The Application of Artificial-Intelligence-Assisted Dental Age Assessment in Children with Growth Delay
Abstract
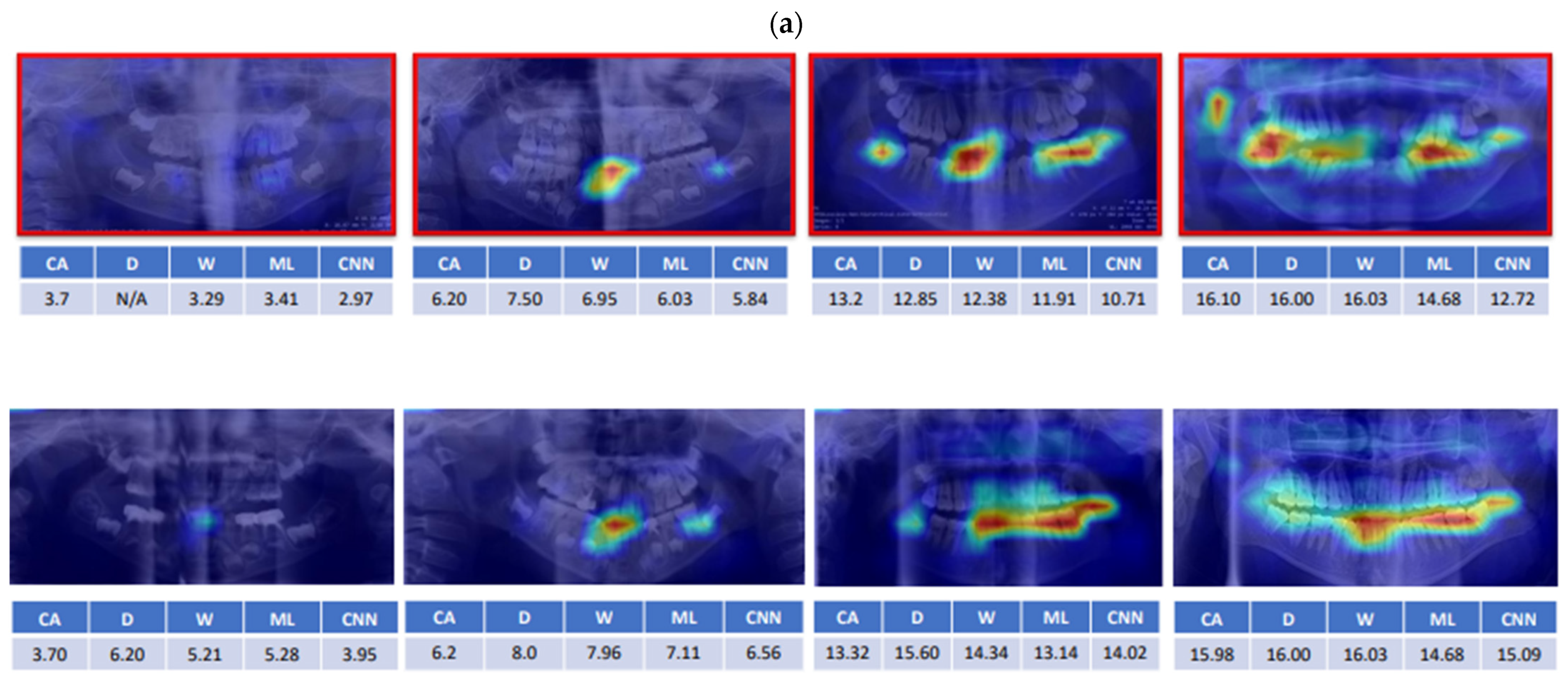
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.1.1. The Images Collected from the Healthy Children
2.1.2. The Images Collected from Children with GD
2.1.3. TDS Recording
2.1.4. The Development of AI-Assisted Modalities
2.1.5. The Training of the ML Algorithm
2.1.6. The Training of the CNN Models
2.1.7. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krekmanova, L.; Carlstedt-Duke, J.; Brönnegård, M.; Marcus, C.; Gröndahl, E.; Modéer, T.; Dahllöf, G. Dental maturity in children of short stature, with or without growth hormone deficiency. Eur. J. Oral Sci. 1997, 105, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Krekmanova, L.; Carlstedt-Duke, J.; Marcus, C.; Dahllöf, G. Dental maturity in children of short stature--a two-year longitudinal study of growth hormone substitution. Acta Odontol. Scand. 1999, 57, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.; Afzal, M.; Qureshi, S.M.; Aziz, S.; Lutfullah, M.; Khan, S.A.; Iqbal, M.; Maqsood, S.U.; Sadiq, N.; Farid, N. Etiology of short stature in children. J. Coll. Physicians Surg. Pak. 2008, 18, 493–497. [Google Scholar] [PubMed]
- Kjellberg, H.; Beiring, M.; Wikland, K.A. Craniofacial morphology, dental occlusion, tooth eruption, and dental maturity in boys of short stature with or without growth hormone deficiency. Eur. J. Oral Sci. 2000, 108, 359–367. [Google Scholar] [CrossRef]
- Chaillet, N.; Nyström, M.; Demirjian, A. Comparison of dental maturity in children of different ethnic origins: International maturity curves for clinicians. J. Forensic Sci. 2005, 50, 1164–1174. [Google Scholar] [CrossRef]
- Shamim, T. Forensic odontology. J. Coll. Physicians Surg. Pak. 2012, 22, 240–245. [Google Scholar]
- Demirjian, A.; Buschang, P.H.; Tanguay, R.; Patterson, D.K. Interrelationships among measures of somatic, skeletal, dental, and sexual maturity. Am. J. Orthod. 1985, 88, 433–438. [Google Scholar] [CrossRef]
- Perinetti, G.; Contardo, L.; Gabrieli, P.; Baccetti, T.; Di Lenarda, R. Diagnostic performance of dental maturity for identification of skeletal maturation phase. Eur. J. Orthod. 2012, 34, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Vallejo-Bolaños, E.; España-López, A.J. The relationship between dental age, bone age and chronological age in 54 children with short familial stature. Int. J. Paediatr. Dent. 1997, 7, 15–17. [Google Scholar] [CrossRef]
- Haavikko, K. Tooth formation age estimated on a few selected teeth. A simple method for clinical use. Procc. Finn. Dent. Soc. 1974, 70, 15–19. [Google Scholar]
- Demirjian, A.; Goldstein, H.; Tanner, J.M. A new system of dental age assessment. Hum. Biol. 1973, 45, 211–227. [Google Scholar] [PubMed]
- Kumar, V.; Hegde, S.K.; Bhat, S.S. The relationship between dental age, bone age and chronological age in children with short stature. Int. J. Contemp. Dent. 2011, 2, 6–11. [Google Scholar]
- Willems, G. A review of the most commonly used dental age estimation techniques. J. Forensic Odonto-Stomatol. 2001, 19, 9–17. [Google Scholar]
- Jayaraman, J.; Wong, H.M.; King, N.M.; Roberts, G.J. The French–Canadian data set of Demirjian for dental age estimation: A systematic review and meta-analysis. J. Forensic Leg. Med. 2013, 20, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, J.; Singh, M. Willems method of dental age estimation in children: A systematic review and meta-analysis. J. Forensic Leg. Med. 2017, 52, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Galibourg, A.; Cussat-Blanc, S.; Dumoncel, J.; Telmon, N.; Monsarrat, P.; Maret, D. Comparison of different machine learning approaches to predict dental age using Demirjian’s staging approach. Int. J. Leg. Med. 2021, 135, 665–675. [Google Scholar] [CrossRef]
- Te-Ju Wu, C.L.T.; Huang, Y.-H.; Fan, T.-Y.; Chen, Y.-P. Efficacy of machine-learning assisted dental age assessment in Han population. Leg. Med. under processing.
- Kim, J.; Bae, W.; Jung, K.-H.; Song, I.-S. Development and validation of deep learning-based algorithms for the estimation of chronological age using panoramic dental x-ray images. Proc. Mach. Learn. Res. 2019. Available online: https://openreview.net/revisions?id=BJg4tI2VqV (accessed on 1 May 2022).
- Pan, J.; Shen, C.; Yang, Z.; Fan, L.; Wang, M.; Shen, S.; Tao, J.; Ji, F. A modified dental age assessment method for 5-to 16-year-old eastern Chinese children. Clin. Oral Investig. 2021, 25, 3463–3474. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Levin, B.; Paik, M.C. Statistical Methods for Rates and Proportions; John Wiley Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kuremoto, K.; Okawa, R.; Matayoshi, S.; Kokomoto, K.; Nakano, K. Estimation of dental age based on the developmental stages of permanent teeth in Japanese children and adolescents. Sci. Rep. 2022, 12, 3345. [Google Scholar] [CrossRef]
- Thesleff, I. The genetic basis of tooth development and dental defects. Am. J. Med. Genet. Part A 2006, 140, 2530–2535. [Google Scholar] [CrossRef]
- Sezer, B.; Çarıkçıoğlu, B.; Kargül, B. Dental age and tooth development in children with molar-incisor hypomineralization: A case-control study. Arch. Oral Biol. 2022, 134, 105325. [Google Scholar] [CrossRef]
- Owlia, F.; Akhavan-Karbassi, M.-H.; Rahimi, R. Could molar-incisor hypomineralization (MIH) existence be predictor of short stature? Int. J. Prev. Med. 2020, 11, 101. [Google Scholar] [PubMed]
- Kvaal, S.; Solheim, T. A non-destructive dental method for age estimation. J. Forensic Odonto-Stomatol. 1994, 12, 6–11. [Google Scholar]
- Cameriere, R.; Ferrante, L.; Cingolani, M. Variations in pulp/tooth area ratio as an indicator of age: A preliminary study. J. Forensic Sci. 2004, 49, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Panchbhai, A. Dental radiographic indicators, a key to age estimation. Dentomaxillofacial Radiol. 2011, 40, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Gulsahi, A.; Kulah, C.K.; Bakirarar, B.; Gulen, O.; Kamburoglu, K. Age estimation based on pulp/tooth volume ratio measured on cone-beam CT images. Dentomaxillofacial Radiol. 2018, 47, 20170239. [Google Scholar] [CrossRef]

| Girls | |||||
| Prediction Error | N | Mean (Age) | Std. Deviation (Age) | Std. Error Mean (Age) | p Value |
| CA-D method | 169 | −0.818 | 0.852 | 0.066 | 0.000 * |
| CA-W method | 169 | −0.279 | 0.792 | 0.061 | 0.000 * |
| CA-ML | 169 | 0.039 | 0.736 | 0.057 | 0.488 |
| CA-CNN | 169 | 0.014 | 0.718 | 0.055 | 0.793 |
| Boys | |||||
| Prediction Error | N | Mean (Age) | Std. Deviation (Age) | Std. Error Mean (Age) | p Value |
| CA-D method | 210 | −0.926 | 1.005 | 0.069 | 0.000 * |
| CA-W method | 210 | −0.468 | 0.917 | 0.063 | 0.000 * |
| CA-ML | 210 | −0.050 | 0.770 | 0.053 | 0.345 |
| CA-CNN | 210 | 0.007 | 0.637 | 0.044 | 0.874 |
| Girls | |||||
| Prediction Error | N | Mean (Age) | Std. Deviation (Age) | Std. Error Mean (Age) | p Value |
| CA-D method | 45 | −0.694 | 1.508 | 0.225 | 0.003 * |
| CA-W method | 45 | −0.244 | 1.503 | 0.224 | 0.283 |
| CA-ML | 45 | 0.252 | 1.301 | 0.194 | 0.201 |
| CA-CNN | 45 | 0.466 | 1.044 | 0.156 | 0.005 * |
| Boys | |||||
| Prediction Error | N | Mean (Age) | Std. Deviation (Age) | Std. Error Mean (Age) | p Value |
| CA-D method | 54 | -0.318 | 1.051 | 0.147 | 0.036 * |
| CA-W method | 54 | -0.048 | 1.090 | 0.148 | 0.746 |
| CA-ML | 54 | 0.497 | 0.971 | 0.133 | 0.000 * |
| CA-CNN | 54 | 0.902 | 1.158 | 0.158 | 0.000 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-J.; Tsai, C.-L.; Gao, Q.-Z.; Chen, Y.-P.; Kuo, C.-F.; Huang, Y.-H. The Application of Artificial-Intelligence-Assisted Dental Age Assessment in Children with Growth Delay. J. Pers. Med. 2022, 12, 1158. https://doi.org/10.3390/jpm12071158
Wu T-J, Tsai C-L, Gao Q-Z, Chen Y-P, Kuo C-F, Huang Y-H. The Application of Artificial-Intelligence-Assisted Dental Age Assessment in Children with Growth Delay. Journal of Personalized Medicine. 2022; 12(7):1158. https://doi.org/10.3390/jpm12071158
Chicago/Turabian StyleWu, Te-Ju, Chia-Ling Tsai, Quan-Ze Gao, Yueh-Peng Chen, Chang-Fu Kuo, and Ying-Hua Huang. 2022. "The Application of Artificial-Intelligence-Assisted Dental Age Assessment in Children with Growth Delay" Journal of Personalized Medicine 12, no. 7: 1158. https://doi.org/10.3390/jpm12071158
APA StyleWu, T. -J., Tsai, C. -L., Gao, Q. -Z., Chen, Y. -P., Kuo, C. -F., & Huang, Y. -H. (2022). The Application of Artificial-Intelligence-Assisted Dental Age Assessment in Children with Growth Delay. Journal of Personalized Medicine, 12(7), 1158. https://doi.org/10.3390/jpm12071158






