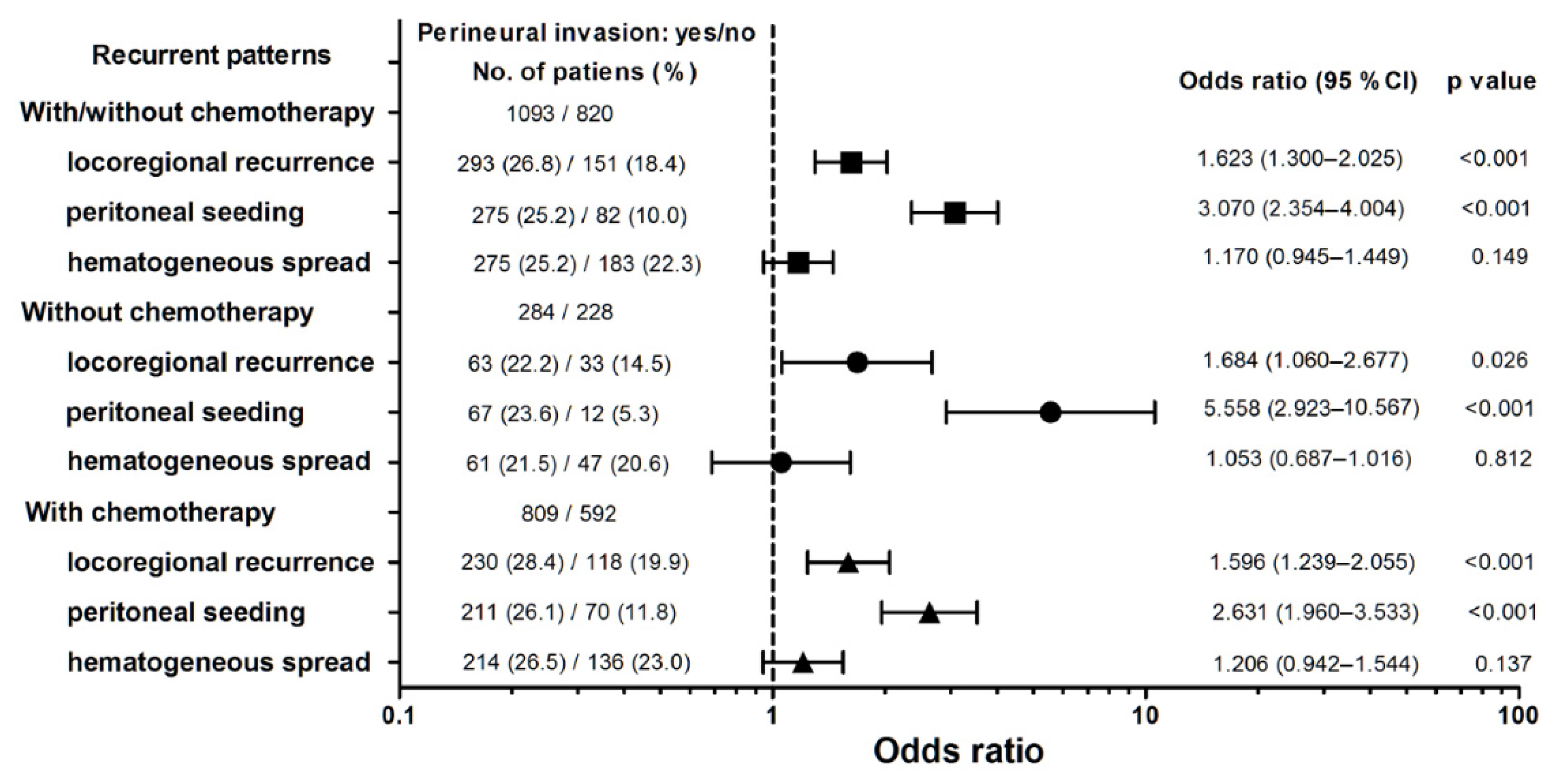
| Chemotherapy | 1401 | 592 (72.2) | 809 (74.0) | 0.373 |
| Factors | Median Survival (Month) | 95% Confidence Interval | 3-Year DFS (%) | 5-Year DFS (%) | p Value |
|---|---|---|---|---|---|
| Age (year) | 0.748 | ||||
| ≤65 (n = 935) | 46.7 | 32.7–60.8 | 52.6 | 46.9 | |
| >65 (n = 978) | 42.8 | 26.1–59.4 | 52.5 | 46.6 | |
| Sex | 0.417 | ||||
| Male (n = 1186) | 41.0 | 30.8–51.2 | 51.4 | 45.6 | |
| Female (n = 727) | 50.3 | 28.1–72.5 | 54.3 | 48.6 | |
| Location | <0.001 | ||||
| Upper (n = 382) | 46.2 | 0.1–98.9 | 52.0 | 47.9 | |
| Middle (n = 344) | 148.4 | NA | 60.4 | 53.4 | |
| Lower (n = 1119) | 42.6 | 32.1–53.0 | 51.7 | 45.6 | |
| Entire (n = 56) | 18.1 | 12.6–23.6 | 31.1 | 26.4 | |
| Other (n = 12) | 10.5 | 3.5–17.5 | 13.3 | 13.3 | |
| Type of gastrectomy | <0.001 | ||||
| Total (n = 608) | 28.0 | 18.6–37.3 | 47.2 | 41.8 | |
| Partial (n = 1305) | 55.0 | 31.6–78.5 | 55.0 | 49.0 | |
| Tumor size (cm) | <0.001 | ||||
| ≤2.4 (n = 258) | NA | 72.7 | 65.5 | ||
| 24.1–3.9 (n = 485) | 122.9 | NA | 59.5 | 52.6 | |
| 3.91–9.4 (n = 1006) | 30.2 | 24.2–36.2 | 47.2 | 42.1 | |
| >9.4 (n = 164) | 14.2 | 8.8–19.6 | 32.0 | 27.7 | |
| T status | <0.001 | ||||
| T1 (n = 64) | NA | 67.3 | 61.9 | ||
| T2 (n = 164) | NA | 74.7 | 65.4 | ||
| T3 (n = 382) | NA | 58.6 | 53.5 | ||
| T4a (n = 1161) | 34.2 | 26.7–41.7 | 49.2 | 43.4 | |
| T4b (n = 142) | 14.0 | 9.6–18.4 | 30.9 | 27.5 | |
| N status | <0.001 | ||||
| N0 (n = 332) | NA | 83.8 | 79.3 | ||
| N1 (n = 308) | NA | 68.8 | 63.1 | ||
| N2 (n = 459) | 139.9 | NA | 58.9 | 53.8 | |
| N3a (n = 494) | 21.8 | 18.9–24.7 | 34.1 | 27.2 | |
| N3b (n = 320) | 11.0 | 9.5–12.4 | 21.6 | 14.9 | |
| Stage (AJCC eighth edition) | <0.001 | ||||
| IIA (n = 203) | NA | 85.0 | 80.5 | ||
| IIB (n = 357) | NA | 75.9 | 69.4 | ||
| IIIA (n = 548) | 165.1 | NA | 60.1 | 55.0 | |
| IIIB (n = 454) | 21.9 | 18.9–24.8 | 34.0 | 28.1 | |
| IIIC (n = 351) | 11.5 | 10.1–12.8 | 20.6 | 14.2 | |
| LN ratio | <0.001 | ||||
| ≤0.05 (n = 566) | NA | 79.4 | 75.2 | ||
| 0.051–0.15 (n = 401) | NA | 62.3 | 56.6 | ||
| 0.151–0.37 (n = 479) | 26.9 | 23.2–30.5 | 42.5 | 34.6 | |
| 0.371–0.53 (n = 217) | 16.1 | 12.6–19.5 | 28.3 | 22.2 | |
| >0.53 (n = 250) | 9.9 | 8.4–11.4 | 14.3 | 8.8 | |
| Histological type | <0.001 | ||||
| Differentiated (n = 710) | 119.4 | NA | 58.9 | 53.6 | |
| Undifferentiated (n = 1203) | 31.9 | 25.0–38.9 | 48.7 | 42.6 | |
| Lymphatic invasion | <0.001 | ||||
| No (n = 650) | NA | 21.1–27.2 | 73.2 | 68.2 | |
| Yes (n = 1263) | 24.1 | 33.3–54.6 | 41.7 | 35.5 | |
| Vascular invasion | <0.001 | ||||
| No (n = 1577) | 59.7 | 15.0–104.4 | 55.9 | 50.0 | |
| Yes (n = 336) | 18.3 | 14.9–21.8 | 36.3 | 31.1 | |
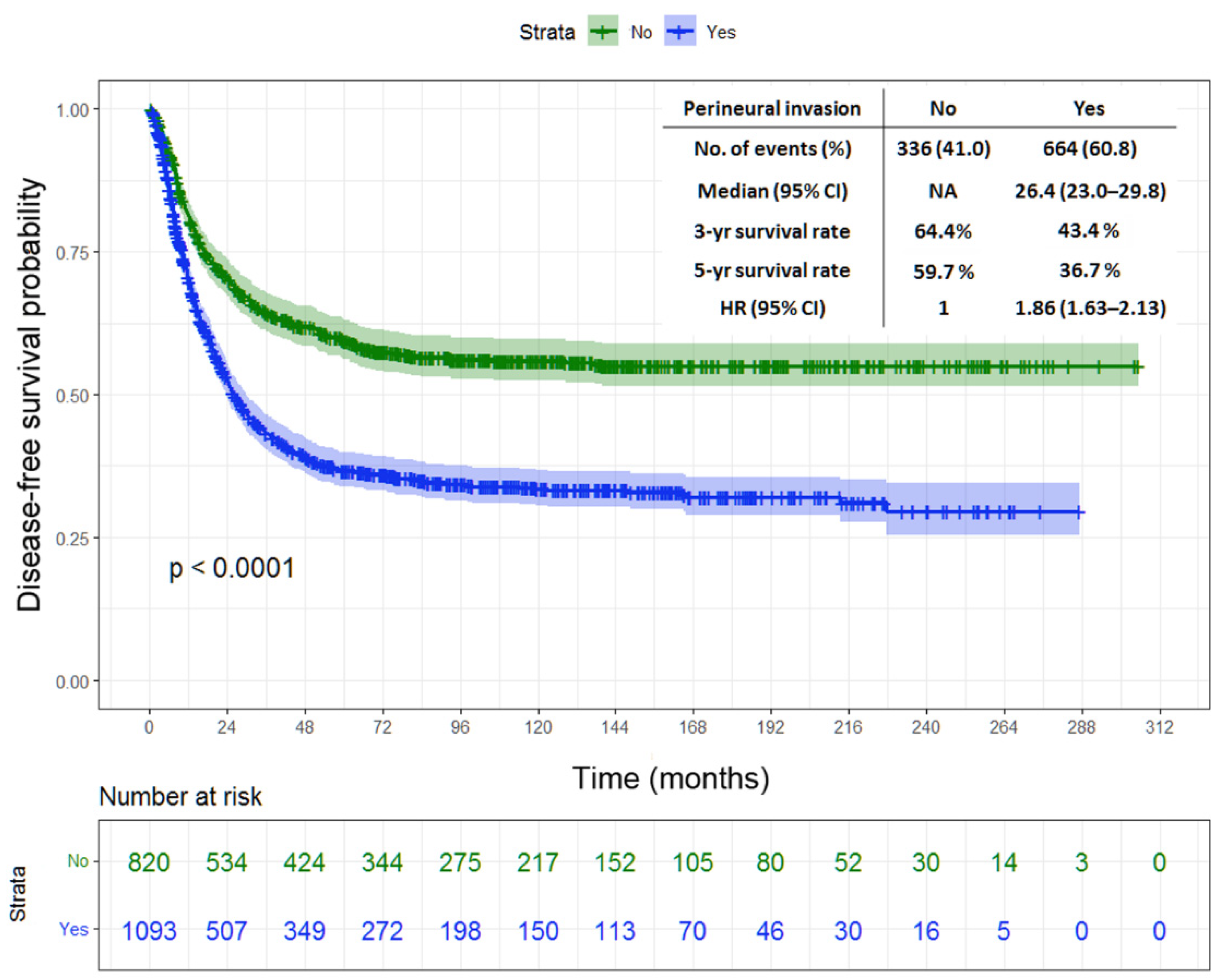
| Perineural invasion | <0.001 | ||||
| No (n = 820) | NA | 64.4 | 59.7 | ||
| Yes (n = 1093) | 26.4 | 23.0–29.8 | 43.4 | 36.7 | |
| Chemotherapy | 0.571 | ||||
| No (n = 512) | 59.3 | NA | 56.0 | 49.7 | |
| Yes (n = 1401) | 41.4 | 31.4–51.3 | 51.5 | 45.9 |
| Factors | Median Survival (Month) | 95% Confidence Interval | 3-Year CSS (%) | 5-Year CSS (%) | p Value |
|---|---|---|---|---|---|
| Age (year) | 0.411 | ||||
| ≤65 (n = 935) | 62.4 | 46.9–78.0 | 60.3 | 50.5 | |
| >65 (n = 978) | 50.4 | 35.9–64.9 | 57.5 | 47.7 | |
| Sex | 0.374 | ||||
| Male (n = 1186) | 52.2 | 39.9–64.4 | 57.6 | 47.9 | |
| Female (n = 727) | 62.7 | 45.1–80.2 | 61.1 | 51.1 | |
| Location | <0.001 | ||||
| Upper (n = 382) | 51.5 | 23.1–79.8 | 57.7 | 48.1 | |
| Middle (n = 344) | 100.4 | - | 66.5 | 55.7 | |
| Lower (n = 1119) | 55.5 | 44.1–66.9 | 58.4 | 48.9 | |
| Entire (n = 56) | 24.3 | 18.7–29.8 | 38.2 | 25.2 | |
| Other (n = 12) | 29.7 | 17.6–41.8 | 14.6 | 14.6 | |
| Type of gastrectomy | <0.001 | ||||
| Total (n = 608) | 38.4 | 28.9–47.9 | 51.5 | 43.4 | |
| Partial (n = 1305) | 66.7 | 51.9–81.4 | 62.3 | 51.7 | |
| Tumor size (cm) | <0.001 | ||||
| ≤2.4 (n = 258) | NA | 79.6 | 67.8 | ||
| 2.41–3.9 (n = 485) | 126.3 | NA | 66.5 | 56.8 | |
| 3.91–9.4 (n = 1006) | 41.6 | 34.6–48.6 | 53.7 | 43.8 | |
| >9.4 (n = 164) | 21.7 | 16.9–26.4 | 35.6 | 28.7 | |
| T status | <0.001 | ||||
| T1 (n = 64) | 241.9 | 0.1–486.8 | 75.3 | 68.0 | |
| T2 (n = 164) | NA | 80.2 | 70.3 | ||
| T3 (n = 382) | 93.6 | NA | 67.4 | 56.2 | |
| T4a (n = 1161) | 43.7 | 35.6–51.7 | 54.9 | 45.1 | |
| T4b (n = 142) | 21.4 | 16.6–26.2 | 37.9 | 30.6 | |
| N status | <0.001 | ||||
| N0 (n = 332) | NA | 86.9 | 81.3 | ||
| N1 (n = 308) | NA | 75.8 | 65.4 | ||
| N2 (n = 459) | 118.9 | 58.6–179.3 | 64.3 | 55.7 | |
| N3a (n = 494) | 31.2 | 28.4–34.0 | 44.0 | 30.4 | |
| N3b (n = 320) | 18.3 | 15.6–21.1 | 27.9 | 18.1 | |
| Stage (AJCC eighth edition) | |||||
| IIA (n = 203) | NA | <0.001 | |||
| IIB (n = 357) | NA | 86.7 | 82.4 | ||
| IIIA (n = 548) | 104.1 | NA | 81.9 | 72.7 | |
| IIIB (n = 454) | 30.5 | 28.1–32.8 | 66.9 | 57.2 | |
| IIIC (n = 351) | 18.2 | 16.0–20.5 | 42.4 | 30.3 | |
| LN ratio | <0.001 | ||||
| ≤0.05 (n = 566) | NA | 83.9 | 77.0 | ||
| 0.051–0.15 (n = 401) | 241.9 | NA | 68.4 | 58.6 | |
| 0.151–0.37 (n = 479) | 38.1 | 32.4–43.9 | 52.2 | 38.3 | |
| 0.371–0.53 (n = 217) | 24.4 | 19.7–29.1 | 35.8 | 25.4 | |
| >0.53 (n = 250) | 16.2 | 14.3–18.1 | 19.1 | 11.2 | |
| Histological type | <0.001 | ||||
| Differentiated (n = 710) | 100.7 | 31.3–170.1 | 65.9 | 56.0 | |
| Undifferentiated (n = 1203) | 44.0 | 36.6–51.5 | 54.8 | 45.1 | |
| Lymphatic invasion | <0.001 | ||||
| No (n = 650) | NA | 78.7 | 70.7 | ||
| Yes (n = 1263) | 33.8 | 30.7–37.0 | 48.6 | 37.9 | |
| Vascular invasion | <0.001 | ||||
| No (n = 1577) | 72.2 | 48.6–95.8 | 62.4 | 52.5 | |
| Yes (n = 336) | 26.7 | 21.4–32.1 | 42.4 | 32.9 | |
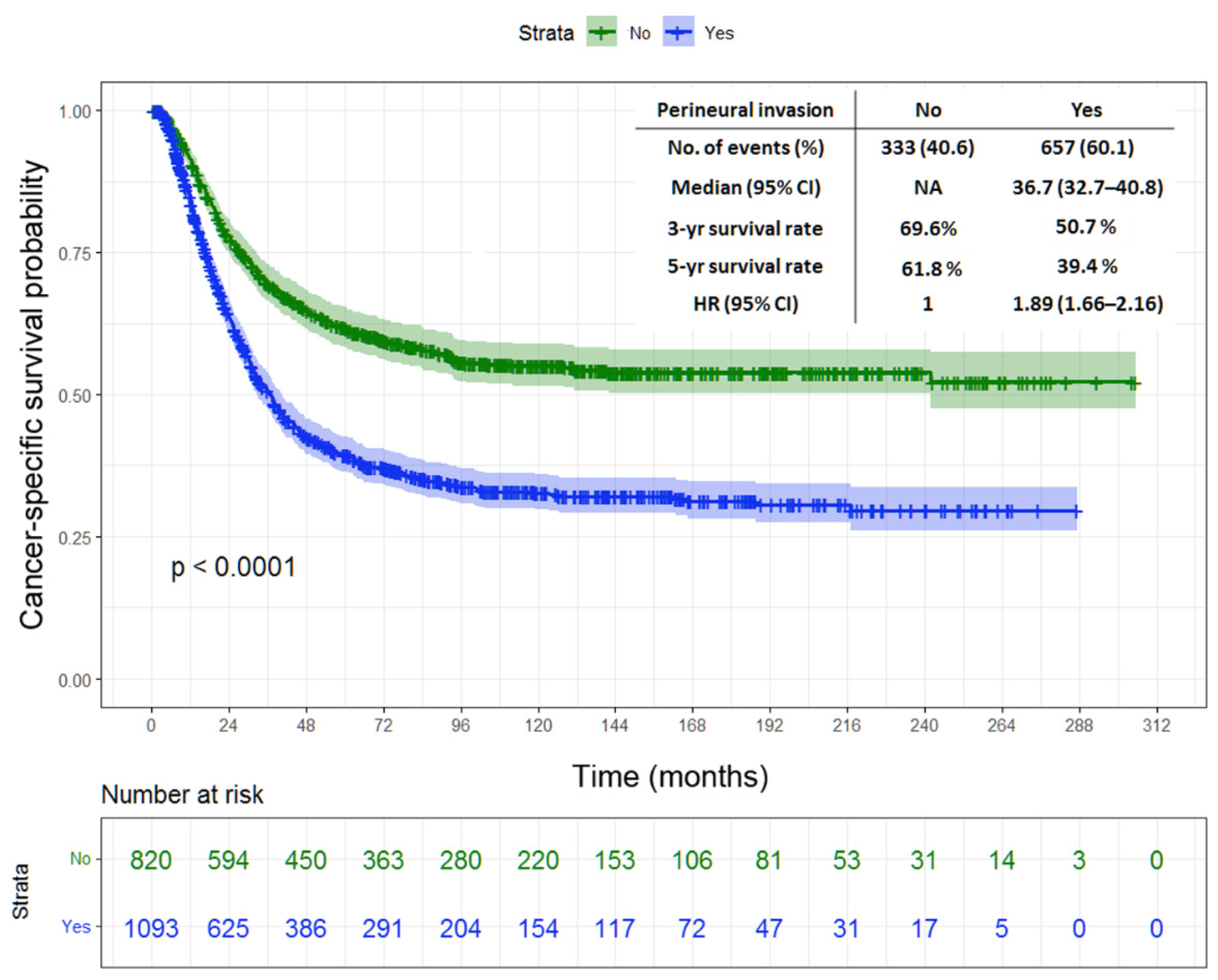
| Perineural invasion | <0.001 | ||||
| No (n = 820) | NA | 69.6 | 61.8 | ||
| Yes (n = 1093) | 36.7 | 32.7–40.8 | 50.7 | 39.4 | |
| Chemotherapy | 0.862 | ||||
| No (n = 512) | 62.6 | 39.4–85.7 | 60.6 | 50.8 | |
| Yes (n = 1401) | 55.5 | 44.0–66.9 | 58.5 | 48.7 |
| Factors | Hazard Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Type of gastrectomy | ||||
| Total/partial | 1.149 | 1.000 | 1.319 | 0.050 |
| Tumor size (cm) | ||||
| 2.41–3.9/≤2.4 | 1.167 | 0.913 | 1.491 | 0.217 |
| 3.91–9.4/≤2.4 | 1.397 | 1.113 | 1.754 | 0.004 |
| >9.4/≤2.4 | 1.581 | 1.177 | 2.124 | 0.002 |
| Stage (AJCC eighth edition) | ||||
| IIB/IIA | 1.598 | 1.108 | 2.306 | 0.012 |
| IIIA/IIA | 1.766 | 1.232 | 2.531 | 0.002 |
| IIIB/IIA | 1.998 | 1.344 | 2.971 | <0.001 |
| IIIC/IIA | 2.283 | 1.493 | 3.492 | <0.001 |
| LN ratio | ||||
| 0.051–0.15/≤0.05 | 1.595 | 1.239 | 2.053 | <0.001 |
| 0.151–0.37/≤0.05 | 2.364 | 1.817 | 3.075 | <0.001 |
| 0.371–0.53/≤0.05 | 3.116 | 2.270 | 4.276 | <0.001 |
| >0.53/≤0.05 | 4.570 | 3.288 | 6.354 | <0.001 |
| Histological type | ||||
| Differentiated/undifferentiated | 1.029 | 0.896 | 1.182 | 0.685 |
| Vascular invasion | ||||
| Yes/no | 1.074 | 0.918 | 1.256 | 0.374 |
| Lymphatic invasion | ||||
| Yes/no | 1.161 | 0.963 | 1.400 | 0.118 |
| Perineural invasion | ||||
| Yes/no | 1.227 | 1.063 | 1.415 | 0.005 |
| Factors | Hazard Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Type of gastrectomy | ||||
| Total/partial | 1.149 | 0.999 | 1.320 | 0.051 |
| Tumor size (cm) | ||||
| 2.41–3.9/≤2.4 | 1.189 | 0.927 | 1.525 | 0.173 |
| 3.91–9.4/2.4 | 1.439 | 1.142 | 1.813 | 0.002 |
| >9.4/≤2.4 | 1.692 | 1.254 | 2.282 | <0.001 |
| Stage (AJCC eighth edition) | ||||
| IIB/IIA | 1.558 | 1.075 | 2.259 | 0.019 |
| IIIA/IIA | 1.796 | 1.249 | 2.583 | 0.002 |
| IIIB/IIA | 2.082 | 1.396 | 3.106 | <0.001 |
| IIIC/IIA | 2.357 | 1.535 | 3.619 | <0.001 |
| LN ratio | ||||
| 0.051–0.15/≤0.05 | 1.547 | 1.201 | 1.993 | <0.001 |
| 0.151–0.37/≤0.05 | 2.249 | 1.728 | 2.928 | <0.001 |
| 0.371–0.53/≤0.05 | 2.943 | 2.145 | 4.039 | <0.001 |
| >0.53/≤0.05 | 4.331 | 3.114 | 6.024 | <0.001 |
| Histological type | ||||
| Differentiated/undifferentiated | 1.058 | 0.921 | 1.215 | 0.427 |
| Vascular invasion | ||||
| Yes/no | 1.062 | 0.906 | 1.243 | 0.459 |
| Lymphatic invasion | ||||
| Yes/no | 1.168 | 0.969 | 1.408 | 0.104 |
| Perineural invasion | ||||
| Yes/no | 1.231 | 1.066 | 1.421 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-F.; Wang, S.-Y.; Le, P.-H.; Chen, T.-H.; Kuo, C.-J.; Lin, C.-J.; Chou, W.-C.; Yeh, T.-S.; Hsu, J.-T. Prognostic Significance of Perineural Invasion in Patients with Stage II/III Gastric Cancer Undergoing Radical Surgery. J. Pers. Med. 2022, 12, 962. https://doi.org/10.3390/jpm12060962
Chen Y-F, Wang S-Y, Le P-H, Chen T-H, Kuo C-J, Lin C-J, Chou W-C, Yeh T-S, Hsu J-T. Prognostic Significance of Perineural Invasion in Patients with Stage II/III Gastric Cancer Undergoing Radical Surgery. Journal of Personalized Medicine. 2022; 12(6):962. https://doi.org/10.3390/jpm12060962
Chicago/Turabian StyleChen, Yi-Fu, Shan-Yu Wang, Puo-Hsien Le, Tsung-Hsing Chen, Chia-Jung Kuo, Chun-Jung Lin, Wen-Chi Chou, Ta-Sen Yeh, and Jun-Te Hsu. 2022. "Prognostic Significance of Perineural Invasion in Patients with Stage II/III Gastric Cancer Undergoing Radical Surgery" Journal of Personalized Medicine 12, no. 6: 962. https://doi.org/10.3390/jpm12060962
APA StyleChen, Y.-F., Wang, S.-Y., Le, P.-H., Chen, T.-H., Kuo, C.-J., Lin, C.-J., Chou, W.-C., Yeh, T.-S., & Hsu, J.-T. (2022). Prognostic Significance of Perineural Invasion in Patients with Stage II/III Gastric Cancer Undergoing Radical Surgery. Journal of Personalized Medicine, 12(6), 962. https://doi.org/10.3390/jpm12060962








