Association of Dipstick Proteinuria with Long-Term Mortality among Patients with Hypertensive Crisis in the Emergency Department
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Proteinuria Measurement
2.3. Data Collection and Outcomes
2.4. Statistical Analysis
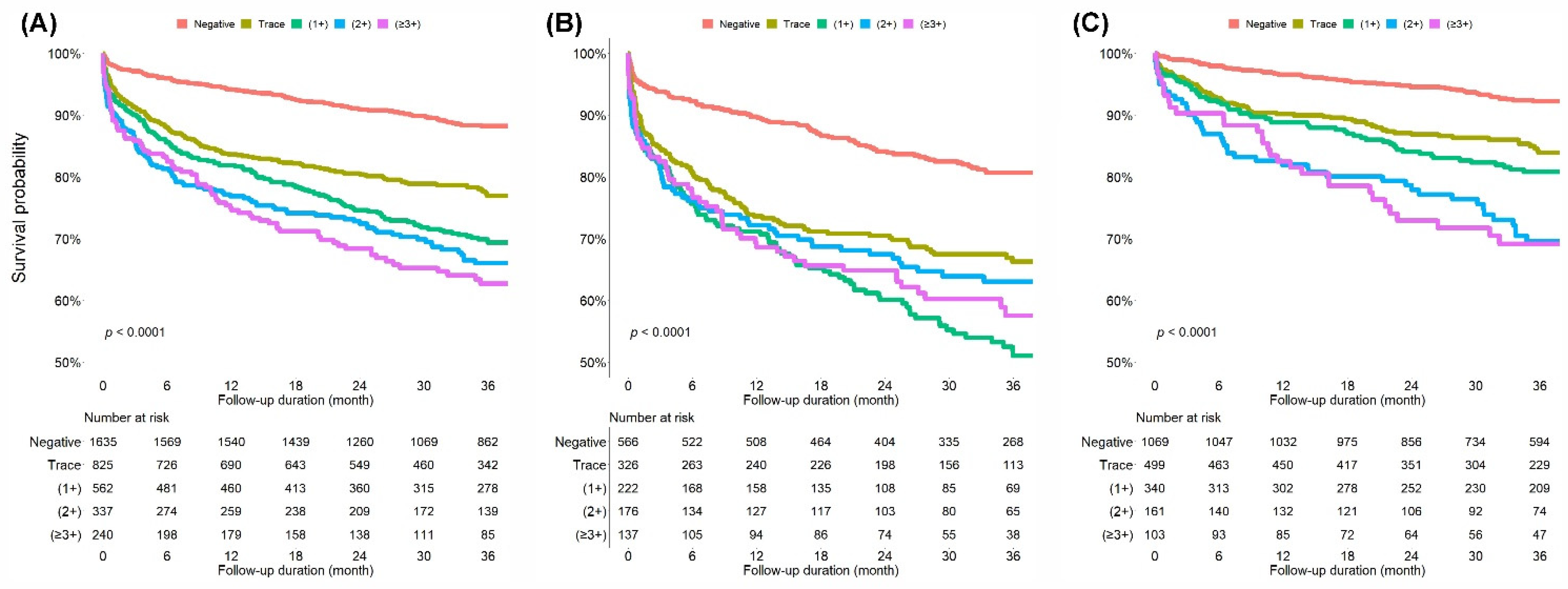
3. Results
3.1. Baseline Characteristics
3.2. Outcomes of the Index Visit and During the Follow-Up Period
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Van den Born, B.-J.H.; Lip, G.Y.H.; Brguljan-Hitij, J.; Cremer, A.; Segura, J.; Morales, E.; Mahfoud, F.; Amraoui, F.; Persu, A.; Kahan, T.; et al. ESC Council on hypertension position document on the management of hypertensive emergencies. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 5, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Amraoui, F.; Van Der Hoeven, N.V.; Van Valkengoed, I.G.; Vogt, L.; Van Den Born, B.J. Mortality and cardiovascular risk in patients with a history of malignant hypertension: A case-control study. J. Clin. Hypertens. 2014, 16, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Kim, B.S.; Lyu, M.; Kim, H.J.; Lee, J.H.; Park, J.K.; Lim, Y.H.; Shin, J. Clinical Characteristics and Predictors of All-Cause Mortality in Patients with Hypertensive Urgency at an Emergency Department. J. Clin. Med. 2021, 10, 4314. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, H.J.; Lyu, M.; Kim, W.D.; Lee, Y.; Kim, M.; Lee, S.; Park, J.K.; Shin, J.; Shin, H.; et al. Clinical characteristics, practice patterns, and outcomes of patients with acute severe hypertension visiting the emergency department. J. Hypertens. 2021, 39, 2506–2513. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Ninomiya, T.; Kiyohara, Y.; Murakami, Y.; Irie, F.; Sairenchi, T.; Miura, K.; Okamura, T.; Ueshima, H. Prediction of cardiovascular disease mortality by proteinuria and reduced kidney function: Pooled analysis of 39,000 individuals from 7 cohort studies in Japan. Am. J. Epidemiol. 2013, 178, 1–11. [Google Scholar] [CrossRef]
- Hillege, H.L.; Fidler, V.; Diercks, G.F.; van Gilst, W.H.; de Zeeuw, D.; van Veldhuisen, D.J.; Gans, R.O.; Janssen, W.M.; Grobbee, D.E.; de Jong, P.E. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002, 106, 1777–1782. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef]
- Lee, H.Y.; Shin, J.; Kim, G.H.; Park, S.; Ihm, S.H.; Kim, H.C.; Kim, K.I.; Kim, J.H.; Lee, J.H.; Park, J.M.; et al. 2018 Korean Society of Hypertension Guidelines for the management of hypertension: Part II-diagnosis and treatment of hypertension. Clin. Hypertens. 2019, 25, 20. [Google Scholar] [CrossRef]
- Pascual, J.M.; Rodilla, E.; Costa, J.A.; Garcia-Escrich, M.; Gonzalez, C.; Redon, J. Prognostic value of microalbuminuria during antihypertensive treatment in essential hypertension. Hypertension 2014, 64, 1228–1234. [Google Scholar] [CrossRef]
- Kim, W.; Kim, B.S.; Kim, H.J.; Lee, J.H.; Shin, J.; Shin, J.H. Clinical implications of cardiac troponin-I in patients with hypertensive crisis visiting the emergency department. Ann. Med. 2022, 54, 507–515. [Google Scholar] [CrossRef]
- Jeon, C.-H.; Lee, A.-J.; Kim, S.-G.; Suh, H.-S.; Bae, Y.-C. Annual Report on the External Quality Assessment Scheme for Urinalysis and Fecal Occult Blood Testing in Korea (2017). J. Lab. Med. Qual. Assur. 2018, 40, 128–135. [Google Scholar] [CrossRef]
- Zaman, M.J.S.; Sanders, J.; Crook, A.M.; Feder, G.; Shipley, M.; Timmis, A.; Hemingway, H. Cardiothoracic ratio within the “normal” range independently predicts mortality in patients undergoing coronary angiography. Heart Br. Card. Soc. 2007, 93, 491–494. [Google Scholar] [CrossRef][Green Version]
- Hancock, E.W.; Deal, B.J.; Mirvis, D.M.; Okin, P.; Kligfield, P.; Gettes, L.S. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram: Part V: Electrocardiogram Changes Associated With Cardiac Chamber Hypertrophy A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009, 53, 992–1002. [Google Scholar] [CrossRef]
- Oh, H.J.; Kim, C.T.; Ryu, D.-R. Effect of Renin-Angiotensin System Blockade on Mortality in Korean Hypertensive Patients with Proteinuria. Electrolyte Blood Press 2019, 17, 25–35. [Google Scholar] [CrossRef]
- Leoncini, G.; Viazzi, F.; Parodi, D.; Ratto, E.; Vettoretti, S.; Vaccaro, V.; Ravera, M.; Deferrari, G.; Pontremoli, R. Mild Renal Dysfunction and Cardiovascular Risk in Hypertensive Patients. J. Am. Soc. Nephrol. 2004, 15, S88–S90. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef]
- Schrader, J.; Lüders, S.; Kulschewski, A.; Hammersen, F.; Züchner, C.; Venneklaas, U.; Schrandt, G.; Schnieders, M.; Rangoonwala, B.; Berger, J.; et al. Microalbuminuria and tubular proteinuria as risk predictors of cardiovascular morbidity and mortality in essential hypertension: Final results of a prospective long-term study (MARPLE Study). J. Hypertens. 2006, 24, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.; Annemans, L.; Strippoli, G. Proteinuria and clinical outcomes in hypertensive patients. Am. J. Hypertens. 2009, 22, 1137–1147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ota, H.; Takeuchi, T.; Sato, N.; Hasebe, N. Dipstick proteinuria as a surrogate marker of long-term mortality after acute myocardial infarction. J. Cardiol. 2013, 62, 277–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, W.; Liu, Q.; Wang, Q.-y.; Yu, H.; Yu, J. Albuminuria and Dipstick Proteinuria for Predicting Mortality in Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 323. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Wang, A.; Wang, J.; Yang, Y.; Chen, S.; Wu, S.; Zhao, M.; Guo, X.; Zhang, L. Dipstick proteinuria and risk of myocardial infarction and all-cause mortality in diabetes or pre-diabetes: A population-based cohort study. Sci. Rep. 2017, 7, 11986. [Google Scholar] [CrossRef]
- Kitamura, A.; Yamagishi, K.; Imano, H.; Kiyama, M.; Cui, R.; Ohira, T.; Umesawa, M.; Muraki, I.; Sankai, T.; Saito, I.; et al. Impact of Hypertension and Subclinical Organ Damage on the Incidence of Cardiovascular Disease Among Japanese Residents at the Population and Individual Levels—The Circulatory Risk in Communities Study (CIRCS). Circ. J. 2017, 81, 1022–1028. [Google Scholar] [CrossRef]
- Sehestedt, T.; Jeppesen, J.; Hansen, T.W.; Wachtell, K.; Ibsen, H.; Torp-Petersen, C.; Hildebrandt, P.; Olsen, M.H. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur. Heart J. 2009, 31, 883–891. [Google Scholar] [CrossRef]
- Haller, P.M.; Beer, B.N.; Tonkin, A.M.; Blankenberg, S.; Neumann, J.T. Role of Cardiac Biomarkers in Epidemiology and Risk Outcomes. Clin. Chem. 2020, 67, 96–106. [Google Scholar] [CrossRef]
- De Zeeuw, D.; Parving, H.H.; Henning, R.H. Microalbuminuria as an early marker for cardiovascular disease. J. Am. Soc. Nephrol. JASN 2006, 17, 2100–2105. [Google Scholar] [CrossRef]
- Haas, M.E.; Aragam, K.G.; Emdin, C.A.; Bick, A.G.; Hemani, G.; Davey Smith, G.; Kathiresan, S. Genetic Association of Albuminuria with Cardiometabolic Disease and Blood Pressure. Am. J. Hum. Genet. 2018, 103, 461–473. [Google Scholar] [CrossRef]
- Sumida, K.; Nadkarni, G.N.; Grams, M.E.; Sang, Y.; Ballew, S.H.; Coresh, J.; Matsushita, K.; Surapaneni, A.; Brunskill, N.; Chadban, S.J.; et al. Conversion of Urine Protein–Creatinine Ratio or Urine Dipstick Protein to Urine Albumin–Creatinine Ratio for Use in Chronic Kidney Disease Screening and Prognosis. Ann. Intern. Med. 2020, 173, 426–435. [Google Scholar] [CrossRef]
- Kwon, Y.; Han, K.; Kim, Y.H.; Park, S.; Kim, D.H.; Roh, Y.K.; Park, Y.G.; Cho, K.H. Dipstick proteinuria predicts all-cause mortality in general population: A study of 17 million Korean adults. PLoS ONE 2018, 13, e0199913. [Google Scholar] [CrossRef]
- Konta, T.; Kudo, K.; Sato, H.; Ichikawa, K.; Ikeda, A.; Suzuki, K.; Hirayama, A.; Shibata, Y.; Watanabe, T.; Daimon, M.; et al. Albuminuria is an independent predictor of all-cause and cardiovascular mortality in the Japanese population: The Takahata study. Clin. Exp. Nephrol. 2013, 17, 805–810. [Google Scholar] [CrossRef]
- Han, E.N.; Lee, K.B.; Kim, H.; Hyun, Y.Y. Trace Urine Albumin and Mortality: Kangbuk Samsung Health Study. Kidney Blood Press. Res. 2018, 43, 951–958. [Google Scholar] [CrossRef]
- Kunz, R.; Friedrich, C.; Wolbers, M.; Mann, J.F. Meta-analysis: Effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann. Intern. Med. 2008, 148, 30–48. [Google Scholar] [CrossRef]
- Wilmer, W.A.; Rovin, B.H.; Hebert, C.J.; Rao, S.V.; Kumor, K.; Hebert, L.A. Management of glomerular proteinuria: A commentary. J. Am. Soc. Nephrol. JASN 2003, 14, 3217–3232. [Google Scholar] [CrossRef]
- Wakasugi, M.; Kazama, J.; Narita, I.; Iseki, K.; Fujimoto, S.; Moriyama, T.; Yamagata, K.; Konta, T.; Tsuruya, K.; Asahi, K.; et al. Association between Overall Lifestyle Changes and the Incidence of Proteinuria: A Population-based, Cohort Study. Intern. Med. 2017, 56, 1475–1484. [Google Scholar] [CrossRef]


| All Patients (n = 3599) | Negative (n = 1635) | Dipstick Proteinuria | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Trace (n = 825) | 1+ (n = 562) | 2+ (n = 337) | ≥3+ (n = 240) | ||||
| Age, mean (SD) | 63.1 (16.8) | 60.9 (16.1) | 63.2 (17.6) | 65.0 (17.3) | 67.9 (16.3) | 66.7 (16.3) | <0.001 |
| Female sex, n (%) | 1795 (49.9) | 878 (53.7) | 385 (46.7) | 267 (47.5) | 158 (46.9) | 107 (44.6) | <0.001 |
| Medical history, n (%) | |||||||
| Hypertension | 1935 (55.0) | 789 (49.2) | 420 (52.2) | 322 (59.0) | 226 (69.3) | 178 (74.8) | <0.001 |
| Diabetes mellitus | 931 (26.6) | 271 (16.9) | 204 (25.7) | 185 (34.3) | 138 (42.3) | 133 (56.4) | <0.001 |
| Dyslipidemia | 357 (10.3) | 168 (10.6) | 81 (10.3) | 47 (8.7) | 31 (9.7) | 30 (12.9) | 0.955 |
| Ischemic stroke | 327 (9.4) | 111 (7.0) | 73 (9.3) | 69 (12.8) | 42 (13.0) | 32 (13.7) | <0.001 |
| Hemorrhagic stroke | 108 (3.1) | 32 (2.0) | 30 (3.8) | 26 (4.8) | 11 (3.5) | 9 (3.9) | 0.008 |
| Coronary artery disease | 315 (9.1) | 125 (7.9) | 66 (8.4) | 50 (9.3) | 44 (13.7) | 30 (12.9) | <0.001 |
| Heart failure | 160 (4.6) | 33 (2.1) | 35 (4.5) | 29 (5.4) | 32 (9.9) | 31 (13.3) | <0.001 |
| Chronic kidney disease | 188 (5.4) | 21 (1.3) | 29 (3.7) | 33 (6.1) | 41 (12.8) | 64 (27.2) | <0.001 |
| Social history, n (%) | |||||||
| Cigarette smoking | 751 (29.1) | 330 (29.0) | 184 (31.4) | 108 (26.2) | 68 (25.9) | 61 (32.3) | 0.771 |
| Alcohol consumption | 946 (36.1) | 467 (40.5) | 211 (35.6) | 132 (31.6) | 81 (30.5) | 55 (28.6) | <0.001 |
| Triage vitals, mean (SD) | |||||||
| SBP, mmHg | 191.4 (22.7) | 189.1 (20.2) | 190.9 (23.4) | 191.9 (23.7) | 197.4 (26.1) | 198.8 (25.4) | <0.001 |
| DBP, mmHg | 108.1 (17.8) | 107.4 (16.4) | 108.6 (18.0) | 108.7 (17.8) | 109.5 (21.3) | 107.0 (20.9) | 0.163 |
| Laboratory tests | 3559 (98.9) | 1611 (98.5) | 820 (99.4) | 555 (98.8) | 335 (99.4) | 238 (99.2) | 0.181 |
| Serum creatinine, mg/dL, mean (SD) | 1.08 (1.18) | 0.82 (0.48) | 0.94 (0.61) | 1.13 (0.92) | 1.50 (1.56) | 2.63 (3.10) | <0.001 |
| eGFR, mL/min/1.73 m2, mean (SD) | 80.1 (28.3) | 89.6 (21.5) | 82.8 (25.2) | 74.1 (29.6) | 63.2 (30.6) | 47.4 (31.9) | <0.001 |
| Troponin-I, ng/mL, mean (SD) | 0.16 (1.99) | 0.04 (0.25) | 0.14 (1.61) | 0.22 (1.20) | 0.51 (5.38) | 0.27 (1.59) | 0.010 |
| BNP, pg/mL, mean (SD) | 354 (700) | 149 (366) | 279 (525) | 421 (666) | 569 (845) | 945 (1260) | <0.001 |
| Chest radiography done, n (%) | 3393 (94.3) | 1529 (93.5) | 781 (94.7) | 527 (93.8) | 323 (95.8) | 233 (97.1) | 0.021 |
| Cardiomegaly a, n (%) | 480 (14.1) | 185 (12.1) | 112 (14.3) | 74 (14.0) | 61 (19.0) | 48 (20.6) | <0.001 |
| ECG done, n (%) | 3123 (86.8) | 1417 (86.7) | 696 (84.4) | 469 (83.5) | 313 (92.9) | 228 (95.0) | 0.001 |
| LVH b, n (%) | 425 (13.6) | 159 (11.2) | 95 (13.7) | 76 (16.3) | 59 (19.0) | 36 (15.8) | <0.001 |
| Acute HMOD, n (%) | 1427 (39.6) | 566 (34.6) | 326 (39.5) | 222 (39.5) | 176 (52.2) | 137 (57.1) | <0.001 |
| All Patients (n = 3599) | Negative (n = 1635) | Dipstick Proteinuria | p-Value for Trend | ||||
|---|---|---|---|---|---|---|---|
| Trace (n = 825) | 1+ (n = 562) | 2+ (n = 337) | ≥3+ (n = 240) | ||||
| Outcomes of the index ED visit, n (%) | |||||||
| Admission | 2199 (61.1) | 869 (53.1) | 515 (62.4) | 367 (65.3) | 259 (76.9) | 189 (78.8) | <0.001 |
| Discharge | 1108 (30.8) | 627 (38.3) | 248 (30.1) | 146 (26.0) | 49 (14.5) | 38 (15.8) | <0.001 |
| Discharge against medical advice | 288 (8.0) | 139 (8.5) | 62 (7.5) | 48 (8.5) | 28 (8.3) | 11 (4.6) | 0.176 |
| Death in the ED | 4 (0.1) | 0 (0) | 0 (0) | 1 (0.2) | 1 (0.3) | 2 (0.8) | 0.001 |
| Revisit to ED, n (%) | |||||||
| 1-month revisits | 267 (9.1) | 109 (8.2) | 60 (9.1) | 50 (11.2) | 33 (11.8) | 15 (7.4) | 0.193 |
| 3-month revisit | 480 (16.4) | 185 (13.9) | 104 (15.7) | 87 (19.5) | 61 (21.8) | 43 (21.1) | <0.001 |
| 1-year revisit | 832 (28.4) | 355 (26.7) | 173 (26.1) | 131 (29.3) | 95 (33.9) | 78 (38.2) | <0.001 |
| Readmission, n (%) | |||||||
| 1-month readmission | 150 (5.1) | 62 (4.7) | 33 (5.0) | 27 (6.0) | 20 (7.1) | 8 (3.9) | 0.354 |
| 3-month readmission | 239 (8.2) | 95 (7.1) | 49 (7.4) | 40 (8.9) | 36 (12.8) | 19 (9.3) | 0.008 |
| 1-year readmission | 364 (12.4) | 146 (11.0) | 72 (10.8) | 54 (12.0) | 55 (19.5) | 37 (18.1) | <0.001 |
| Mortality, n (%) | |||||||
| 1-month mortality | 177 (4.9) | 32 (2.0) | 46 (5.6) | 40 (7.1) | 33 (9.8) | 26 (10.8) | <0.001 |
| 3-month mortality | 256 (7.1) | 47 (2.9) | 72 (8.7) | 56 (10.0) | 47 (13.9) | 34 (14.2) | <0.001 |
| 1-year mortality | 471 (13.1) | 95 (5.8) | 135 (16.4) | 102 (18.1) | 78 (23.1) | 61 (25.4) | <0.001 |
| 3-year mortality | 712 (19.8) | 177 (10.8) | 179 (21.7) | 163 (29.0) | 108 (32.0) | 85 (35.4) | <0.001 |
| Grade of Dipstick Proteinuria | Unadjusted | Model 1 * | Model 2 † | Model 3 ‡ |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Negative | REF | REF | REF | REF |
| Trace | 2.19 (1.78–2.69) | 1.91 (1.55–2.35) | 1.86 (1.51–2.30) | 1.91 (1.53–2.37) |
| (1+) | 2.98 (2.41–3.69) | 2.35 (1.90–2.91) | 2.25 (1.81–2.80) | 2.32 (1.85–2.91) |
| (2+) | 3.45 (2.71–4.38) | 2.49 (1.96–3.17) | 2.38 (1.85–3.05) | 2.40 (1.86–3.10) |
| (≥3+) | 3.88 (3.00–5.03) | 2.73 (2.10–3.54) | 2.64 (2.01–3.46) | 2.40 (1.78–3.24) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.S.; Yu, M.-Y.; Park, J.-K.; Shin, J.; Shin, J.-H. Association of Dipstick Proteinuria with Long-Term Mortality among Patients with Hypertensive Crisis in the Emergency Department. J. Pers. Med. 2022, 12, 971. https://doi.org/10.3390/jpm12060971
Kim BS, Yu M-Y, Park J-K, Shin J, Shin J-H. Association of Dipstick Proteinuria with Long-Term Mortality among Patients with Hypertensive Crisis in the Emergency Department. Journal of Personalized Medicine. 2022; 12(6):971. https://doi.org/10.3390/jpm12060971
Chicago/Turabian StyleKim, Byung Sik, Mi-Yeon Yu, Jin-Kyu Park, Jinho Shin, and Jeong-Hun Shin. 2022. "Association of Dipstick Proteinuria with Long-Term Mortality among Patients with Hypertensive Crisis in the Emergency Department" Journal of Personalized Medicine 12, no. 6: 971. https://doi.org/10.3390/jpm12060971
APA StyleKim, B. S., Yu, M.-Y., Park, J.-K., Shin, J., & Shin, J.-H. (2022). Association of Dipstick Proteinuria with Long-Term Mortality among Patients with Hypertensive Crisis in the Emergency Department. Journal of Personalized Medicine, 12(6), 971. https://doi.org/10.3390/jpm12060971






