Abstract
Cardiovascular disease (CVD) represents the most important cause of mortality and morbidity worldwide. There is heterogeneity in the epidemiology and management of CVD between male and female patients. In the specific case of acute aortic dissection (AAD), women, at the time of diagnosis, are older than men and complain less frequently of an abrupt onset of pain with delayed presentation to the emergency department. Furthermore, a history of hypertension and chronic obstructive pulmonary disease is more common among women. In type A AAD, women more often experience pleural effusion and coronary artery compromise, but experience less neurological and malperfusion symptoms. They undergo less frequent surgical treatment and have higher overall in-hospital mortality. Conversely, in type B AAD no significant differences were shown for in-hospital mortality between the two genders. However, it should be highlighted that further studies are needed in order to develop AAD gender specific preventive, diagnostic and therapeutic strategies.
1. Introduction
Cardiovascular disease (CVD) represents the most important cause of mortality and morbidity worldwide [1,2]. Interestingly, there is evidence of heterogeneity in the mechanism, risk factors, clinical characteristics, and the short- and long-term outcomes of CVD between women and men [3]. Furthermore, women are under-represented in cardiovascular clinical trials, which has reduced the elaboration of gender-specific approaches that could improve guideline recommendations and physicians’ adherence [4]. The aim of the present report was to highlight gender differences in patients with acute aortic dissection (AAD), including risk factors, presenting clinical features, diagnosis, management and outcomes. Pregnancy-related AAD is also discussed.
2. AAD
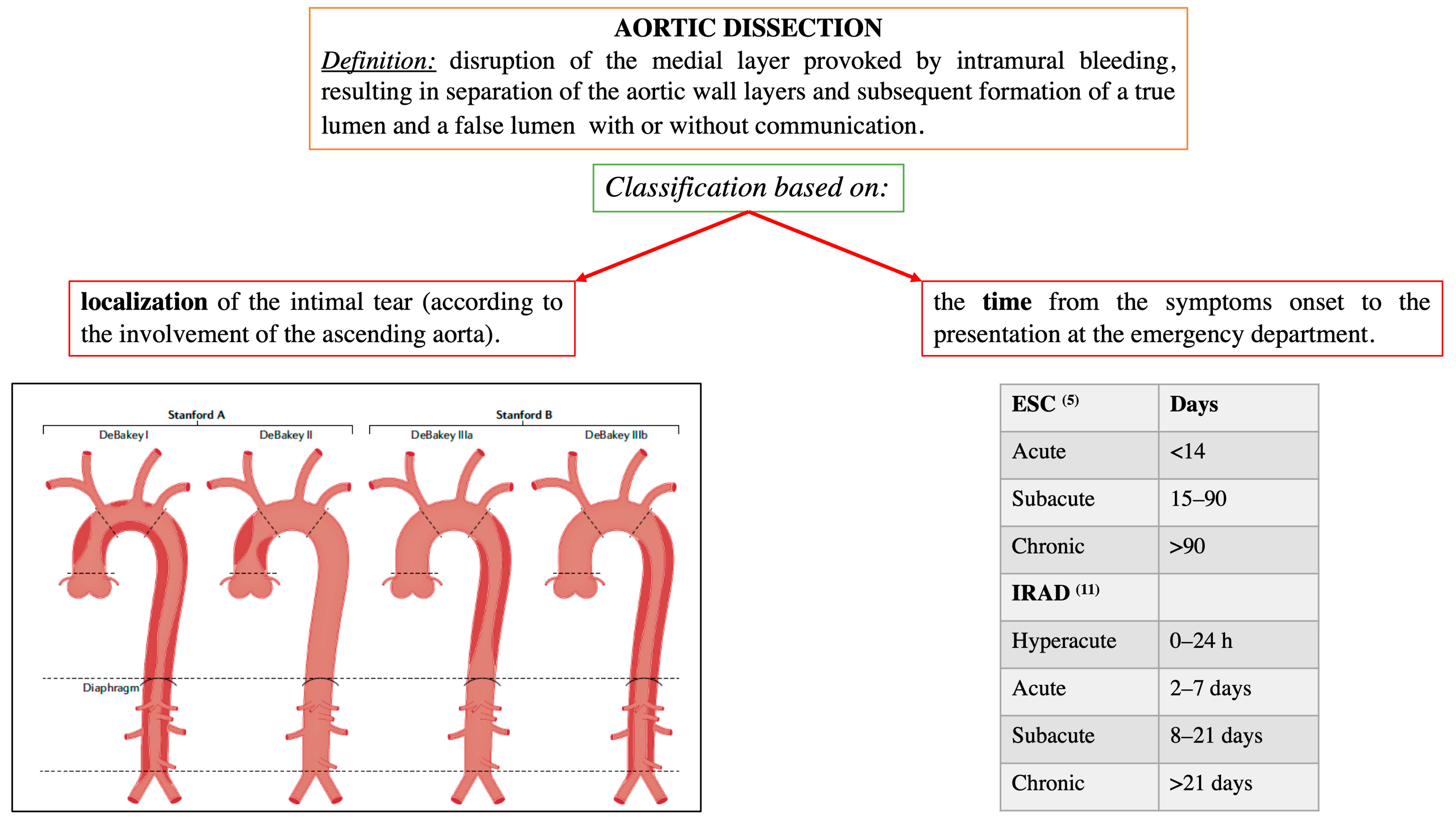
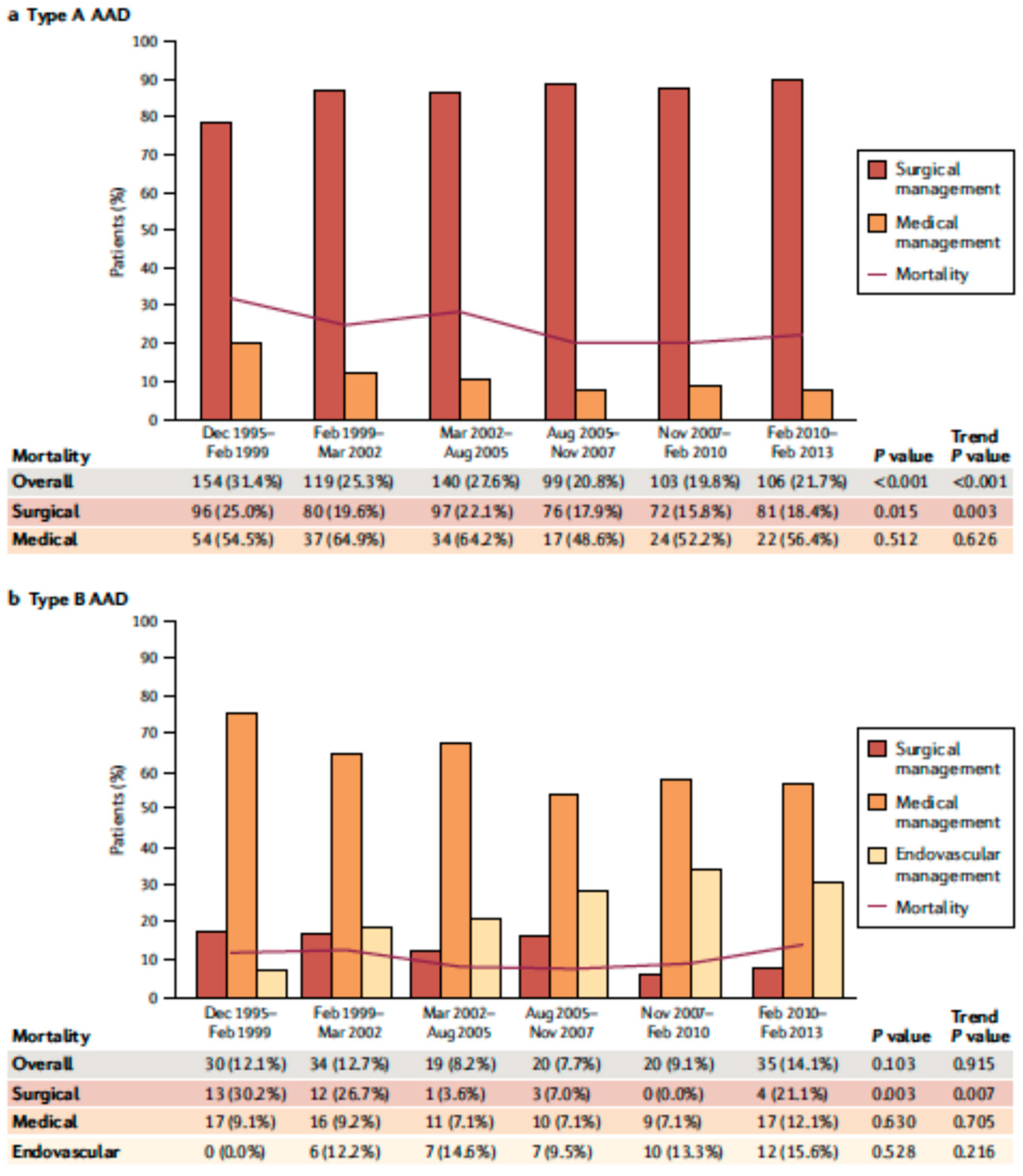
AAD represents a life-threatening emergency, needing prompt diagnosis and appropriate therapeutic interventions (Figure 1) [5,6]. The real incidence of AAD is arduous to define, due to pre-hospital mortality and/or missing diagnoses. Population-based studies show an incidence of 2.6 to 3.5 cases per 100,000 person-years (67% Type A; 33% Type B) [7,8]. Men are more affected than women (9.1 vs. 5.4 per 100,000 men and women, respectively; p < 0.001) [9,10]. Interestingly, women are aged more than men at the time of AAD onset [11]. Due to non-specific symptoms and signs, the diagnosis is often delayed [11]. Therefore, a high index of suspicion is crucial to make the diagnosis. In this regard a specific integrated diagnostic algorithm has been designed, including clinical risk assessment along with biomarkers (D-dimer) and imaging techniques (computed tomography being by far the most utilized) (Figure 2 and Figure 3) [12,13]. Urgent surgery is recommended for type A AAD, while medical treatment is usual for uncomplicated Type B AAD [5,6,14]. Thoracic endovascular aortic repair (TEVAR) is generally indicated in the case of complicated type B AAD [5]. The International Registry of Acute Aortic Dissection (IRAD) highlighted that in-hospital mortality for type A AAD has a downward trend from 31% to 22% (p < 0.001), over 17 years (December 1995 to February 2013), principally related to decreased surgical mortality (25% to 18%; p < 0.003) [15]. However, no changes in-hospital mortality rates for type B AAD (12% to 14%) were observed (Figure 4) [16,17]. Given that AAD is a life-long condition, involving the whole aortic system (holistic concept), patients should obtain optimal blood pressure (≤120/80 mmHg) and heart rate (≤60 bpm) control and imaging follow up (MRI or CT) in order to prevent aorta-related death and major complications [5,6,10,17].
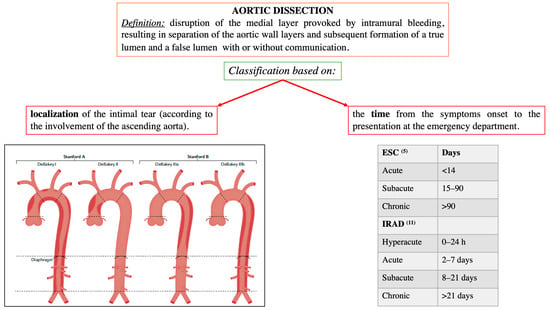
Figure 1.
Localization and time-based definitions of aortic dissection, according to the European and American guidelines [5,6], and the International Registry of Acute Aortic Dissection (IRAD) [11]. Modified by Bossone et al., Nat Rev Cardiol. 2021 May;18(5):331–348 [10].
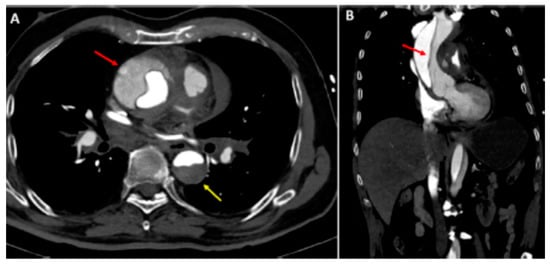
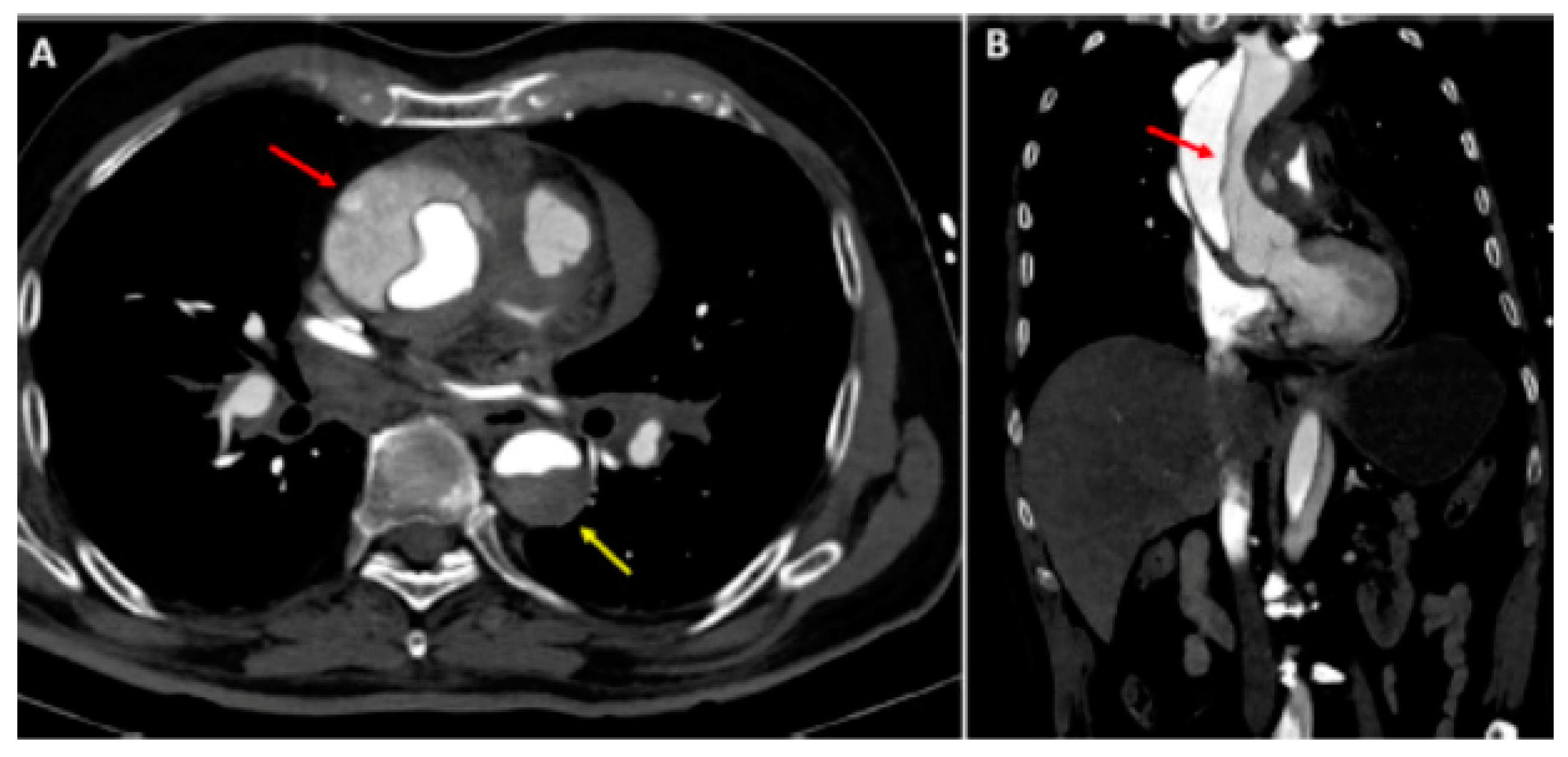
Figure 2.
Type A aortic dissection: (A) Computed tomography axial view. Ascending aorta (red arrow) and descending aorta (yellow arrow) dissection; (B) dissection extending into the right subclavian artery (sagittal view). Reprinted/adapted with permission from Ref. [18]. Copyright year 2021, with permission from Elsevier.
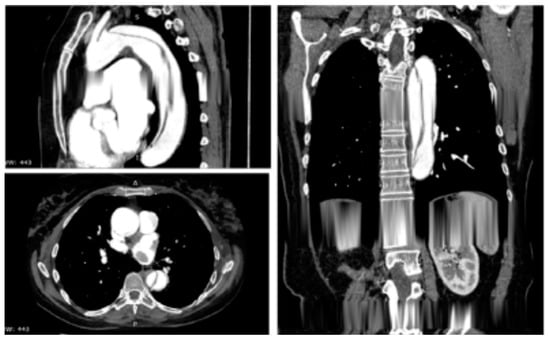
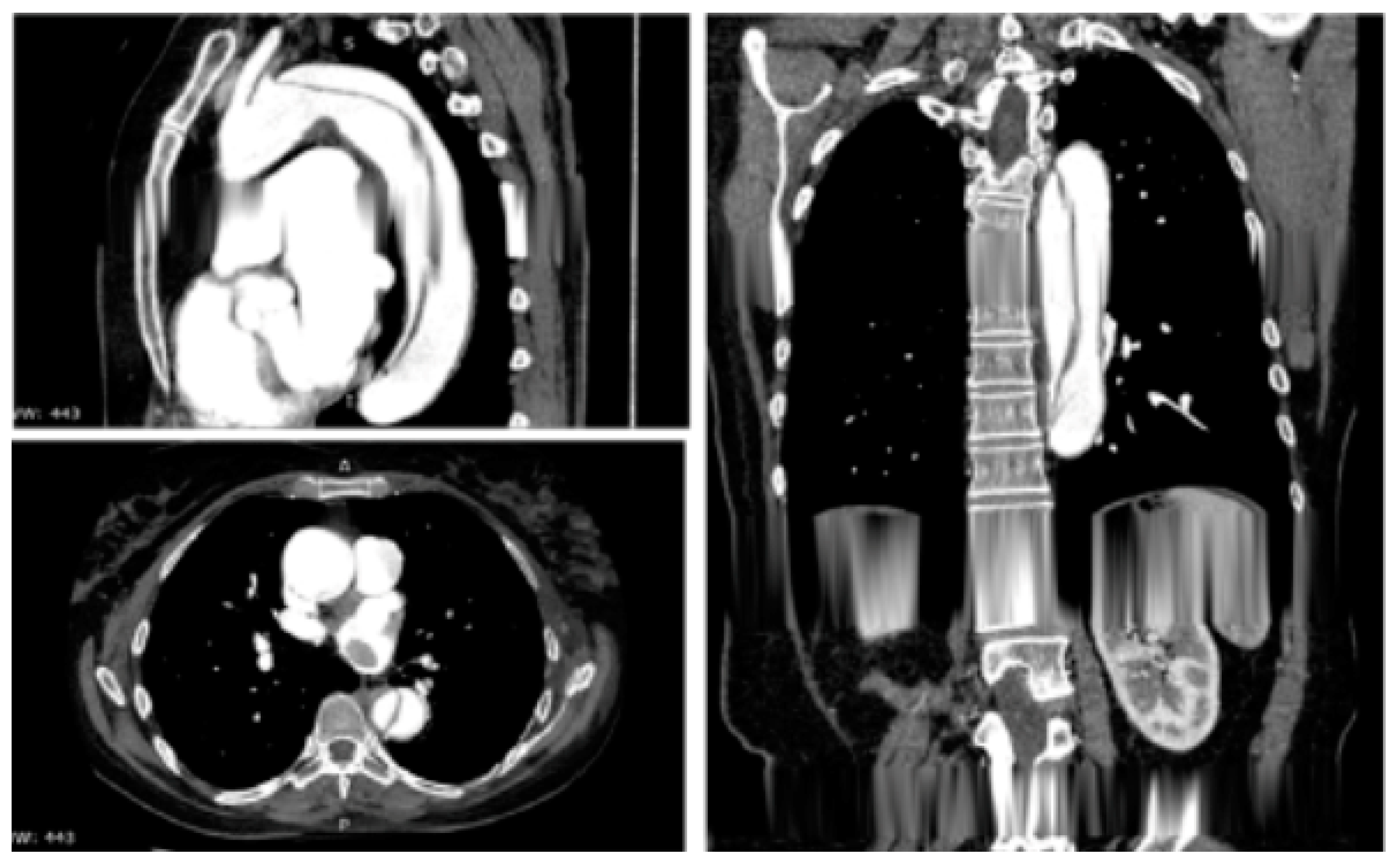
Figure 3.
Contrast-enhanced computed tomography scan reconstruction of type B acute aortic dissection. Reprinted/adapted with permission from Ref. [12]. Copyright year 2020, with permission from Elsevier.
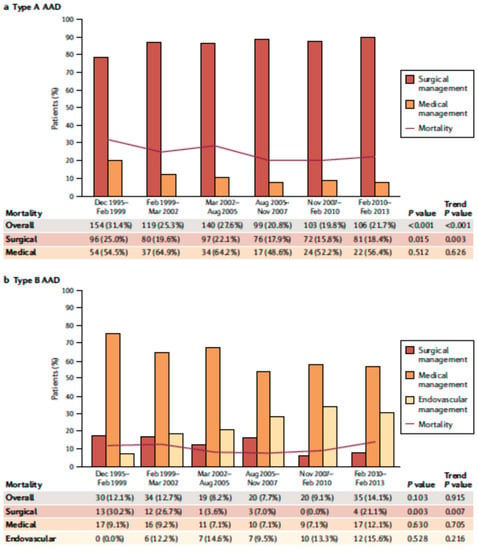
Figure 4.
Over time type A (a) and type B (b) acute aortic dissection in-hospital mortality. Modified by Bossone et al., Nat Rev Cardiol. 2021 May;18(5): 331–348 [10].
3. Impact of Gender in AAD
3.1. Type A AAD
In major population-based studies and registries, women were less frequently affected by type A AAD than men (31 to 48% vs. 52 to 69% respectively) [19,20,21,22,23,24,25]). Furthermore, at time of diagnosis, women were older than men (an average of 6–7 years older) [19,20,21,22,23,24,25].
In this regard, more than 50% of women experienced type A AAD at ≥70 years of age [19,20,21,22,23,24,25,26]. However, Liu et al. [27], in a Chinese population-based study (884 patients; 76.1% male, mean age 51.4 ± 11.8 years) did not show a significant gender difference in age (women 51.4 ± 11.8 vs. men 55.1 ± 12.5 years; p = 0.10).
The German Registry for Acute Aortic Dissection Type A (GERAADA; 56 centers; 3380 patients; 1234 (37%) women vs. 2146 (63%) men), showed that women were older than men (65.5 ± 12.7 vs. 59.2 ± 13.3 years; p < 0.001) [23]. The extension of AAD to the abdominal aorta was more frequent in men than in women (43% vs. 39%; p = 0.01) [23]. Visceral and renal malperfusion were more frequently diagnosed in men (women 32.8% vs. men 38.5%, p = 0.001) [23]. While aortic roots replacement was more frequent in men (21.6 vs. 17.7%; p < 0.001), distinct aortic-arch-repair approaches were distributed similarly in both genders (p = 0.094). Thirty-day mortality (women 16.3% vs. men 16.6%; p = 0.177), as well as the incidence of hemiplegia or hemiparesis after surgery (women 11.5% vs. men 10.1%; p = 0.240), did not differ between the two genders [23].
In the Nordic Consortium for Acute Type A Aortic Dissection (NORCAAD; 1154 patients with type A AAD surgical treatment; January 2005-December 2014) women represented 32%, and were significantly aged (65 ± 11 vs. 60 ± 12 years; p < 0.001), more often had hypertension (58 vs. 48%; p = 0.001) and chronic obstructive pulmonary disease (COPD) and had lower body mass index (26 ± 5 vs. 27 ± 4 kg/m2; p < 0.001), compared with men (8.3% vs. 4.9%; p = 0.03) [20]. Hypothermic cardiac arrest time and operation time were shorter among women (343 ± 133 vs. 374 ± 134.8 min; p < 0.001). There was no difference in intraoperative death or 30-day mortality between the genders (men 6.7% vs. women 9.1%; p = 0.17) [20].
In a recent metanalysis of nine studies comparing clinical outcomes according to gender in type A AAD patients treated surgically [28], women and men showed similar in-hospital/30-day mortality (RR = 1.04; 95% CI, 0.85–1.28; p = 0.67), risk of post-surgical stroke (RR = 1.07; 95% CI, 0.91–1.25; p = 0.43), and dialysis (RR = 0.84; 95% CI, 0.59–1.19; p = 0.32).On the other hand, a lower risk of reintervention for bleeding (RR = 0.84; 95% CI, 0.75–0.94; p < 0.01) was shown in women [28].
Table 1 and Table 2 showed clinical characteristics and outcomes of type A AAD of the major population based studied.

Table 1.
Clinical presentation of AAD, according to type (A, B and A + B) of the major population-based studies.

Table 2.
Short- and long-term outcomes of AAD, according to type (A, B and A+B) of the major population-based studies.
3.2. Type B AAD
Few studies have investigated the impact of gender in type B AAD [26,27,29,30]. Liang et al. [29] identified, from 2009 to 2012, 9855 (women 43.6%, n = 4293) patients with type B AAD. Women experienced AAD at a later age, had more comorbidities (heart failure, COPD, diabetes, rheumatologic disorders), and were more often medically managed compared to men (87.4 vs. 81.8%; p < 0.001) [29]. There were no differences in gender of unadjusted in-hospital mortality rates (women 11.6% vs. men 10.7%; p = 0.2) [29]. In an adjusted propensity-weighted regression analysis, gender did not significantly influence in-hospital mortality (OR = 0.91; 95% confidence interval [CI] 0.79–1.00; p =0 0.2) or stroke rates (OR = 0.91; 95% CI 0.51–1.57; p = 0.7), but women were less likely to have acute renal injury during hospitalization (OR = 0.68; 95% CI 0.60–0.70; p < 0.001) and more likely to experience cardiac events when undergoing open repair (OR = 1.45; 95% CI 1.01–2.11; p = 0.04) [29]. In particular, elderly women (>70 years old) experienced less acute renal failure (OR = 0.72; 95%CI 0.62–0.85; p < 0.001) but had higher odds of acute cardiac events compared to elderly men (OR = 1.20; 95% CI 1.04–1.50; p = 0.02) [29].
Takahashi et al. [30] analyzed data about 2372 (695 women, 29.3%) patients with type B AAD, enrolled in the Tokyo Acute Super-Network Registry. Women were older than men and presented later at the emergency department. Women showed a higher proportion of intramural hematoma (63.7% vs. 53.7%, p < 0.001) and were more medically treated (90.9% vs. 86.3%, p = 0.002), with higher in-hospital mortality (5.3% vs. 2.7%, p = 0.002) [30]. At multivariate analysis, female gender was not associated with higher in-hospital mortality (OR 1.67 [95% CI, 0.96–2.91]) [30].
4. Insights into Gender Related AAD from the IRAD
The IRAD, established in 1996 at the University of Michigan, Ann Arbor, USA, is an observational registry involving 53 highly specialized aortic centers around the world, aiming to assess diagnoses, management, and outcomes of AAD [15,16,31].
According to IRAD data [11] (Table 3), AAD was more frequent in men but women were generally older (overall population n = 1078, 32% women; 49.7% of women were 70 years of age or older vs. 28.6% of men). The type A/B AAD ratio was approximately 2:1 in both genders [11]. Previous cardiac surgery was more common in men, while hypertension was more prevalent in women [11]. Other etiologies or risk factors (atherosclerosis, diabetes, Marfan syndrome, bicuspid aortic valve, cocaine abuse, iatrogenic dissection, previous aortic dissection, previous aortic aneurysm,) were similar between genders [11].

Table 3.
AAD gender-related differences in epidemiological, clinical, treatment and outcomes from International Registry of Acute Aortic Dissection (IRAD) [11,32].
The presentation to hospital, after symptom onset, was significantly more delayed in women than in men (mean absolute difference of 4.7 h), negatively affecting outcomes [11]. Although typical presentation with chest pain was similar in women and men, women were less likely to report an abrupt onset of pain (p = 0.004) [11]. Congestive heart failure (p = 0.03) and coma/neurologic alterations (p = 0.05) were more common in women [11]. Electrocardiographic findings were similar in the two groups. CT was the most utilized imaging in both genders (>70%; 80.6% in men and 76.6% in women) [11]. Tomographic findings suggestive of periaortic hematoma (p = 0.03), pericardial effusion (38.8% vs. 28.6%; p = 0.001), pleural effusion (26.1% vs. 14.7%; p < 0.001) and coronary artery involvement (10.8% vs. 6.9%; p = 0.05), were more frequent among women [11]. Initial medical management with intravenous beta-blockers was less used in women than in men (62.1% in men vs. 55.6% in women; p = 0.05) [11].
There was no difference in surgical techniques for both type A and B AAD, however more women were medically treated than men (35% in men vs. 45.7% in women; p = 0.001) [11]. In-hospital complications, such as hypotension (0.001) and cardiac tamponade (p = 0.007) occurred more among women [11]. On the other hand, limb ischemia was more common in men (p = 0.04) [11].
Women showed higher overall in-hospital mortality (type A AAD + type B AAD) than men (p = 0.001) [11]. Women had lower survival than men for type A AAD (log rank p = 0.01) but not for type B AAD (log rank p = 0.47) [11]. Furthermore, women showed the greatest in-hospital mortality for surgically treated type A AAD (31.9% mortality in women vs. 21.9% in men, p = 0.013) [11].
No significant gender-related difference was shown for type A AAD medically treated mortality [11]. Interestingly, in the advanced age cohort (>75 years) women with type A AAD were treated more with only medical treatment than men (31.4% vs. 14.0%; p = 0.04). In the analysis stratified by age (age < 50, 50 to 65, 66 to 75, and >75 years), major differences in mortality between gender were shown in the 66- to 75-year age group (36% vs. 16%; p = 0.001) [11]. Older age and less typical symptoms at onset of AAD have been proposed as possible factors contributing to poorer outcomes in women.
A more recent analysis of an IRAD-Interventional Cohort (IVC) [32], consisting of more than 2823 type A AAD patients treated with endovascular, surgical, or hybrid procedures has partly confirmed the previous IRAD data about gender differences (Table 1). Of particular interest was the fact that, overall, in-hospital mortality was 16.7% in women (n = 162) and 13.8% in men (n = 256, p = 0.039). The frequency of postoperative complications was similar between genders, except for acute kidney injury, which was lower in women (17.7% vs. 21.2%, p = 0.029) [32].
However, five-years survival (82.6% in women vs. 85.9% in men) and reintervention (87.8% in women vs. 87.6% in men) were similar between genders [32]. Furthermore, the authors found that operative approaches were different between genders. As a note, complete arch replacement (OR = 7.3; 95% CI 2.07–25; p = 0.002) along with age (OR = 1.04; 95% CI 1.03–1.05; p < 0.001), renal failure (OR = 2.68; 95% CI 1.91–3.74; p < 0.001), coma (OR = 13.38; 95% CI 7.87–22.73; p < 0.001), limb ischemia (OR = 1.87; 95% CI 1.23–2.86; p = 0.003), and cardiopulmonary bypass time (OR = 1.01; 95% CI 1.01–1.01; p < 0.001) were independent predictors of in-hospital mortality in both genders [32].
In summary, data from IRAD highlighting women as compared to men show women to have the following: (a) older age, higher incidence of a history of hypertension and more delayed presentation to hospital; (b) more complications, such as periaortic hematoma, pericardial effusion, pleural effusion and coronary artery involvement; (c) higher overall and surgical type A AAD in-hospital mortality.
5. AAD in Pregnancy
Among 9707 AAD patients (3341 women [34.4%]) enrolled in the IRAD registry from 1998 to 2019, 29 women (0.3%, mean age, 32 ± 6 years) experienced pregnancy-related AAD, 13 (45%) had type A AAD and 16 (55%) had type B AAD [33,34,35,36]. AAD occurred in 15 pregnant women (4 in the first and 11 in the third trimester) and in 12 during the post-partum period. Twenty women (69%) had predisposing conditions: 13 (65%) Marfan syndrome, 2 (10%) Loeys-Dietz syndrome, 2 (10%) BAV, 2 (10%) family history of aortic disease and 1 (5%) familial thoracic aortic aneurysm [35]. In type A AAD, the mean aortic diameters at diagnosis were 54.5 (±5) at sinus of Valsalva and 54.7 (±7) at ascending aorta and in type B AAD was 32.5 (±5) at descending aorta [33]. Twenty-eight women (97%) survived at hospital discharge [mortality rate of 3% (1/29)] [33].
In The NHLBI National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC) [37], among 94 women with Marfan syndrome, 10 (10.6%) experienced aortic complications during or post pregnancy (4 type A and 3 type BAAD). Regarding postpartum, 5 of 7 AAD including all 3 type B, experienced complications. Only 5 of 8 women were conscious of their Marfan syndrome diagnosis [37].
In the Nationwide Inpatient Sample of more than 10 million pregnancies and 41,000 AADs from 1998 to 2008, 44 cases of AAD in pregnancy were described, representing 0.1% of all cases of AAD. Only 7 of the 44 cases had Marfan syndrome, with 2 other women having other congenital anomalies [38]. The in-hospital mortality was of 6.8% for AAD during pregnancy vs. 15.4% of all AAD [38].
Kamel et al. [39] described 36 cases of pregnancy-related AAD, out of 6,566,826 pregnancies from 2005 to 2013. Aortic complications occurred in 5.5 (95% confidence interval [CI], 4.0–7.8) per million patients during pregnancy and in postpartum, compared with 1.4 (95% CI, 0.7–2.9) per million during the equivalent period 1 year later [38]. Pregnancy was associated with a significantly higher risk of AAD (incidence rate ratio, 4.0; 95% CI, 2.0–8.2) compared to the control period 1 year later [39].
Thus, AAD is a rare, but serious, complication of pregnancy [in the majority of cases type A AAD (57–80%)] [40,41,42,43]. There is no evidence of increased in-hospital mortality in pregnancy-related AAD. However, it should be underlined that the recognition of predisposing conditions (namely aortopathies, often not diagnosed until the acute event) and the monitoring of the aorta diameters throughout pregnancy and in post-partum, may reduce complication rates and improve outcomes.
6. Conclusions
A comprehensive understanding of gender differences in AAD is lacking. For both type A and B AAD, women, at the time of diagnosis, are older than men and complain less frequently about abrupt onset of pain, with delayed presentation to the emergency department. Furthermore, a history of hypertension and chronic obstructive pulmonary disease is more common among women. In type A AAD, women more often experience pleural effusion and coronary artery compromise but experience less neurological and malperfusion symptoms. They undergo less frequent surgical treatment and have higher overall in-hospital mortality. On the other hand, in type B AAD no significant differences are registered for in-hospital mortality between the two genders. A greater knowledge of gender differences in AAD risk factors, clinical presentation and treatment may improve diagnostic accuracy, along with short- and long-term prognosis. However, it should be highlighted that further studies are needed in order to develop AAD gender-tailored preventive, diagnostic and therapeutic strategies.
Author Contributions
Conceptualization, E.B.; methodology, E.B. and A.C.; validation, E.B. and K.A.E.; writing—original draft preparation, A.C.; writing—review and editing, E.B.; supervision, K.A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M.; et al. Global, Regional, and National Burden of Stroke, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [Green Version]
- Zipes, D.P.; Libby, P.; Bonow, R.O.; Mann, D.L.; Tomaselli, G.F. Braunwald’s Heart Disease a Textbook of Cardiovascular Medicine; Chapter Book; Elsevier/Saunders: Philadelphia, PA, USA, 2019. [Google Scholar]
- Connelly, P.J.; Azizi, Z.; Alipour, P.; Delles, C.; Pilote, L.; Raparelli, V. The Importance of Gender to Understand Sex Differences in Cardiovascular Disease. Can. J. Cardiol. 2021, 37, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Bairey Merz, C.N.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.; et al. The Lancet Women and Cardiovascular Disease Commission: Reducing the Global Burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the Diagnosis and Treatment of Aortic Diseases: Document Covering Acute and Chronic Aortic Diseases of the Thoracic and Abdominal Aorta of the Adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients with Thoracic Aortic Disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J. Am. Coll. Cardiol. 2010, 55, e27–e129. [Google Scholar] [CrossRef] [Green Version]
- Clouse, W.D.; Hallett, J.W.; Schaff, H.V.; Spittell, P.C.; Rowland, C.M.; Ilstrup, D.M.; Melton, L.J. Acute Aortic Dissection: Population-Based Incidence Compared with Degenerative Aortic Aneurysm Rupture. Mayo Clin. Proc. 2004, 79, 176–180. [Google Scholar] [CrossRef]
- Mészáros, I.; Mórocz, J.; Szlávi, J.; Schmidt, J.; Tornóci, L.; Nagy, L.; Szép, L. Epidemiology and Clinicopathology of Aortic Dissection. Chest 2000, 117, 1271–1278. [Google Scholar] [CrossRef]
- Smedberg, C.; Steuer, J.; Leander, K.; Hultgren, R. Sex Differences and Temporal Trends in Aortic Dissection: A Population-Based Study of Incidence, Treatment Strategies, and Outcome in Swedish Patients during 15 Years. Eur. Heart J. 2020, 41, 2430–2438. [Google Scholar] [CrossRef]
- Bossone, E.; Eagle, K.A. Epidemiology and Management of Aortic Disease: Aortic Aneurysms and Acute Aortic Syndromes. Nature reviews. Cardiology 2021, 18, 331–348. [Google Scholar] [CrossRef]
- Nienaber, C.A.; Fattori, R.; Mehta, R.H.; Richartz, B.M.; Evangelista, A.; Petzsch, M.; Cooper, J.V.; Januzzi, J.L.; Ince, H.; Sechtem, U.; et al. Gender-Related Differences in Acute Aortic Dissection. Circulation 2004, 109, 3014–3021. [Google Scholar] [CrossRef] [Green Version]
- Bossone, E.; Ranieri, B.; Romano, L.; Russo, V.; Barbuto, L.; Cocchia, R.; Pezzullo, F.; Amato, C.; Vriz, O.; Di Tommaso, L.; et al. Acute Aortic Syndromes: Diagnostic and Therapeutic Pathways. Heart Fail. Clin. 2020, 16, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Mauro, C.; Vriz, O.; Romano, L.; Citro, R.; Russo, V.; Ranieri, B.; Alamro, B.; Aladmawi, M.; Granata, R.; Galzerano, D.; et al. Imaging Cardiovascular Emergencies: Real World Clinical Cases. Heart Fail. Clin. 2020, 16, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; LaBounty, T.M.; Eagle, K.A. Acute Aortic Syndromes: Diagnosis and Management, an Update. Eur. Heart J. 2018, 39, 739–749d. [Google Scholar] [CrossRef]
- Evangelista, A.; Isselbacher, E.M.; Bossone, E.; Gleason, T.G.; Eusanio, M.D.; Sechtem, U.; Ehrlich, M.P.; Trimarchi, S.; Braverman, A.C.; Myrmel, T.; et al. Insights from the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation 2018, 137, 1846–1860. [Google Scholar] [CrossRef]
- Pape, L.A.; Awais, M.; Woznicki, E.M.; Suzuki, T.; Trimarchi, S.; Evangelista, A.; Myrmel, T.; Larsen, M.; Harris, K.M.; Greason, K.; et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends from the International Registry of Acute Aortic Dissection. J. Am. Coll. Cardiol. 2015, 66, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Isselbacher, E.M.; Bonaca, M.P.; Di Eusanio, M.; Froehlich, J.; Bossone, E.; Sechtem, U.; Pyeritz, R.; Patel, H.; Khoynezhad, A.; Eckstein, H.-H.; et al. Recurrent Aortic Dissection: Observations from the International Registry of Aortic Dissection. Circulation 2016, 134, 1013–1024. [Google Scholar] [CrossRef]
- Bossone, E.; Czerny, M.; Lerakis, S.; Rodríguez-Palomares, J.; Kukar, N.; Ranieri, B.; Russo, V.; Punzo, B.; Cocchia, R.; Cademartiri, F.; et al. Imaging and Biomarkers in Acute Aortic Syndromes: Diagnostic and Prognostic Implications. Curr. Probl. Cardiol. 2021, 46, 100654. [Google Scholar] [CrossRef]
- Fukui, T.; Tabata, M.; Morita, S.; Takanashi, S. Gender Differences in Patients Undergoing Surgery for Acute Type A Aortic Dissection. J. Thorac. Cardiovasc. Surg. 2015, 150, 581–587.e1. [Google Scholar] [CrossRef]
- Chemtob, R.A.; Hjortdal, V.; Ahlsson, A.; Gunn, J.; Mennander, A.; Zindovic, I.; Olsson, C.; Pivodic, A.; Hansson, E.C.; Jeppsson, A.; et al. Effects of Sex on Early Outcome Following Repair of Acute Type A Aortic Dissection: Results from The Nordic Consortium for Acute Type A Aortic Dissection (NORCAAD). Aorta 2019, 7, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Conway, B.D.; Stamou, S.C.; Kouchoukos, N.T.; Lobdell, K.W.; Hagberg, R.C. Effects of Gender on Outcomes and Survival Following Repair of Acute Type A Aortic Dissection. Int. J. Angiol. 2015, 24, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, C.; Salem, M.A.; Puehler, T.; Hoffmann, G.; Lutter, G.; Cremer, J.; Haneya, A. Sex-Specific Risk Factors for Early Mortality and Survival after Surgery of Acute Aortic Dissection Type a: A Retrospective Observational Study. J. Cardiothorac. Surg. 2020, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Rylski, B.; Georgieva, N.; Beyersdorf, F.; Busch, C.; Boening, A.; Haunschild, J.; Etz, C.D.; Luehr, M.; Kallenbach, K.; German Registry for Acute Aortic Dissection Type A Working Group of the German Society of Thoracic, Cardiac, and Vascular Surgery. Gender-Related Differences in Patients with Acute Aortic Dissection Type A. J. Thorac. Cardiovasc. Surg. 2021, 162, 528–535.e1. [Google Scholar] [CrossRef] [PubMed]
- Sabashnikov, A.; Heinen, S.; Deppe, A.C.; Zeriouh, M.; Weymann, A.; Slottosch, I.; Eghbalzadeh, K.; Popov, A.F.; Liakopoulos, O.; Rahmanian, P.B.; et al. Impact of Gender on Long-Term Outcomes after Surgical Repair for Acute Stanford a Aortic Dissection: A Propensity Score Matched Analysis. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 702–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Asai, T.; Kinoshita, T. Clinical Differences between Men and Women Undergoing Surgery for Acute Type A Aortic Dissection. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 944–950. [Google Scholar] [CrossRef]
- Maitusong, B.; Sun, H.P.; Xielifu, D.; Mahemuti, M.; Ma, X.; Liu, F.; Xie, X.; Azhati, A.; Zhou, X.R.; Ma, Y.T. Sex-Related Differences Between Patients with Symptomatic Acute Aortic Dissection. Medicine 2016, 95, e3100. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, X.Z.; Wang, Y.; He, R.X.; Yang, L.; Jing, Q.M.; Liu, H.W. Correlation between Sex and Prognosis of Acute Aortic Dissection in the Chinese Population. Chin. Med. J. 2018, 131, 1430–1435. [Google Scholar] [CrossRef]
- Lawrence, K.W.; Yin, K.; Connelly, H.L.; Datar, Y.; Brydges, H.; Balasubramaniyan, R.; Karlson, K.J.; Edwards, N.M.; Dobrilovic, N. Sex-Based Outcomes in Surgical Repair of Acute Type A Aortic Dissection: A Meta-Analysis and Meta-Regression. J. Thorac. Cardiovasc. Surg. 2022; in press. [Google Scholar] [CrossRef]
- Liang, N.L.; Genovese, E.A.; Al-Khoury, G.E.; Hager, E.S.; Makaroun, M.S.; Singh, M.J. Effects of Gender Differences on Short-Term Outcomes in Patients with Type B Aortic Dissection. Ann. Vasc. Surg. 2017, 38, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Yoshino, H.; Akutsu, K.; Shimokawa, T.; Ogino, H.; Kunihara, T.; Usui, M.; Watanabe, K.; Kawata, M.; Masuhara, H.; et al. Sex-Related Differences in Clinical Features and In-Hospital Outcomes of Type B Acute Aortic Dissection: A Registry Study. J. Am. Heart Assoc. 2022, 11, e024149. [Google Scholar] [CrossRef]
- Hagan, P.G.; Nienaber, C.A.; Isselbacher, E.M.; Bruckman, D.; Karavite, D.J.; Russman, P.L.; Evangelista, A.; Fattori, R.; Suzuki, T.; Oh, J.K.; et al. The International Registry of Acute Aortic Dissection (IRAD): New Insights into an Old Disease. Jama 2000, 283, 897–903. [Google Scholar] [CrossRef]
- Huckaby, L.V.; Sultan, I.; Trimarchi, S.; Leshnower, B.; Chen, E.P.; Brinster, D.R.; Myrmel, T.; Estrera, A.L.; Montgomery, D.G.; Korach, A.; et al. Sex-Based Aortic Dissection Outcomes from the International Registry of Acute Aortic Dissection. Ann. Thorac. Surg. 2022, 113, 498–505. [Google Scholar] [CrossRef]
- Braverman, A.C.; Mittauer, E.; Harris, K.M.; Evangelista, A.; Pyeritz, R.E.; Brinster, D.; Conklin, L.; Suzuki, T.; Fanola, C.; Ouzounian, M.; et al. Clinical Features and Outcomes of Pregnancy-Related Acute Aortic Dissection. JAMA Cardiol. 2021, 6, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Cauldwell, M.; Steer, P.J.; Curtis, S.L.; Mohan, A.; Dockree, S.; Mackillop, L.; Parry, H.M.; Oliver, J.; Sterrenberg, M.; Wallace, S.; et al. Maternal and Fetal Outcomes in Pregnancies Complicated by Marfan Syndrome. Heart 2019, 105, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- den Hartog, A.W.; Franken, R.; Zwinderman, A.H.; Timmermans, J.; Scholte, A.J.; van den Berg, M.P.; de Waard, V.; Pals, G.; Mulder, B.J.M.; Groenink, M. The Risk for Type B Aortic Dissection in Marfan Syndrome. J. Am. Coll. Cardiol. 2015, 65, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Braverman, A.C.; Moon, M.R.; Geraghty, P.; Willing, M.; Bach, C.; Kouchoukos, N.T. Pregnancy after Aortic Root Replacement in Loeys-Dietz Syndrome: High Risk of Aortic Dissection. Am. J. Med. Genet. Part A 2016, 170, 2177–2180. [Google Scholar] [CrossRef]
- Roman, M.J.; Pugh, N.L.; Hendershot, T.P.; Devereux, R.B.; Dietz, H.; Holmes, K.; Eagle, K.A.; LeMaire, S.A.; Milewicz, D.M.; Morris, S.A.; et al. Aortic Complications Associated with Pregnancy in Marfan Syndrome: The NHLBI National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC). J. Am. Heart Assoc. 2016, 5, e004052. [Google Scholar] [CrossRef] [Green Version]
- Sawlani, N.; Shroff, A.; Vidovich, M.I. Aortic Dissection and Mortality Associated with Pregnancy in the United States. J. Am. Coll. Cardiol. 2015, 65, 1600–1601. [Google Scholar] [CrossRef] [Green Version]
- Kamel, H.; Roman, M.J.; Pitcher, A.; Devereux, R.B. Pregnancy and the Risk of Aortic Dissection or Rupture: A Cohort-Crossover Analysis. Circulation 2016, 134, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Wolfe, D.S.; Taub, C.C. Cardiovascular Outcomes of Pregnancy in Marfan’s Syndrome Patients: A Literature Review. Congenit. Heart Dis. 2018, 13, 203–209. [Google Scholar] [CrossRef]
- Ma, W.G.; Zhu, J.M.; Chen, Y.; Qiao, Z.Y.; Ge, Y.P.; Li, C.N.; Zheng, J.; Liu, Y.M.; Sun, L.Z. Aortic Dissection during Pregnancy and Postpartum in Patients with Marfan Syndrome: A 21-Year Clinical Experience in 30 Patients. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2020, 58, 294–301. [Google Scholar] [CrossRef]
- Mimoun, L.; Detaint, D.; Hamroun, D.; Arnoult, F.; Delorme, G.; Gautier, M.; Milleron, O.; Meuleman, C.; Raoux, F.; Boileau, C.; et al. Dissection in Marfan Syndrome: The Importance of the Descending Aorta. Eur. Heart J. 2011, 32, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Schoenhoff, F.S.; Jungi, S.; Czerny, M.; Roost, E.; Reineke, D.; Matyas, G.; Steinmann, B.; Schmidli, J.; Kadner, A.; Carrel, T. Acute Aortic Dissection Determines the Fate of Initially Untreated Aortic Segments in Marfan Syndrome. Circulation 2013, 127, 1569–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).