Robotic Mediastinal Tumor Resections: Position and Port Placement
Abstract
:1. Introduction
2. Anterior Mediastinal Tumor
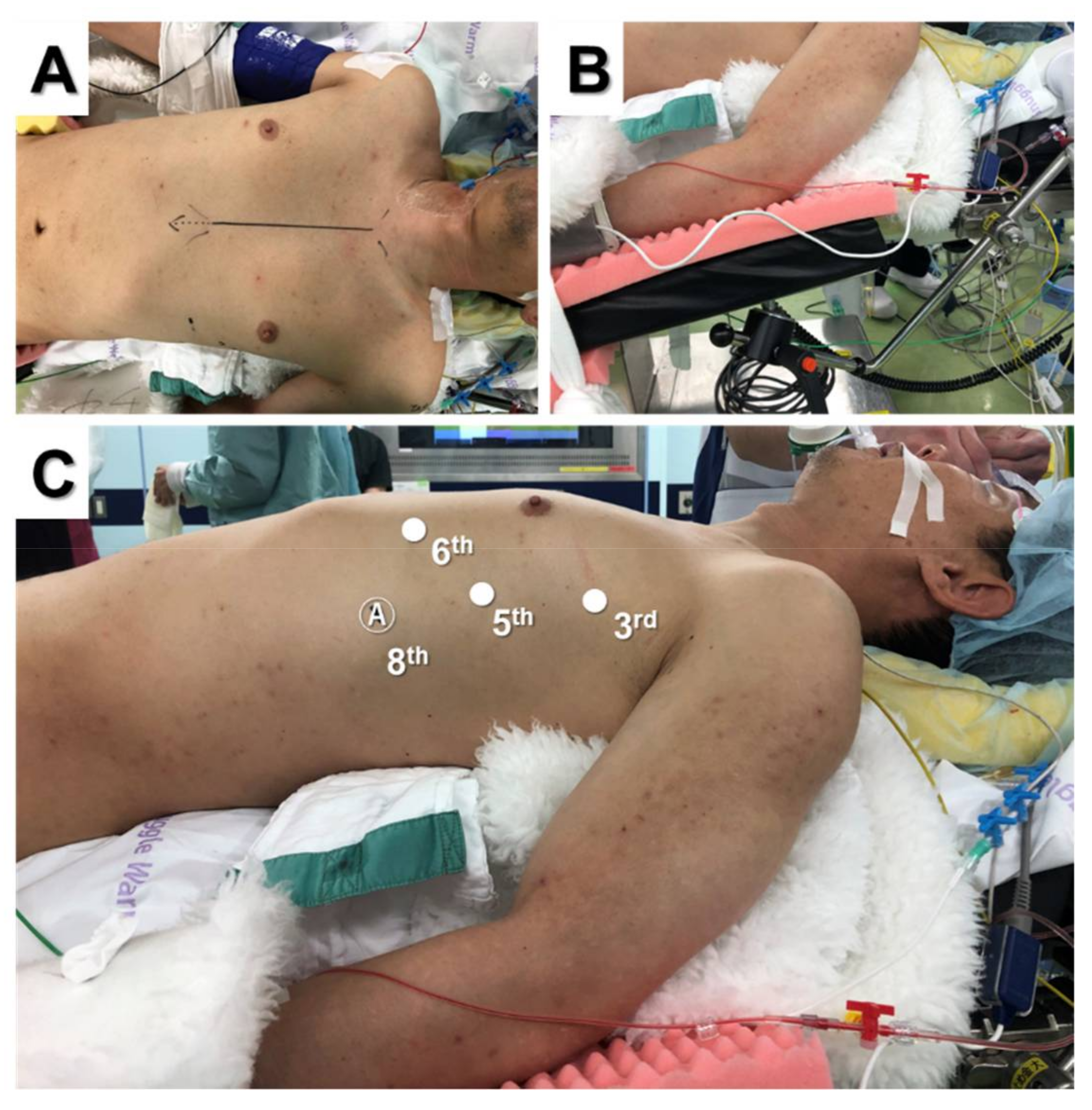
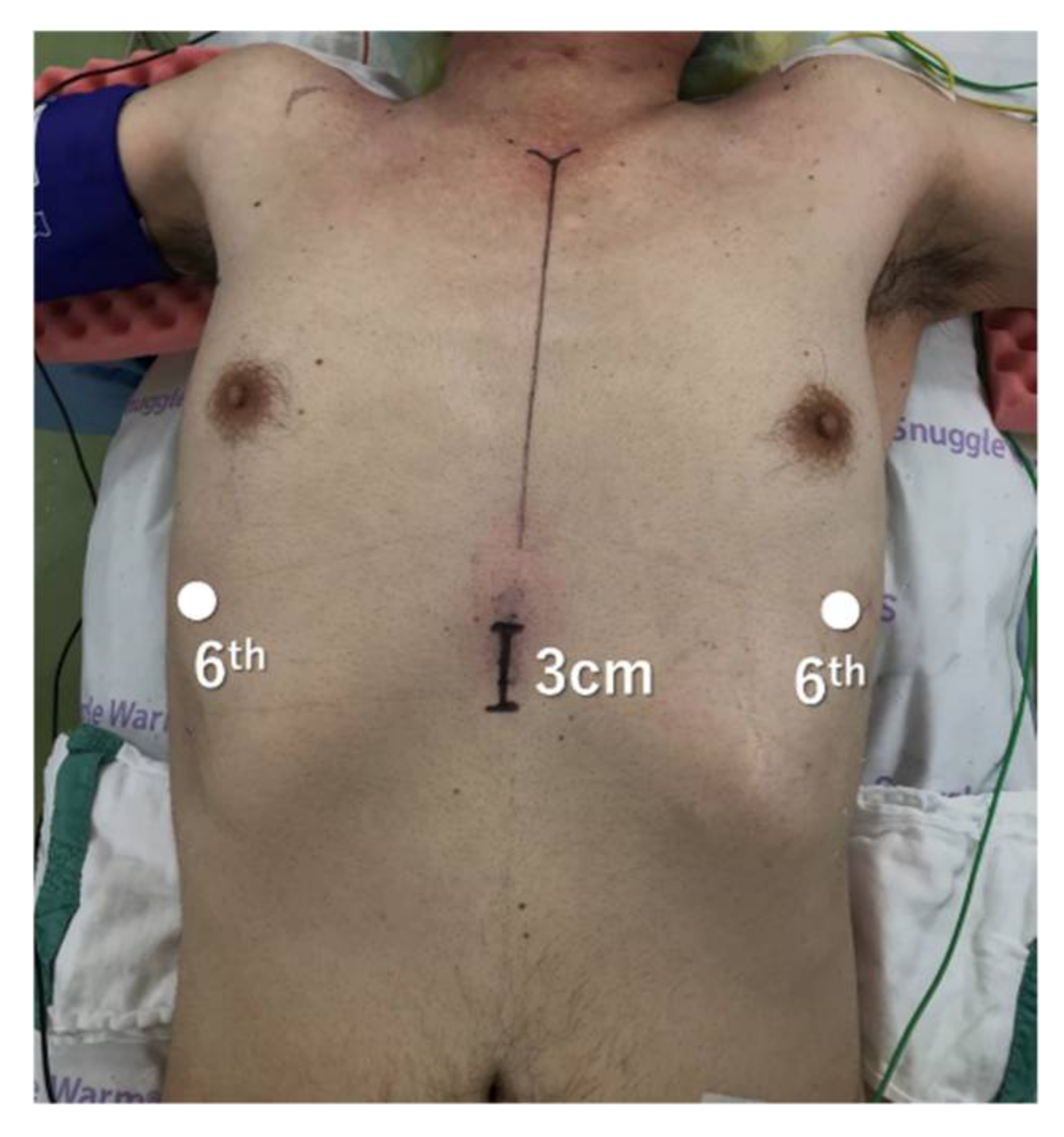
2.1. Total and Extended Thymectomy
2.2. Lateral Versus Subxiphoid Approach
2.3. Partial Thymectomy
3. Posterior Mediastinal Tumor
3.1. Posterior Tumor Resection
3.2. Dumbbell Tumor Resection
4. Superior Mediastinal Tumor
5. Middle Mediastinal Tumor
6. Literature Summary and Preferred Position and Approach for RATS Mediastinal Tumor Resection
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pennathur, A.; Qureshi, I.; Schuchert, M.J.; Dhupar, R.; Ferson, P.F.; Gooding, W.E.; Christie, N.A.; Gilbert, S.; Shende, M.; Awais, O.; et al. Comparison of surgical techniques for early-stage thymoma: Feasibility of minimally invasive thymectomy and comparison with open resection. J. Thorac. Cardiovasc. Surg. 2011, 141, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Tantai, J.C.; Ge, X.X.; Li, W.; Feng, J.; Cheng, M.; Shi, J.X.; Zhao, H. Surgical techniques for early-stage thymoma: Video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J. Thorac. Cardiovasc. Surg. 2014, 147, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Chen, C.; Wang, Y.; Wei, Y.; Fu, J.; Zhang, P.; Liu, Y.; Zhang, R.; Chen, K.; Yu, Z.; et al. Video-assisted thoracoscopic surgery versus open surgery for Stage I thymic epithelial tumours: A propensity score-matched study. Eur. J. Cardio-Thorac. Surg. 2018, 54, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, I.; Hashizume, M.; Shimada, M.; Tomikawa, M.; Sugimachi, K. Video-assisted thoracoscopic extirpation of a posterior mediastinal mass using the da Vinci computer enhanced surgical system. Ann. Thorac. Surg. 2002, 74, 1235–1237. [Google Scholar] [CrossRef]
- Alvarado, C.E.; Worrell, S.G.; Bachman, K.C.; Jiang, B.; Janko, M.; Gray, K.E.; Argote-Greene, L.M.; Linden, P.A.; Towe, C.W. Robotic Approach Has Improved Outcomes for Minimally Invasive Resection of Mediastinal Tumors. Ann. Thorac. Surg. 2021, 113, 1853–1858. [Google Scholar] [CrossRef]
- Shen, C.; Li, J.; Li, J.; Che, G. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for treatment of patients with thymoma: A systematic review and meta-analysis. Thorac. Cancer 2022, 13, 151–161. [Google Scholar] [CrossRef]
- Raja, S.M.; Guptill, J.T.; McConnell, A.; Al-Khalidi, H.R.; Hartwig, M.G.; Klapper, J.A. Perioperative Outcomes of Thymectomy in Myasthenia Gravis: A Thoracic Surgery Database Analysis. Ann. Thorac. Surg. 2022, 113, 904–910. [Google Scholar] [CrossRef]
- Kang, C.H.; Na, K.J.; Park, S.; Park, I.K.; Kim, Y.T. Long-Term Outcomes of Robotic Thymectomy in Patients with Thymic Epithelial Tumors. Ann. Thorac. Surg. 2021, 112, 430–435. [Google Scholar] [CrossRef]
- Suda, T.; Kaneda, S.; Hachimaru, A.; Tochii, D.; Maeda, R.; Tochii, S.; Takagi, Y. Thymectomy via a subxiphoid approach: Single-port and robot-assisted. J. Thorac. Dis. 2016, 8, S265–S271. [Google Scholar]
- Zhang, H.; Chen, L.; Zheng, Y.; Wang, Z.; Geng, Y.; Wang, F.; Liu, D.; He, A.; Li, J.; Wang, Y. Robot-assisted thymectomy via subxiphoid approach: Technical details and early outcomes. J. Thorac. Dis. 2018, 10, 1677–1682. [Google Scholar] [CrossRef]
- Park, S.Y.; Han, K.N.; Hong, J.I.; Kim, H.K.; Kim, D.J.; Choi, Y.H. Subxiphoid approach for robotic single-site-assisted thymectomy. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2020, 58, i34–i38. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.H.; Na, K.J.; Song, J.W.; Bae, S.Y.; Park, S.; Park, I.K.; Kim, Y.T. The robotic thymectomy via the subxiphoid approach: Technique and early outcomes. Eur. J. Cardio-Thorac. Surg. 2020, 58, i39–i43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, F.; Qiu, G.; Li, Z.; Peng, L.; Wang, X.; Xie, S.; Chen, L.Q.; Wang, Y. Safety and feasibility of a modularized procedure for trans-subxiphoid robotic extended thymectomy. Surg. Endosc. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Savitt, M.A.; Gao, G.; Furnary, A.P.; Swanson, J.; Gately, H.L.; Handy, J.R. Application of robotic-assisted techniques to the surgical evaluation and treatment of the anterior mediastinum. Ann. Thorac. Surg. 2005, 79, 450–455, discussion 455. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Hao, L.; Demin, L.; Guohua, D.; Hua, J.; Yi, S. Da Vinci robot-assisted system for thymectomy: Experience of 55 patients in China. Int. J. Med. Robot 2014, 10, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Chen, X.; Huang, J.; Lin, H.; Mao, F.; Zhao, X.; Luo, Q.; Ding, Z. A comparison of three approaches for the treatment of early-stage thymomas: Robot-assisted thoracic surgery, video-assisted thoracic surgery, and median sternotomy. J. Thorac. Dis. 2017, 9, 1997–2005. [Google Scholar] [CrossRef]
- Li, X.-K.; Xu, Y.; Cong, Z.-Z.; Zhou, H.; Wu, W.-J.; Shen, Y. Comparison of the progression-free survival between robot-assisted thymectomy and video-assisted thymectomy for thymic epithelial tumors: A propensity score matching study. J. Thorac. Dis. 2020, 12, 4033–4043. [Google Scholar] [CrossRef]
- Marcuse, F.; Hochstenbag, M.; De Baets, M.H.V.; Bootsma, G.; Maat, A.; Hoeijmakers, J.G.J.; Keijzers, M.; Abdul Hamid, M.; De Ruysscher, D.; Maessen, J.G. Robotic Thymectomy for Thymomas: A Retrospective Follow-up Study in The Netherlands. Ann. Thorac. Surg. 2021, in press. [Google Scholar] [CrossRef]
- Geraci, T.C.; Ferrari-Light, D.; Pozzi, N.; Cerfolio, R.J. Midterm Results for Robotic Thymectomy for Malignant Disease. Ann. Thorac. Surg. 2021, 111, 1675–1681. [Google Scholar] [CrossRef]
- Freeman, R.K.; Ascioti, A.J.; Van Woerkom, J.M.; Vyverberg, A.; Robison, R.J. Long-term follow-up after robotic thymectomy for nonthymomatous myasthenia gravis. Ann. Thorac. Surg. 2011, 92, 1018–1022. [Google Scholar] [CrossRef]
- Marulli, G.; Rea, F.; Melfi, F.; Schmid, T.A.; Ismail, M.; Fanucchi, O.; Augustin, F.; Swierzy, M.; Di Chiara, F.; Mussi, A.; et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: A multicenter European study. J. Thorac. Cardiovasc. Surg. 2012, 144, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Schneiter, D.; Tomaszek, S.; Kestenholz, P.; Hillinger, S.; Opitz, I.; Inci, I.; Weder, W. Minimally invasive resection of thymomas with the da Vinci® Surgical System. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2013, 43, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Azenha, L.F.; Deckarm, R.; Minervini, F.; Dorn, P.; Lutz, J.; Kocher, G.J. Robotic vs. Transsternal Thymectomy: A Single Center Experience over 10 Years. J. Clin. Med. 2021, 10, 4991. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Zirafa, C.C.; Ceccarelli, I.; Guida, M.; Davini, F.; Maestri, M.; Morganti, R.; Ricciardi, R.; Hung Key, T.; Melfi, F. Robotic thymectomy for thymoma in patients with myasthenia gravis: Neurological and oncological outcomes. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2021, 60, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Wilshire, C.L.; Blitz, S.L.; Fuller, C.C.; Rückert, J.C.; Li, F.; Cerfolio, R.J.; Ghanim, A.F.; Onaitis, M.W.; Sarkaria, I.S.; Wigle, D.A.; et al. Minimally invasive thymectomy for myasthenia gravis favours left-sided approach and low severity class. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2021, 60, 898–905. [Google Scholar] [CrossRef]
- Bongiolatti, S.; Salvicchi, A.; Puzhlyiakov, V.; Cipollini, F.; Viggiano, D.; Gonfiotti, A.; Voltolini, L. Long-Term Outcomes of Robot-Assisted Radical Thymectomy for Large Thymomas: A Propensity Matched Analysis. Int. J. Med. Robot 2022, e2439. [Google Scholar] [CrossRef]
- Rea, F.; Marulli, G.; Bortolotti, L.; Feltracco, P.; Zuin, A.; Sartori, F. Experience with the "da Vinci" robotic system for thymectomy in patients with myasthenia gravis: Report of 33 cases. Ann. Thorac. Surg. 2006, 81, 455–459. [Google Scholar] [CrossRef]
- Rückert, J.C.; Ismail, M.; Swierzy, M.; Sobel, H.; Rogalla, P.; Meisel, A.; Wernecke, K.D.; Rückert, R.I.; Müller, J.M. Thoracoscopic thymectomy with the da Vinci robotic system for myasthenia gravis. Ann. N. Y. Acad. Sci. 2008, 1132, 329–335. [Google Scholar] [CrossRef]
- Raza, B.; Dhamija, A.; Abbas, G.; Toker, A. Robotic thymectomy for myasthenia gravis surgical techniques and outcomes. J. Thorac. Dis. 2020, 13, 6187–6194. [Google Scholar] [CrossRef]
- Suda, T.; Tochii, D.; Tochii, S.; Takagi, Y. Trans-subxiphoid robotic thymectomy. Interact. CardioVasc. Thorac. Surg. 2015, 20, 669–671. [Google Scholar] [CrossRef]
- Ashton, R.C., Jr.; McGinnis, K.M.; Connery, C.P.; Swistel, D.G.; Ewing, D.R.; DeRose, J.J., Jr. Totally endoscopic robotic thymectomy for myasthenia gravis. Ann. Thorac. Surg. 2003, 75, 569–571. [Google Scholar] [CrossRef]
- Bodner, J.; Wykypiel, H.; Greiner, A.; Kirchmayr, W.; Freund, M.C.; Margreiter, R.; Schmid, T. Early experience with robot-assisted surgery for mediastinal masses. Ann. Thorac. Surg. 2004, 78, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Asaf, B.B.; Pulle, M.V.; Puri, H.V.; Sethi, N.; Bishnoi, S. Myasthenia is a poor prognostic factor for perioperative outcomes after robotic thymectomy for thymoma. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2021, 59, 807–813. [Google Scholar] [CrossRef]
- Park, J.H.; Na, K.J.; Kang, C.H.; Park, S.; Park, I.K.; Kim, Y.T. Robotic subxiphoid thymectomy versus lateral thymectomy: A propensity-score-matched comparison. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2022, ezac288. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Sakamaki, H. The technical aspects of a midline robotic thymectomy. JTCVS Technol. 2020, 4, 368–370. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Bryant, A.S.; Minnich, D.J. Operative techniques in robotic thoracic surgery for inferior or posterior mediastinal pathology. J. Thorac. Cardiovasc. Surg. 2012, 143, 1138–1143. [Google Scholar] [CrossRef]
- Fukui, M.; Watanabe, Y.; Matsunaga, T.; Ueno, H.; Hattori, A.; Takamochi, K.; Suzuki, K. Port Placement in Robotic Thoracic Surgery for Inferior Mediastinal Tumors. Ann. Thorac. Surg. 2022, 113, e145–e148. [Google Scholar] [CrossRef]
- Okazaki, M.; Takigawa, T.; Suzawa, K.; Toyooka, S. Robot-assisted intrathoracic procedure for dumbbell tumour in the prone position. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 643–645. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Qiu, G.; Wang, Y. Robot-assisted thoracic surgery for apex-located neurogenic tumors. Asian J. Surg. 2022, 45, 662–663. [Google Scholar] [CrossRef]
- Pacchiarotti, G.; Wang, M.Y.; Kolcun, J.P.G.; Chang, K.H.; Al Maaieh, M.; Reis, V.S.; Nguyen, D.M. Robotic paravertebral schwannoma resection at extreme locations of the thoracic cavity. Neurosurg. Focus 2017, 42, E17. [Google Scholar] [CrossRef]



| Lateral | Subxiphoid | p-Value | |
|---|---|---|---|
| (n = 13) | (n = 16) | ||
| Patient characteristics | |||
| Age (years old) | 61.9 ± 14.8 | 59.1 ± 13.7 | 0.6023 |
| Gender (male/female) | 5/8 | 8/8 | 0.5344 |
| Tumor size | 3.1 ± 1.9 | 3.5 ± 1.9 | 0.6623 |
| Procedure | 0.0788 | ||
| Extended thymectomy | 2 | 7 | |
| Total thymectomy | 11 | 9 | |
| Perioperative outcomes | |||
| Operative time (min) | 180.4 ± 61.6 | 185.6 ± 49.5 | 0.8011 |
| Console time (min) | 123.2 ± 60.0 | 110.8 ± 44.6 | 0.5280 |
| Operative time–Console time (min) | 57.2 ± 8.9 | 74.8 ± 10.5 | <0.0001 |
| Intraoperative bleeding (mL) | 21.9 ± 23.6 | 14.4 ± 17.5 | 0.3312 |
| Intraoperative complications | 0 (0%) | 0 (0%) | - |
| Postoperative complications | 1 (7.7%) | 0 (0%) | 0.2589 |
| Maximum size of incision (cm) | 2.3 ± 0.6 | 3.0 ± 0.0 | 0.0001 |
| WBC POD5 (×103/mm3) | 5.72 ± 1.01 | 6.19 ± 1.98 | 0.4555 |
| CRP POD5 (mg/dL) | 2.25 ± 2.03 | 3.59 ± 1.92 | 0.0799 |
| NRS POD5 | 1.0 ± 0.6 | 0.8 ± 1.0 | 0.5157 |
| Tumor Location | Author | Country | Year | Total | MG | Thymoma | Approach | Position | Ports | Conversion (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Anterior | Rückert | Germany | 2008 | 106 | 95 | 12 | Left | Semi-supine | 3 | 1.1 |
| Anterior | Freeman | USA | 2011 | 75 | 75 | 0 | Left | Supine | 3 | 1.1 |
| Anterior | Schneiter | Switzerland | 2013 | 58 | 25 | 20 | Left | Semi-supine | 3 | 0.0 |
| Anterior | Marulli | Italy | 2013 | 79 | 45 | 79 | Left | Semi-supine | 3 | 1.3 |
| Anterior | Jun | China | 2014 | 55 | n.a. | 21 | Right (>Left) | Supine | 4 | 0.0 |
| Anterior | Qian | China | 2017 | 51 | 4 | 51 | Right (>Left) | Semi-supine | 4 | 0.0 |
| Anterior | Kang | Korea | 2020 | 110 | 18 | 67 | Subxiphoid | Supine | 3 | 0.9 |
| Anterior | Li | China | 2020 | 60 | 4 | 55 | Right | Supine | 3 | n.a. |
| Anterior | Azenha | Switzerland | 2021 | 81 | n.a. | 34 | Left (>Right) | n.a. | 3 | 0.0 |
| Anterior | Marcuse | Netherlands | 2021 | 130 | 89 | 130 | Right (>Left) | Supine | 3 | 7.7 |
| Anterior | Romano | Italy | 2021 | 53 | 34 | 53 | Left (>Right) | Supine | 3 | 1.9 |
| Anterior | Wilshire | USA | 2021 | 123 | 26 | 123 | Left (>Right) | Semi-supine | 3 | n.a. |
| Anterior | Kang | Korea | 2021 | 158 | 27 | 132 | Subxiphiod/Left/Right | Supine | 3 | 1.3 |
| Anterior | Kumar | India | 2021 | 111 | 68 | 111 | Left/Right | Supine | 3 | 6.3 |
| Anterior | Geraci | USA | 2021 | 84 | n.a. | 84 | Right (>Left) | Semi-supine | 3 | 2.3 |
| Anterior | Zhang | China | 2022 | 87 | 87 | 53 | Subxiphoid | Supine | 4 | 0.0 |
| Anterior | Bongiolatti | Italy | 2022 | 54 | 13 | 54 | Left (>Right) | Semi-supine | 3 | 11.1 |
| Anterior | Park | Korea | 2022 | 389 | 44 | 198 | Subxiphiod/Left (>Right) | Supine | 3 | 0.5 |
| Superior | Wang | China | 2021 | 15 | - | - | Lateral | Lateral decubitus | 3 | 0.0 |
| Posterior | Cerfolio | USA | 2012 | 75 | - | - | Lateral | Lateral decubitus | 4 | 1.3 |
| Posterior | Li | China | 2020 | 58 | - | - | Lateral | Lateral decubitus | 3 | 3.4 |
| Tumor Location | Procedure | Preferred Position | Preferred Approach |
|---|---|---|---|
| Anterior mediastinum | Extended thymectomy | Supine | Subxiphoid |
| Total thymectomy | Supine | Subxiphoid or lateral | |
| Partial thymectomy | Supine | Lateral | |
| Superior mediastinum | Tumor resection | Lateral decubitus | Lateral |
| Middle mediastinum | Tumor resection | Lateral decubitus | Lateral |
| Posterior mediastinum | Tumor resection | Lateral decubitus | Lateral |
| Dumbbell tumor resection | Prone | Lateral |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okazaki, M.; Shien, K.; Suzawa, K.; Sugimoto, S.; Toyooka, S. Robotic Mediastinal Tumor Resections: Position and Port Placement. J. Pers. Med. 2022, 12, 1195. https://doi.org/10.3390/jpm12081195
Okazaki M, Shien K, Suzawa K, Sugimoto S, Toyooka S. Robotic Mediastinal Tumor Resections: Position and Port Placement. Journal of Personalized Medicine. 2022; 12(8):1195. https://doi.org/10.3390/jpm12081195
Chicago/Turabian StyleOkazaki, Mikio, Kazuhiko Shien, Ken Suzawa, Seiichiro Sugimoto, and Shinichi Toyooka. 2022. "Robotic Mediastinal Tumor Resections: Position and Port Placement" Journal of Personalized Medicine 12, no. 8: 1195. https://doi.org/10.3390/jpm12081195
APA StyleOkazaki, M., Shien, K., Suzawa, K., Sugimoto, S., & Toyooka, S. (2022). Robotic Mediastinal Tumor Resections: Position and Port Placement. Journal of Personalized Medicine, 12(8), 1195. https://doi.org/10.3390/jpm12081195






