Translational Value of Tumor-Associated Lymphangiogenesis in Cholangiocarcinoma
Abstract
1. Introduction
2. The Lymphatic System in the Liver
3. Lymphangiogenesis in Embryonic Development and in Liver Disease
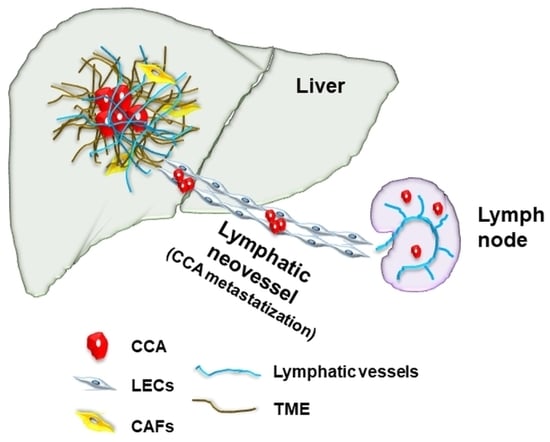
4. Tumor-Associated Lymphangiogenesis: Clinical Significance
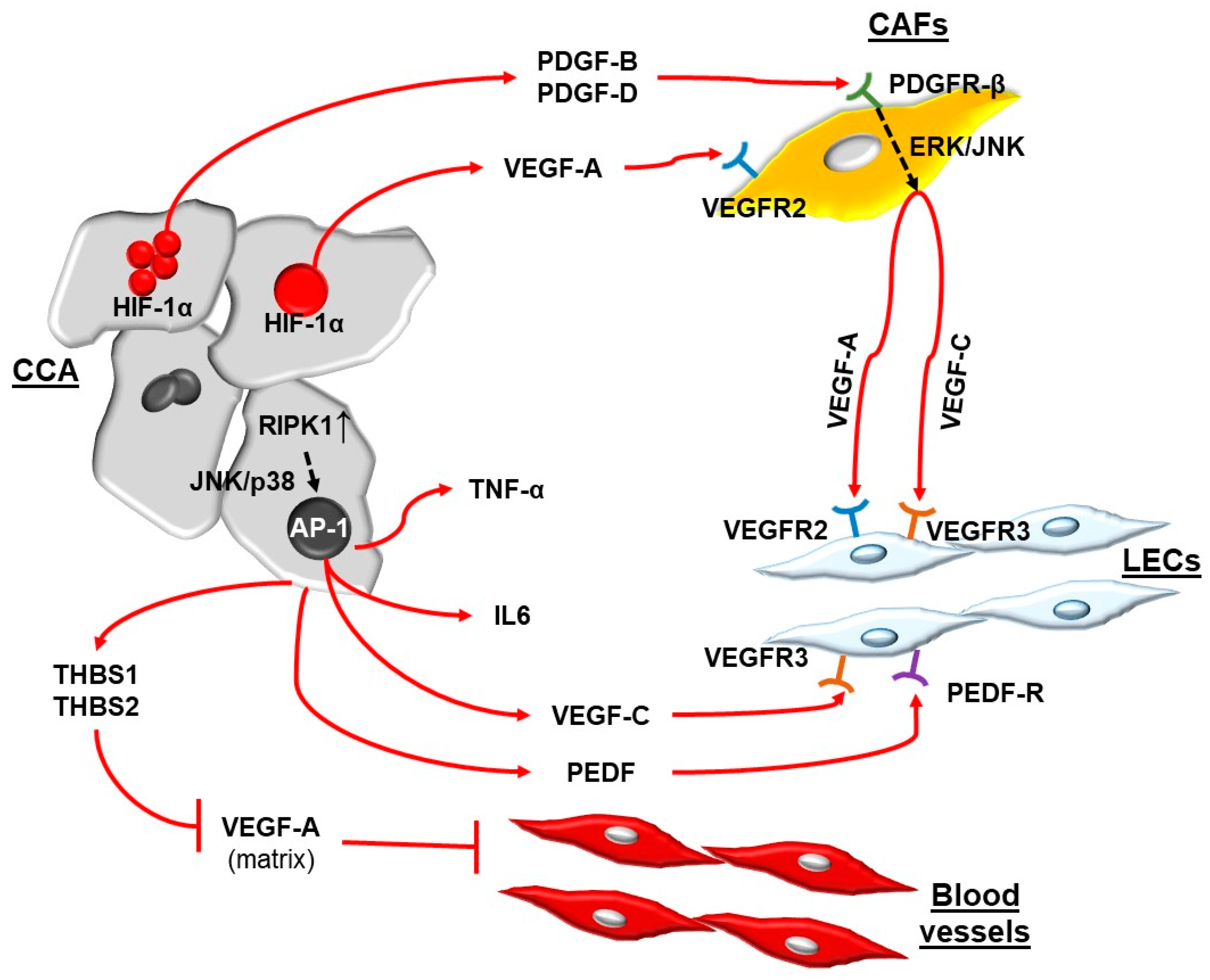
5. Signals Directing Tumor-Associated Lymphangiogenesis and Cell Types Involved
6. Therapeutic Opportunities for Targeting Tumor-Associated Lymphangiogenesis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, D.A.; Brandi, G. Detecting and targeting NTRK gene fusions in cholangiocarcinoma: News and perspectives. Expert Rev. Precis. Med. Drug Dev. 2021, 6, 225–227. [Google Scholar] [CrossRef]
- Cillo, U.; Fondevila, C.; Donadon, M.; Gringeri, E.; Mocchegiani, F.; Schlitt, H.J.; Ijzermans, J.N.M.; Vivarelli, M.; Zieniewicz, K.; Olde Damink, S.W.M.; et al. Surgery for cholangiocarcinoma. Liver Int. 2019, 39, 143–155. [Google Scholar] [CrossRef]
- Tawarungruang, C.; Khuntikeo, N.; Chamadol, N.; Laopaiboon, V.; Thuanman, J.; Thinkhamrop, K.; Kelly, M.; Thinkhamrop, B. Survival after surgery among patients with cholangiocarcinoma in Northeast Thailand according to anatomical and morphological classification. BMC Cancer 2021, 21, 497. [Google Scholar] [CrossRef]
- Izquierdo-Sanchez, L.; Lamarca, A.; La Casta, A.; Buettner, S.; Utpatel, K.; Klümpen, H.J.; Adeva, J.; Vogel, A.; Lleo, A.; Fabris, L.; et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J. Hepatol. 2022, 76, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Yokoyama, T.; Takesue, Y.; Hiyama, E.; Yokoyama, Y.; Kanehiro, T.; Uemura, K.; Matsuura, Y. Long-term survival of peripheral intrahepatic cholangiocarcinoma with metastasis to the para-aortic lymph nodes. Surgery 2000, 127, 105–106. [Google Scholar] [CrossRef]
- Nooijen, L.E.; Banales, J.M.; de Boer, M.T.; Braconi, C.; Folseraas, T.; Forner, A.; Holowko, W.; Hoogwater, F.; Klümpen, H.J.; Groot Koerkamp, B.; et al. and ENSCCA Group. Impact of Positive Lymph Nodes and Resection Margin Status on the Overall Survival of Patients with Resected Perihilar Cholangiocarcinoma: The ENSCCA Registry. Cancers 2022, 14, 2389. [Google Scholar] [CrossRef]
- Gunasekaran, G.; Bekki, Y.; Lourdusamy, V.; Schwartz, M. Surgical Treatments of Hepatobiliary Cancers. Hepatology 2021, 73, 128–136. [Google Scholar] [CrossRef]
- Rea, D.J.; Heimbach, J.K.; Rosen, C.B.; Haddock, M.G.; Alberts, S.R.; Kremers, W.K.; Gores, G.J.; Nagorney, D.M. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann. Surg. 2005, 242, 451–458, discussion 458–461. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Karaman, S.; Detmar, M. Mechanisms of lymphatic metastasis. J. Clin. Investig. 2014, 124, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Vaahtomeri, K.; Alitalo, K. Lymphatic Vessels in Tumor Dissemination versus Immunotherapy. Cancer Res. 2020, 80, 3463–3465. [Google Scholar] [CrossRef] [PubMed]
- Paduch, R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol. 2016, 39, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Iwakiri, Y. Lymphatics in the liver. Curr. Opin. Immunol. 2018, 53, 137–142. [Google Scholar] [CrossRef]
- Jeong, J.; Tanaka, M.; Iwakiri, Y. Hepatic lymphatic vascular system in health and disease. J. Hepatol. 2022, 11, 206–218. [Google Scholar] [CrossRef]
- Iwakiri, Y. The lymphatic system: A new frontier in hepatology. Hepatology 2016, 64, 706–707. [Google Scholar] [CrossRef]
- Tanaka, M.; Iwakiri, Y. The Hepatic Lymphatic Vascular System: Structure, Function, Markers, and Lymphangiogenesis. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 733–749. [Google Scholar] [CrossRef]
- Magari, S. Hepatic lymphatic system: Structure and function. J. Gastroenterol. Hepatol. 1990, 5, 82–93. [Google Scholar] [CrossRef]
- Fu, J.; Gerhardt, H.; McDaniel, J.M.; Xia, B.; Liu, X.; Ivanciu, L.; Ny, A.; Hermans, K.; Silasi-Mansat, R.; McGee, S.; et al. Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J. Clin. Investig. 2008, 118, 3725–3737. [Google Scholar] [CrossRef]
- Escobedo, N.; Oliver, G. Lymphangiogenesis: Origin, Specification, and Cell Fate Determination. Annu. Rev. Cell Dev. Biol. 2016, 32, 677–691. [Google Scholar] [CrossRef]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Sáinz-Jaspeado, M.; Claesson-Welsh, L. Cytokines regulating lymphangiogenesis. Curr. Opin. Immunol. 2018, 53, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, B.A.J.; Finlon, J.M.; Gillen, A.E.; Kriss, M.S.; Riemondy, K.A.; Fu, R.; Schuyler, R.P.; Hesselberth, J.R.; Rosen, H.R.; Burchill, M.A. Chronic Liver Disease in Humans Causes Expansion and Differentiation of Liver Lymphatic Endothelial Cells. Front. Immunol. 2019, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Lukacs-Kornek, V. The Role of Lymphatic Endothelial Cells in Liver Injury and Tumor Development. Front. Immunol. 2016, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.; Gasheva, O.; Alpini, G.; Zawieja, D.; Gashev, A.; Glaser, S. The Role of Lymphatics in Cholestasis: A Comprehensive Review. Semin. Liver Dis. 2020, 40, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kondo, R.; Iwakiri, Y. The lymphatic system in alcohol-associated liver disease. Clin. Mol. Hepatol. 2020, 26, 633–638. [Google Scholar] [CrossRef]
- Nakamoto, S.; Ito, Y.; Nishizawa, N.; Goto, T.; Kojo, K.; Kumamoto, Y.; Watanabe, M.; Majima, M. Lymphangiogenesis and accumulation of reparative macrophages contribute to liver repair after hepatic ischemia-reperfusion injury. Angiogenesis 2020, 23, 395–410. [Google Scholar] [CrossRef]
- Burchill, M.A.; Finlon, J.M.; Goldberg, A.R.; Gillen, A.E.; Dahms, P.A.; McMahan, R.H.; Tye, A.; Winter, A.B.; Reisz, J.A.; Bohrnsen, E.; et al. Oxidized low-density lipoprotein drives dysfunction of the liver lymphatic system. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 573–595. [Google Scholar] [CrossRef]
- Hsu, S.J.; Zhang, C.; Jeong, J.; Lee, S.I.; McConnell, M.; Utsumi, T.; Iwakiri, Y. Enhanced Meningeal Lymphatic Drainage Ameliorates Neuroinflammation and Hepatic Encephalopathy in Cirrhotic Rats. Gastroenterology 2021, 160, 1315–1329.e13. [Google Scholar] [CrossRef]
- Stacker, S.A.; Achen, M.G.; Jussila, L.; Baldwin, M.E.; Alitalo, K. Lymphangiogenesis and cancer metastasis. Nat. Rev. Cancer 2002, 2, 573–583. [Google Scholar] [CrossRef]
- Cadamuro, M.; Brivio, S.; Mertens, J.; Vismara, M.; Moncsek, A.; Milani, C.; Fingas, C.; Malerba, M.C.; Nardo, G.; Dall’Olmo, L.; et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J. Hepatol. 2019, 70, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Padera, T.P.; Kadambi, A.; di Tomaso, E.; Carreira, C.M.; Brown, E.B.; Boucher, Y.; Choi, N.C.; Mathisen, D.; Wain, J.; Mark, E.J.; et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 2002, 296, 1883–1886. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Mohammed, S. Breast cancer metastasis and the lymphatic system (Review). Oncol. Lett. 2005, 10, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Thelen, A.; Scholz, A.; Benckert, C.; Schröder, M.; Weichert, W.; Wiedenmann, B.; Neuhaus, P.; Jonas, S. Microvessel density correlates with lymph node metastases and prognosis in hilar cholangiocarcinoma. J. Gastroenterol. 2008, 43, 959–966. [Google Scholar] [CrossRef]
- Sha, M.; Jeong, S.; Wang, X.; Tong, Y.; Cao, J.; Sun, H.Y.; Xia, L.; Xu, N.; Xi, Z.F.; Zhang, J.J.; et al. Tumor-associated lymphangiogenesis predicts unfavorable prognosis of intrahepatic cholangiocarcinoma. BMC Cancer 2019, 19, 208. [Google Scholar] [CrossRef]
- Du, L.C.; Chen, X.C.; Wang, D.; Wen, Y.J.; Wang, C.T.; Wang, X.M.; Kan, B.; Wei, Y.Q.; Zhao, X. VEGF-D-induced draining lymphatic enlargement and tumor lymphangiogenesis promote lymph node metastasis in a xenograft model of ovarian carcinoma. Reprod. Biol. Endocrinol. 2014, 12, 14. [Google Scholar] [CrossRef][Green Version]
- Watanabe, M.; Tanaka, H.; Ohira, M.; Yoshii, M.; Sakurai, K.; Toyokawa, T.; Kubo, N.; Yamamoto, A.; Muguruma, K.; Yamashita, Y.; et al. Intranodal lymphangiogenesis precedes development of lymph node metastasis and accelerates progression of gastric cancer. J. Gastrointest. Surg. 2014, 18, 481–490. [Google Scholar] [CrossRef]
- Ueda, A.; Matsumoto, T.; Komuro, Y. Lymphangiogenesis is a predictor of nodal metastasis in extramammary Paget’s disease. Histopathology 2011, 58, 870–874. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, S.; Zhang, D.; Gong, M.; Cai, Y.; Zou, L. Intratumoral and peritumoral lymphatic vessel density both correlate with lymph node metastasis in breast cancer. Sci. Rep. 2017, 7, 40364. [Google Scholar] [CrossRef]
- van Pul, K.M.; Fransen, M.F.; van de Ven, R.; de Gruijl, T.D. Immunotherapy Goes Local: The Central Role of Lymph Nodes in Driving Tumor Infiltration and Efficacy. Front. Immunol. 2021, 12, 643291. [Google Scholar] [CrossRef]
- Villanueva, L.; Lwin, Z.; Chung, H.C.; Gomez-Roca, C.; Longo, F.; Yanez, E.; Senellart, H.; Doherty, M.; García-Corbacho, J.; Hendifar, A.E.; et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study. J. Clin. Oncol. 2021, 39, 321. [Google Scholar] [CrossRef]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Shibasaki, F. Hypoxia-inducible factor as an angiogenic master switch. Front. Pediatr. 2015, 3, 33. [Google Scholar] [CrossRef]
- Thelen, A.; Scholz, A.; Weichert, W.; Wiedenmann, B.; Neuhaus, P.; Gessner, R.; Benckert, C.; Jonas, S. Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am. J. Gastroenterol. 2010, 105, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Illades, D.; McMillin, M.; Quinn, M.; DeMorrow, S. Cholangiocarcinoma pathogenesis: Role of the tumor microenvironment. Transl. Gastrointest. Cancer 2012, 1, 71–80. [Google Scholar] [PubMed]
- Aishima, S.; Nishihara, Y.; Iguchi, T.; Taguchi, K.; Taketomi, A.; Maehara, Y.; Tsuneyoshi, M. Lymphatic spread is related to VEGF-C expression and D2-40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Mod. Pathol. 2008, 21, 256–364. [Google Scholar] [CrossRef]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef]
- Rizvi, S.; Mertens, J.C.; Bronk, S.F.; Hirsova, P.; Dai, H.; Roberts, L.R.; Kaufmann, S.H.; Gores, G.J. Platelet-derived growth factor primes cancer-associated fibroblasts for apoptosis. J. Biol. Chem. 2014, 289, 22835–22849. [Google Scholar] [CrossRef]
- Cadamuro, M.; Nardo, G.; Indraccolo, S.; Dall’Olmo, L.; Sambado, L.; Moserle, L.; Franceschet, I.; Colledan, M.; Massani, M.; Stecca, T.; et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 2013, 58, 1042–1053. [Google Scholar] [CrossRef]
- Li, C.Z.; Lin, Y.X.; Huang, T.C.; Pan, J.Y.; Wang, G.X. Receptor-Interacting Protein Kinase 1 Promotes Cholangiocarcinoma Proliferation and Lymphangiogenesis through the Activation Protein 1 Pathway. Onco Targets 2019, 12, 9029–9040. [Google Scholar] [CrossRef]
- Liu, X.Y.; Lai, F.; Yan, X.G.; Jiang, C.C.; Guo, S.T.; Wang, C.Y.; Croft, A.; Tseng, H.Y.; Wilmott, J.S.; Scolyer, R.A.; et al. RIP1 Kinase Is an Oncogenic Driver in Melanoma. Cancer Res. 2015, 75, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Chen, X.; Wang, X.; Li, X.; Du, Q.; Hong, H.; Tang, N.; She, F.; Chen, Y. Expression of the RIP-1 gene and its role in growth and invasion of human gallbladder carcinoma. Cell. Physiol. Biochem. 2014, 34, 1152–1165. [Google Scholar] [CrossRef] [PubMed]
- Bist, P.; Leow, S.C.; Phua, Q.H.; Shu, S.; Zhuang, Q.; Loh, W.T.; Nguyen, T.H.; Zhou, J.B.; Hooi, S.C.; Lim, L.H. Annexin-1 interacts with NEMO and RIP1 to constitutively activate IKK complex and NF-κB: Implication in breast cancer metastasis. Oncogene 2011, 30, 3174–3185. [Google Scholar] [CrossRef]
- Alishekevitz, D.; Gingis-Velitski, S.; Kaidar-Person, O.; Gutter-Kapon, L.; Scherer, S.D.; Raviv, Z.; Merquiol, E.; Ben-Nun, Y.; Miller, V.; Rachman-Tzemah, C.; et al. Macrophage-Induced Lymphangiogenesis and Metastasis following Paclitaxel Chemotherapy Is Regulated by VEGFR3. Cell Rep. 2016, 17, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Perez, R.E.; Rezaiekhaligh, M.H.; Mabry, S.M.; Ekekezie, I.I. Polarized migration of lymphatic endothelial cells is critically dependent on podoplanin regulation of Cdc42. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 300, L32–L42. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.; Flores-Borja, F.; Nassiri, S.; Miranda, E.; Lawler, K.; Grigoriadis, A.; Monypenny, J.; Gillet, C.; Owen, J.; Gordon, P.; et al. Integrin-Mediated Macrophage Adhesion Promotes Lymphovascular Dissemination in Breast Cancer. Cell Rep. 2019, 27, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Bieniasz-Krzywiec, P.; Martín-Pérez, R.; Ehling, M.; García-Caballero, M.; Pinioti, S.; Pretto, S.; Kroes, R.; Aldeni, C.; Di Matteo, M.; Prenen, H.; et al. Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell Metab. 2019, 30, 917–936.e10. [Google Scholar] [CrossRef]
- Oka, M.; Iwata, C.; Suzuki, H.I.; Kiyono, K.; Morishita, Y.; Watabe, T.; Komuro, A.; Kano, M.R.; Miyazono, K. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood 2008, 111, 4571–4579. [Google Scholar] [CrossRef]
- Obulkasim, H.; Shi, X.; Wang, J.; Li, J.; Dai, B.; Wu, P.; Wang, S.; Wang, X.; Ding, Y. Podoplanin is an important stromal prognostic marker in perihilar cholangiocarcinoma. Oncol. Lett. 2018, 15, 137–146. [Google Scholar] [CrossRef]
- Tait, C.R.; Jones, P.F. Angiopoietins in tumours: The angiogenic switch. J. Pathol. 2004, 204, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.W.; Thurston, G.; Hackett, S.F.; Renard, R.; Wang, Q.; McClain, J.; Martin, C.; Witte, C.; Witte, M.H.; Jackson, D.; et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev. Cell 2002, 3, 411–423. [Google Scholar] [CrossRef]
- Fagiani, E.; Lorentz, P.; Kopfstein, L.; Christofori, G. Angiopoietin-1 and -2 exert antagonistic functions in tumor angiogenesis, yet both induce lymphangiogenesis. Cancer Res. 2011, 71, 5717–5727. [Google Scholar] [CrossRef] [PubMed]
- Morisada, T.; Oike, Y.; Yamada, Y.; Urano, T.; Akao, M.; Kubota, Y.; Maekawa, H.; Kimura, Y.; Ohmura, M.; Miyamoto, T.; et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood 2005, 105, 4649–4656. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ye, F. Role of Angiopoietins in Development of Cancer and Neoplasia Associated with Viral Infection. Cells 2020, 9, 457. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, G.; Hau, H.M.; Dietel, C.; Benzing, C.; Krenzien, F.; Brandl, A.; Englisch, J.P.; Wiltberger, G.; Schierle, K.; Robson, S.C.; et al. Prognostic significance of TIE2-expressing monocytes in hilar cholangiocarcinoma. J. Surg. Oncol. 2016, 114, 91–98. [Google Scholar] [CrossRef]
- Tang, D.; Nagano, H.; Yamamoto, H.; Wada, H.; Nakamura, M.; Kondo, M.; Ota, H.; Yoshioka, S.; Kato, H.; Damdinsuren, B.; et al. Angiogenesis in cholangiocellular carcinoma: Expression of vascular endothelial growth factor, angiopoietin-1/2, thrombospondin-1 and clinicopathological significance. Oncol. Rep. 2006, 15, 525–532. [Google Scholar] [CrossRef]
- Carpino, G.; Cardinale, V.; Di Giamberardino, A.; Overi, D.; Donsante, S.; Colasanti, T.; Amato, G.; Mennini, G.; Franchitto, M.; Conti, F.; et al. Thrombospondin 1 and 2 along with PEDF inhibit angiogenesis and promote lymphangiogenesis in intrahepatic cholangiocarcinoma. J. Hepatol. 2021, 75, 1377–1386. [Google Scholar] [CrossRef]
- Lawler, P.R.; Lawler, J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb. Perspect. Med. 2012, 2, a006627. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, P.P.; Peng, R.; Zhou, H. MiR-19a enhances cell proliferation, migration, and invasiveness through enhancing lymphangiogenesis by targeting thrombospondin-1 in colorectal cancer. Biochem. Cell Biol. 2019, 97, 731–739. [Google Scholar] [CrossRef]
- Halin, S.; Rudolfsson, S.H.; Doll, J.A.; Crawford, S.E.; Wikström, P.; Bergh, A. Pigment epithelium-derived factor stimulates tumor macrophage recruitment and is downregulated by the prostate tumor microenvironment. Neoplasia 2010, 12, 336–345. [Google Scholar] [CrossRef]
- Roy, S.; Kumaravel, S.; Banerjee, P.; White, T.K.; O’Brien, A.; Seelig, C.; Chauhan, R.; Ekser, B.; Bayless, K.J.; Alpini, G.; et al. Tumor Lymphatic Interactions Induce CXCR2-CXCL5 Axis and Alter Cellular Metabolism and Lymphangiogenic Pathways to Promote Cholangiocarcinoma. Cells 2021, 10, 3093. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Zawieja, S.D.; Wang, W.; Lee, Y.; Wang, Y.J.; von der Weid, P.Y.; Zawieja, D.C.; Muthuchamy, M. Lipopolysaccharide modulates neutrophil recruitment and macrophage polarization on lymphatic vessels and impairs lymphatic function in rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H2042–H2057. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; De la Cruz, E.; Gu, X.; Balint, L.; Oxendine-Burns, M.; Terrones, T.; Ma, W.; Kuo, H.H.; Lantz, C.; Bansal, T.; et al. Lymphoangiocrine signals promote cardiac growth and repair. Nature 2020, 588, 705–711. [Google Scholar] [CrossRef]
- Bengala, C.; Bertolini, F.; Malavasi, N.; Boni, C.; Aitini, E.; Dealis, C.; Zironi, S.; Depenni, R.; Fontana, A.; Del Giovane, C.; et al. Sorafenib in patients with advanced biliary tract carcinoma: A phase II trial. Br. J. Cancer 2010, 102, 68–72. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, M.; Han, Q.; Tan, Y.; Sun, Y.U.; Bouvet, M.; Singh, S.R.; Ye, J.; Hoffman, R.M. Pazopanib Inhibits Tumor Growth, Lymph-node Metastasis and Lymphangiogenesis of an Orthotopic Mouse of Colorectal Cancer. Cancer Genom. Proteom. 2020, 17, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.; Funahashi, Y.; Uenaka, T.; Watanabe, T.; Tsuruoka, A.; Asada, M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin. Cancer Res. 2008, 14, 5459–5465. [Google Scholar] [CrossRef]
- Kodera, Y.; Katanasaka, Y.; Kitamura, Y.; Tsuda, H.; Nishio, K.; Tamura, T.; Koizumi, F. Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3. Breast Cancer Res. 2011, 13, R66. [Google Scholar] [CrossRef]
- Dufies, M.; Giuliano, S.; Ambrosetti, D.; Claren, A.; Ndiaye, P.D.; Mastri, M.; Moghrabi, W.; Cooley, L.S.; Ettaiche, M.; Chamorey, E.; et al. Sunitinib Stimulates Expression of VEGFC by Tumor Cells and Promotes Lymphangiogenesis in Clear Cell Renal Cell Carcinomas. Cancer Res. 2017, 77, 1212–1226. [Google Scholar] [CrossRef]
- Sun, W.; Patel, A.; Normolle, D.; Patel, K.; Ohr, J.; Lee, J.J.; Bahary, N.; Chu, E.; Streeter, N.; Drummond, S. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2019, 25, 902–909. [Google Scholar] [CrossRef]
- Schmieder, R.; Hoffmann, J.; Becker, M.; Bhargava, A.; Müller, T.; Kahmann, N.; Ellinghaus, P.; Adams, R.; Rosenthal, A.; Thierauch, K.H.; et al. Regorafenib (BAY 73-4506): Antitumor and antimetastatic activities in preclinical models of colorectal cancer. Int. J. Cancer 2014, 135, 1487–1496. [Google Scholar] [CrossRef]
- Bock, F.; Onderka, J.; Dietrich, T.; Bachmann, B.; Kruse, F.E.; Paschke, M.; Zahn, G.; Cursiefen, C. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Hozak, R.R.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.E.; Portnoy, D.C.; Prausová, J.; et al. Analysis of angiogenesis biomarkers for ramucirumab efficacy in patients with metastatic colorectal cancer from RAISE, a global, randomized, double-blind, phase III study. Ann. Oncol. 2018, 29, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Falchook, G.S.; Goldman, J.W.; Desai, J.; Leitch, I.; Hong, D.S.; Subbiah, V.; Kurzrock, R.; Rosen, L.S. A first-in-human phase i study of vgx-100, a selective anti-vegf-c antibody, alone and in combination with bevacizumab in patients with advanced solid tumors. J. Clin. Oncol. 2014, 32, 2524. [Google Scholar] [CrossRef]
- Rinderknecht, M.; Villa, A.; Ballmer-Hofer, K.; Neri, D.; Detmar, M. Phage-derived fully human monoclonal antibody fragments to human vascular endothelial growth factor-C block its interaction with VEGF receptor-2 and 3. PLoS ONE 2010, 5, e11941. [Google Scholar] [CrossRef]
- Saif, M.W.; Knost, J.A.; Chiorean, E.G.; Kambhampati, S.R.; Yu, D.; Pytowski, B.; Qin, A.; Kauh, J.S.; O’Neil, B.H. Phase 1 study of the anti-vascular endothelial growth factor receptor 3 monoclonal antibody LY3022856/IMC-3C5 in patients with advanced and refractory solid tumors and advanced colorectal cancer. Cancer Chemother. Pharm. 2016, 78, 815–824. [Google Scholar] [CrossRef]
- Jimenez, X.; Lu, D.; Brennan, L.; Persaud, K.; Liu, M.; Miao, H.; Witte, L.; Zhu, Z. A recombinant, fully human, bispecific antibody neutralizes the biological activities mediated by both vascular endothelial growth factor receptors 2 and 3. Mol. Cancer 2005, 4, 427–434. [Google Scholar] [CrossRef]
- Zhang, D.; Li, B.; Shi, J.; Zhao, L.; Zhang, X.; Wang, C.; Hou, S.; Qian, W.; Kou, G.; Wang, H.; et al. Suppression of tumor growth and metastasis by simultaneously blocking vascular endothelial growth factor (VEGF)-A and VEGF-C with a receptor-immunoglobulin fusion protein. Cancer Res. 2010, 70, 2495–2503. [Google Scholar] [CrossRef]
- Lin, J.; Lalani, A.S.; Harding, T.C.; Gonzalez, M.; Wu, W.W.; Luan, B.; Tu, G.H.; Koprivnikar, K.; VanRoey, M.J.; He, Y.; et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005, 65, 6901–6909. [Google Scholar] [CrossRef]
- Monk, B.J.; Poveda, A.; Vergote, I.; Raspagliesi, F.; Fujiwara, K.; Bae, D.S.; Oaknin, A.; Ray-Coquard, I.; Provencher, D.M.; Karlan, B.Y.; et al. Final results of a phase 3 study of trebananib plus weekly paclitaxel in recurrent ovarian cancer (TRINOVA-1): Long-term survival, impact of ascites, and progression-free survival-2. Gynecol. Oncol. 2016, 143, 27–34. [Google Scholar] [CrossRef]
- Wang, C.; Chu, M. Advances in Drugs Targeting Lymphangiogenesis for Preventing Tumor Progression and Metastasis. Front. Oncol. 2022, 11, 783309. [Google Scholar] [CrossRef]
- Dwyer, B.J.; Jarman, E.J.; Gogoi-Tiwari, J.; Ferreira-Gonzalez, S.; Boulter, L.; Guest, R.V.; Kendall, T.J.; Kurian, D.; Kilpatrick, A.M.; Robson, A.J.; et al. TWEAK/Fn14 signalling promotes cholangiocarcinoma niche formation and progression. J. Hepatol. 2021, 74, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Boulter, L.; Guest, R.V.; Kendall, T.J.; Wilson, D.H.; Wojtacha, D.; Robson, A.J.; Ridgway, R.A.; Samuel, K.; Van Rooijen, N.; Barry, S.T.; et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J. Clin. Investig. 2015, 125, 1269–1285. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, L.; Cadamuro, M.; Spirlì, C.; Fiorotto, R.; Lecchi, S.; Morell, C.M.; Popov, Y.; Scirpo, R.; De Matteis, M.; Amenduni, M.; et al. Macrophage recruitment by fibrocystin-defective biliary epithelial cells promotes portal fibrosis in congenital hepatic fibrosis. Hepatology 2016, 63, 965–982. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.C.; Fingas, C.D.; Christensen, J.D.; Smoot, R.L.; Bronk, S.F.; Werneburg, N.W.; Gustafson, M.P.; Dietz, A.B.; Roberts, L.R.; Sirica, A.E.; et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013, 73, 897–907. [Google Scholar] [CrossRef]
- Hwang, I.; Kim, J.W.; Ylaya, K.; Chung, E.J.; Kitano, H.; Perry, C.; Hanaoka, J.; Fukuoka, J.; Chung, J.Y.; Hewitt, S.M. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J. Transl. Med. 2020, 18, 443. [Google Scholar] [CrossRef]

| Marker | Function | Structure |
|---|---|---|
| Podoplanin | Mucin-like transmembrane glycoprotein involved in fetal development, platelet aggregation, and migration of T cells and dendritic cells | Transmembrane receptor |
| VEGFR-3 | Cognate receptor for VEGF-C and VEGF-D, involved in normal and tumoral lymphangiogenesis, and in stabilization of lymphatic vessels | Tyrosine kinase receptor |
| Lyve1 | Type I integral membrane glycoprotein, acting as receptor for immobilized and soluble hyaluronan. It is involved in LEC trafficking | Hyaluronan receptor |
| Prox1 | Homeobox transcription factor involved in corneal and lymphatic vessel determination during fetal development, and in stabilization of lymphatic vessels in adults | Transcription factor |
| Nrp-2 | Transmembrane glycoprotein able to bind different ligands. It can act as co-receptor for VEGF-C by binding VEGFR-3 | Transmembrane receptor |
| CCL21 | Specifically expressed by LECs, it mediates the trafficking of immune cells (dendritic cells, T cells and neutrophils) expressing its cognate receptor CCR7 | CC-chemokine |
| β-Chemokine receptor D6 | Receptor expressed by lymphatic endothelium able to bind several ligands (i.e., MCP-1, MCP-3, MIP-1α) | CC-chemokine receptor |
| Desmoplakin | Large desmosomal plaque protein involved in cell adhesion due to its bridging action between desmosomes and desmin filaments | Anchor protein |
| Integrin α9 | Heterodimeric integral membrane specifically binding β1 subunit controlling lymphatic valve formation and lymphatic vessel stabilization | Cell adhesion receptor |
| MRC1 | Type I transmembrane receptor binding to L-selectin and involved in trafficking of lymphocytes | L-selectin receptor |
| Type | Name | Target | Tumor/Disease | Phase | Refs |
|---|---|---|---|---|---|
| TKI | Sorafenib | VEGFRs, PDGFRs, c-Kit, RET, BRAF, FGFRs | HCC, CRC, RCC, thyroid cancer, recurrent glioblastoma | Approved | [74] |
| Pazopanib | VEGFRs, PDGFRs, c-Kit, FGFRs | Advanced/metastatic RCC, CRC, advanced STS | Approved | [75] | |
| Lenvatinib | VEGFRs | Thyroid cancer, RCC | Approved | [76] | |
| Sunitinib | VEGFRs, PDGFRs, c-Kit, RET, CD114, CD135 | Pancreatic neuroendocrine tumors, RCC, imatinib-resistant GIST | Approved | [77,78] | |
| Regorafenib | VEGFRs, TIE2, PDGFR-β, FGFR, KIT, RET, RAF | HCC, RCC, STS, GIST | Approved | [79,80] | |
| Antiangiogenetic mAbs/decoy receptors | Bevacizumab | VEGF-A | Metastatic CRC, breast carcinoma, lung carcinomas, advanced/metastatic RCC, ovarian epithelial carcinoma, primary peritoneal carcinoma, cervix carcinoma | Approved | [81] |
| Ramucirumab | VEGFR-2 | advanced gastric cancer, gastro-esophageal junction adenocarcinoma | Approved | [82] | |
| VGX-100 | VEGF-C | Advanced solid tumors | Phase I | [83] | |
| Single chain fragment (scVf) | VEGF-C | Advanced solid tumors | Preclinical | [84] | |
| IMC-3C5 | VEGFR-3 | Mesothelioma, thymic carcinoma | Phase II | [85,86] | |
| VEGFR-31-ig | VEGFR-3 | HCC | Preclinical | [87,88] | |
| Trebananib | Ang-1/Ang-2 | Angiosarcoma, ovarian cancer, endometrial cancer, RCC, solid tumors | Phase I | [89] | |
| CVX-060 | Ang-2 | Advanced RCC | Phase Ib/II | [90] | |
| AMG780 | Ang-1/Ang-2/Tie-2 | Advanced solid tumors | Phase I | [90] | |
| Nesvacumab | Ang-2 | Solid tumors, diabetic macular edema | Phase I | [90] | |
| Other targets | 2H5 | MCP-1 | CCA | Preclincal | [91] |
| GW-2580 | CSFR1 | Neuroinflammation | Preclinical | [92] | |
| Liposomal clodronate (LIP-CLOD) | Macrophage depletion | CCA, CHF | Preclinical | [92,93] | |
| Navitoclax | Bcl-2 | Lymphomas, advanced solid tumors | Phase I/II | [31,94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadamuro, M.; Romanzi, A.; Guido, M.; Sarcognato, S.; Cillo, U.; Gringeri, E.; Zanus, G.; Strazzabosco, M.; Simioni, P.; Villa, E.; et al. Translational Value of Tumor-Associated Lymphangiogenesis in Cholangiocarcinoma. J. Pers. Med. 2022, 12, 1086. https://doi.org/10.3390/jpm12071086
Cadamuro M, Romanzi A, Guido M, Sarcognato S, Cillo U, Gringeri E, Zanus G, Strazzabosco M, Simioni P, Villa E, et al. Translational Value of Tumor-Associated Lymphangiogenesis in Cholangiocarcinoma. Journal of Personalized Medicine. 2022; 12(7):1086. https://doi.org/10.3390/jpm12071086
Chicago/Turabian StyleCadamuro, Massimiliano, Adriana Romanzi, Maria Guido, Samantha Sarcognato, Umberto Cillo, Enrico Gringeri, Giacomo Zanus, Mario Strazzabosco, Paolo Simioni, Erica Villa, and et al. 2022. "Translational Value of Tumor-Associated Lymphangiogenesis in Cholangiocarcinoma" Journal of Personalized Medicine 12, no. 7: 1086. https://doi.org/10.3390/jpm12071086
APA StyleCadamuro, M., Romanzi, A., Guido, M., Sarcognato, S., Cillo, U., Gringeri, E., Zanus, G., Strazzabosco, M., Simioni, P., Villa, E., & Fabris, L. (2022). Translational Value of Tumor-Associated Lymphangiogenesis in Cholangiocarcinoma. Journal of Personalized Medicine, 12(7), 1086. https://doi.org/10.3390/jpm12071086