Quantification of Idua Enzymatic Activity Combined with Observation of Phenotypic Change in Zebrafish Embryos Provide a Preliminary Assessment of Mutated idua Correlated with Mucopolysaccharidosis Type I
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Fish Embryos
2.3. Knockdown of z-idua in Zebrafish Embryos
2.4. LC-MS/MS Assay and Calibration of DS and HS
2.5. RNA Extraction and Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
2.6. Plasmid Constructs Containing idua cDNA and Its Mutated Nucleotides
2.7. Overexpression of z-Idua mRNA
2.8. Enzyme Activity of z-Idua Contained in Zebrafish Embryos
2.9. Cartilage Staining
2.10. Histological Examination, Frozen Section, and Immunostaining of Zebrafish Embryos
3. Results
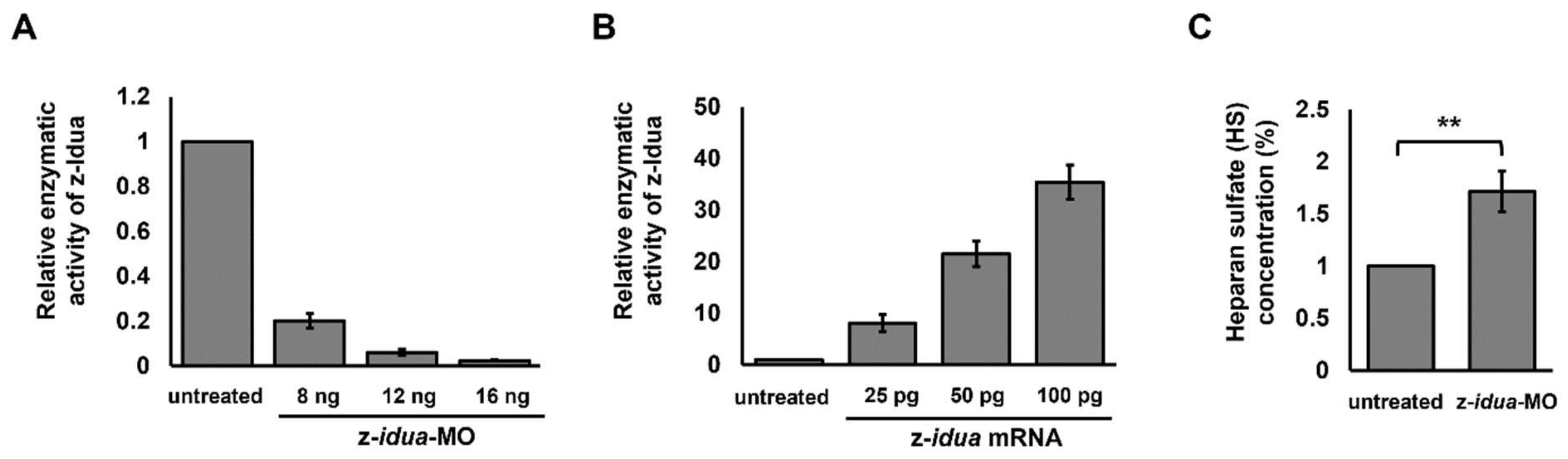
3.1. The Dosage of z-idua mRNA Injected into Embryos Was Positively Correlated with the Enzymatic Activity of IDUA Detected in Zebrafish Embryos
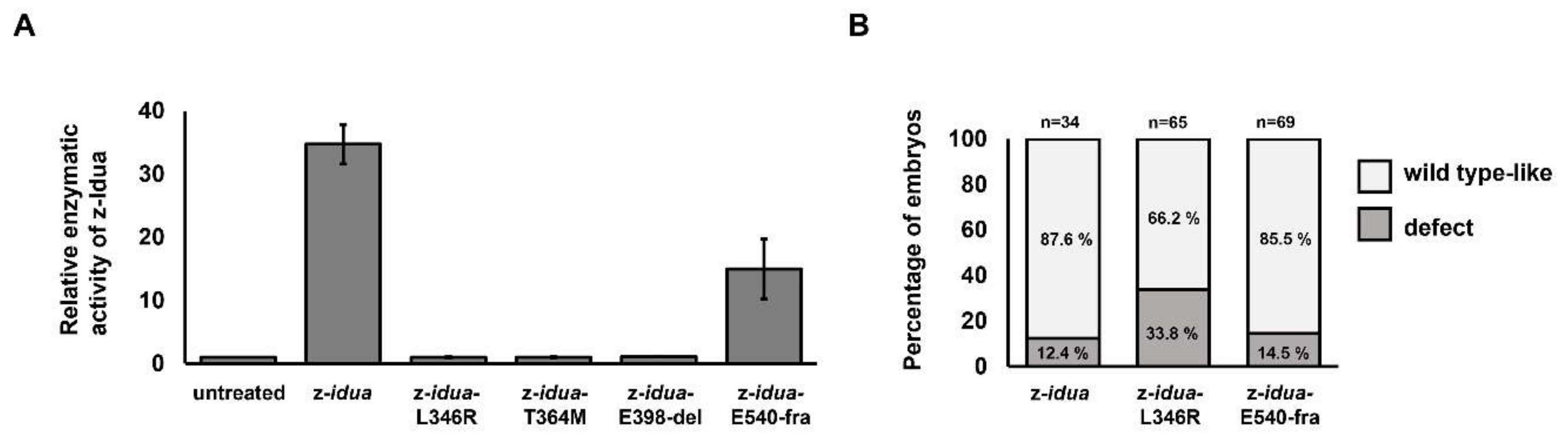
3.2. Using Zebrafish Embryos Served as an In Vivo Platform to Analyze the Enzymatic Activity of Mutant z-Idua
3.3. Effect of Overexpression of Two Zebrafish Mutated idua mRNAs, Corresponding to Human Mutated IDUA Genes, on the Morphological Change in Zebrafish Embryos
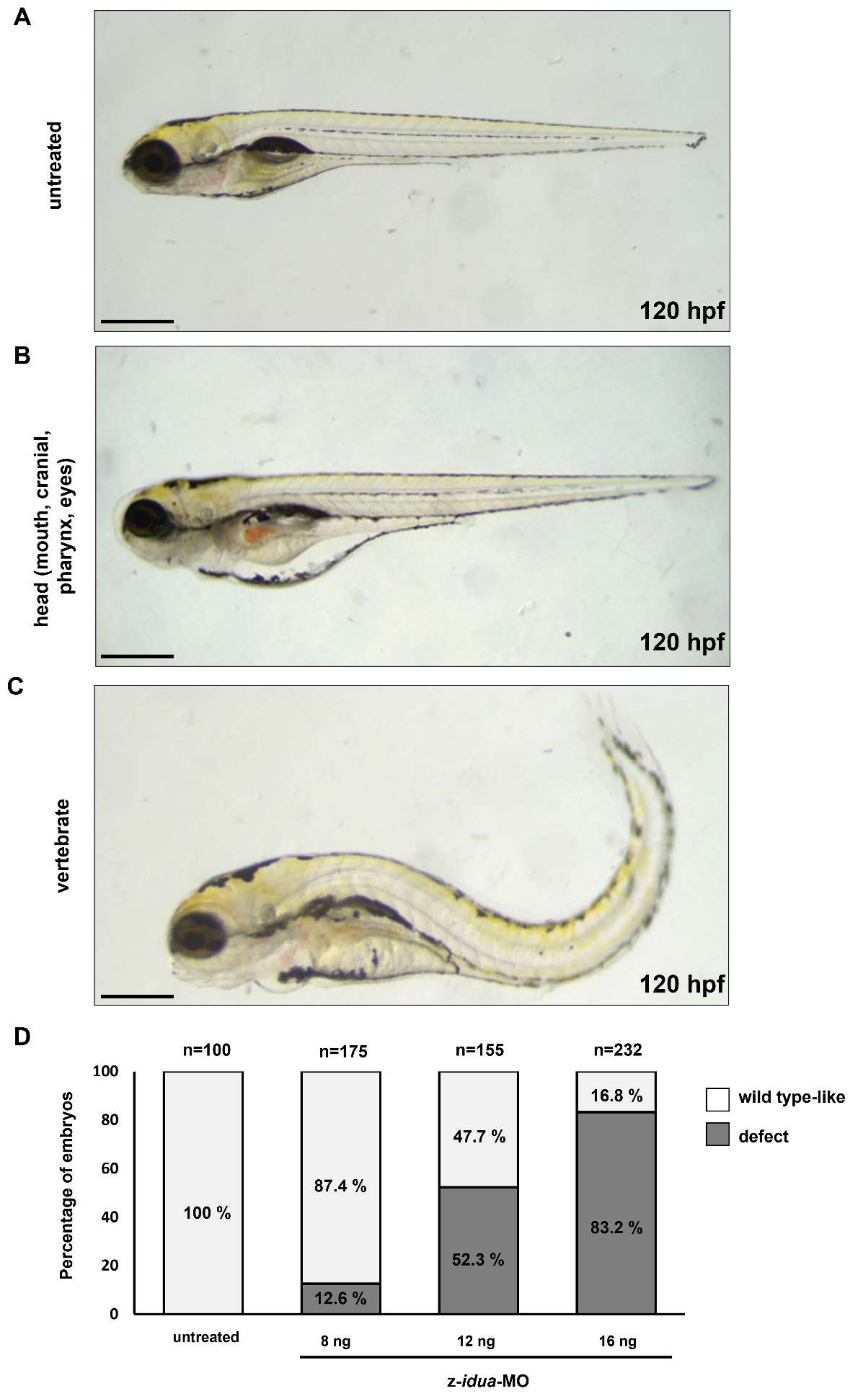
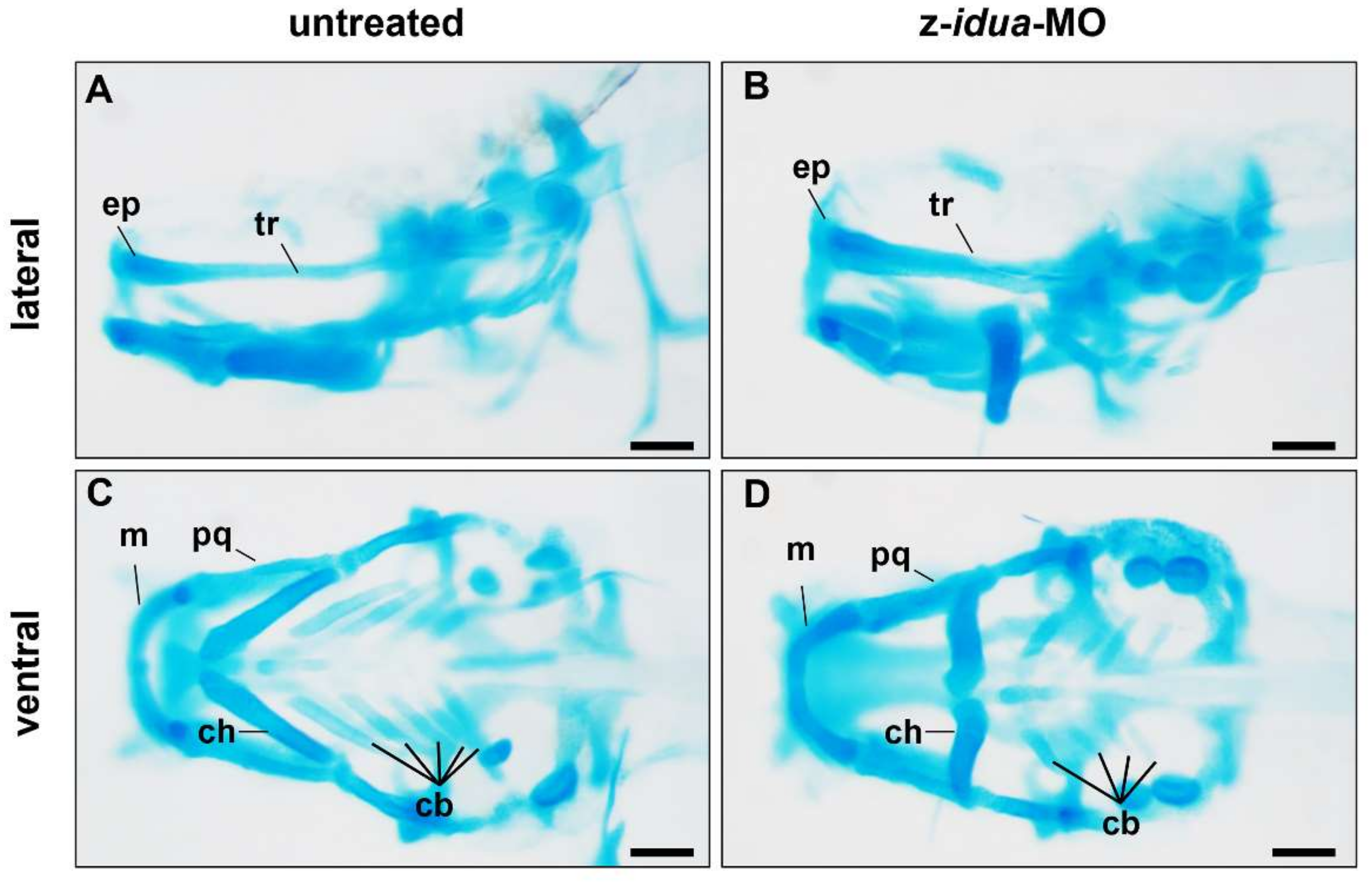
3.4. Knockdown of z-Idua in Zebrafish Embryos Caused Developmental Defects
4. Discussion
4.1. Molecular Strategies Used to Determine Gene Function in Zebrafish Embryos Could Also Be Employed to Identify Gene Function of Mutated Human IDUA
4.2. Endogenous z-Idua Enzymatic Activity Did Not Increase in Embryos Injected with Mutant mRNAs Having Null Enzymatic Function
4.3. The Occurrence of Morphological Defect Was Correlated with the Overexpression of Mutant z-Idua Having Null Enzymatic Function in Embryos
4.4. Accumulated HS, but Not DS, Was Detected in idua-Knockdown Zebrafish Embryos, Resulting in the Occurrence of Edematous Tissues
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNS | central nervous system |
| MPS | mucopolysaccharidosis |
| LSD | lysosomal storage disorder |
| IDUA | α-l-Iduronidase |
| hpf | hours post-fertilization |
| dpf | days post-fertilization |
| PFA | paraformaldehyde |
| GAGs | glycosaminoglycans |
| HS | heparan sulfate |
| DS | dermatan sulfate |
| MPS I-H | Hurler syndrome |
| MPS I-S | Scheie syndrome |
| MPS I-HS | Hurler–Scheie syndrome |
| HSCT | hematopoietic stem cell transplantation |
| ERT | enzyme replacement therapy |
| IDS | iduronate 2-sulfatase |
| MO | morpholino oligonucleotides |
References
- Kim, C.; Kwak, M.J.; Cho, S.Y.; Ko, A.R.; Rheey, J.; Kwon, J.Y.; Chung, Y.; Jin, D.K. Decreased performance in IDUA knockout mouse mimic limitationsofjointfunction and locomotion in patients with Hurler syndrome. Orphanet J. Rare Dis. 2015, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Parini, R.; Deodato, F.; Di Rocco, M.; Lanino, E.; Locatelli, F.; Messina, C.; Rovelli, A.; Scarpa, M. Open issues in Mucopolysaccharidosis type I-Hurler. Orphanet J. Rare Dis. 2017, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Mckusick, V.A. Heritable disorders of connective tissue. J. Chronic Dis. 1956, 3, 360–389. [Google Scholar] [CrossRef]
- Muenzer, J.; Wraith, J.E.; Clarke, L.A. Mucopolysaccharidosis I: Management and treatment guidelines. Pediatrics 2009, 123, 19–29. [Google Scholar] [CrossRef]
- Soliman, O.I.; Timmermans, R.G.; Nemes, A.; Vletter, W.B.; Wilson, J.H.; ten Cate, F.J.; Geleijnse, M.L. Cardiac abnormalities in adults with the attenuated form of mucopolysaccharidosis type I. J. Inherit. Metab. Dis. 2007, 30, 750–757. [Google Scholar] [CrossRef][Green Version]
- Giugliani, R. Mucopolysacccharidoses: From understanding to treatment, a century of discoveries. Genet. Mol. Biol. 2012, 35, 924–931. [Google Scholar] [CrossRef]
- Kuiper, G.A.; Meijer, O.L.M.; Langereis, E.J.; Wijburg, F.A. Failure to shorten the diagnostic delay in two ultra-orphan diseases (mucopolysaccharidosis types I and III): Potential causes and implications. Orphanet J. Rare Dis. 2018, 13, 2. [Google Scholar] [CrossRef]
- Hampe, C.S.; Wesley, J.; Lund, T.C.; Orchard, P.J.; Polgreen, L.E.; Eisengart, J.B.; McLoon, L.K.; Cureoglu, S.; Schachern, P.; McIvor, R.S. Mucopolysaccharidosis Type I: Current Treatments, Limitations, and Prospects for Improvement. Biomolecules 2021, 11, 189. [Google Scholar] [CrossRef]
- Donati, M.A.; Pasquini, E.; Spada, M.; Polo, G.; Burlina, A. Newborn screening in mucopolysaccharidoses. Ital. J. Pediatr. 2018, 44, 126. [Google Scholar] [CrossRef]
- Clarke, L.A.; Dickson, P.; Ellinwood, N.M.; Klein, T.L. Newborn Screening for Mucopolysaccharidosis I: Moving Forward Learning from Experience. Int. J. Neonatal Screen. 2020, 6, 91. [Google Scholar] [CrossRef]
- Chuang, C.K.; Lee, C.L.; Tu, R.Y.; Lo, Y.T.; Sisca, F.; Chang, Y.H.; Liu, M.Y.; Liu, H.Y.; Chen, H.J.; Kao, S.M.; et al. Nationwide Newborn Screening Program for Mucopolysaccharidoses in Taiwan and an Update of the “Gold Standard” Criteria Required to Make a Confirmatory Diagnosis. Diagnostics 2021, 11, 1583. [Google Scholar] [CrossRef]
- Chuang, C.K.; Lin, H.Y.; Wang, T.J.; Huang, Y.H.; Chan, M.J.; Liao, H.C.; Lo, Y.T.; Wang, L.Y.; Tu, R.Y.; Fang, Y.Y.; et al. Status of newborn screening and follow up investigations for Mucopolysaccharidoses I and II in Taiwan. Orphanet J. Rare Dis. 2018, 13, 84. [Google Scholar] [CrossRef]
- Clarke, L.A.; Giugliani, R.; Guffon, N.; Jones, S.A.; Keenan, H.A.; Munoz-Rojas, M.V.; Okuyama, T.; Viskochil, D.; Whitley, C.B.; Wijburg, F.A.; et al. Genotype-phenotype relationships in mucopolysaccharidosis type I (MPS I): Insights from the International MPS I Registry. Clin. Genet. 2019, 96, 281–289. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chiang, C.Y.; Tsai, H.J. Zebrafish and Medaka: New model organisms for modern biomedical research. J. Biomed. Sci. 2016, 23, 19. [Google Scholar] [CrossRef]
- Rennekamp, A.J.; Peterson, R.T. 15 years of zebrafish chemical screening. Curr. Opin. Chem. Biol. 2015, 24, 58–70. [Google Scholar] [CrossRef]
- Lee, H.C.; Lin, C.Y.; Tsai, H.J. Zebrafish, an In Vivo Platform to Screen Drugs and Proteins for Biomedical Use. Pharmaceuticals 2021, 14, 500. [Google Scholar] [CrossRef]
- Platt, F.M.; d’Azzo, A.; Davidson, B.L.; Neufeld, E.F.; Tifft, C.J. Lysosomal storage diseases. Nat. Rev. Dis. Primers 2018, 4, 27. [Google Scholar] [CrossRef]
- Zhang, T.; Peterson, R.T. Modeling Lysosomal Storage Diseases in the Zebrafish. Front. Mol. Biosci. 2020, 7, 82. [Google Scholar] [CrossRef]
- Moro, E.; Tomanin, R.; Friso, A.; Modena, N.; Tiso, N.; Scarpa, M.; Argenton, F. A novel functional role of iduronate-2-sulfatase in zebrafish early development. Matrix Biol. 2010, 29, 43–50. [Google Scholar] [CrossRef]
- Costa, R.; Urbani, A.; Salvalaio, M.; Bellesso, S.; Cieri, D.; Zancan, I.; Filocamo, M.; Bonaldo, P.; Szabo, I.; Tomanin, R.; et al. Perturbations in cell signaling elicit early cardiac defects in mucopolysaccharidosis type II. Hum. Mol. Genet. 2017, 26, 1643–1655. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lee, C.L.; Chang, C.Y.; Chiu, P.C.; Chien, Y.H.; Niu, D.M.; Tsai, F.J.; Hwu, W.L.; Lin, S.J.; Lin, J.L.; et al. Survival and diagnostic age of 175 Taiwanese patients with mucopolysaccharidoses (1985–2019). Orphanet J. Rare Dis. 2020, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Bellesso, S.; Salvalaio, M.; Lualdi, S.; Tognon, E.; Costa, R.; Braghetta, P.; Giraudo, C.; Stramare, R.; Rigon, L.; Filocamo, M.; et al. FGF signaling deregulation is associated with early developmental skeletal defects in animal models for mucopolysaccharidosis type II (MPSII). Hum. Mol. Genet. 2018, 27, 2262–2275. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lin, H.Y.; Chuang, C.K.; Zhang, P.H.; Tu, R.Y.; Lin, S.P.; Tsai, H.J. Effect of Mutated ids Overexpression on IDS Enzyme Activity and Developmental Phenotypes in Zebrafish Embryos: A Valuable Index for Assessing Critical Point-Mutations Associated with Mucopolysaccharidosis Type II Occurrence in Humans. Diagnostics 2020, 10, 854. [Google Scholar] [CrossRef] [PubMed]
- Douek, A.M.; Amiri Khabooshan, M.; Henry, J.; Stamatis, S.A.; Kreuder, F.; Ramm, G.; Anko, M.L.; Wlodkowic, D.; Kaslin, J. An Engineered sgsh Mutant Zebrafish Recapitulates Molecular and Behavioural Pathobiology of Sanfilippo Syndrome A/MPS IIIA. Int. J. Mol. Sci. 2021, 22, 5948. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lin, S.P.; Chuang, C.K.; Niu, D.M.; Chen, M.R.; Tsai, F.J.; Chao, M.C.; Chiu, P.C.; Lin, S.J.; Tsai, L.P.; et al. Incidence of the mucopolysaccharidoses in Taiwan, 1984–2004. Am. J. Med. Genet. A 2009, 149A, 960–964. [Google Scholar] [CrossRef]
- Nelson, J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum. Genet. 1997, 101, 355–358. [Google Scholar] [CrossRef]
- Baehner, F.; Schmiedeskamp, C.; Krummenauer, F.; Miebach, E.; Bajbouj, M.; Whybra, C.; Kohlschutter, A.; Kampmann, C.; Beck, M. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J. Inherit. Metab. Dis. 2005, 28, 1011–1017. [Google Scholar] [CrossRef]
- Jurecka, A.; Lugowska, A.; Golda, A.; Czartoryska, B.; Tylki-Szymanska, A. Prevalence rates of mucopolysaccharidoses in Poland. J. Appl. Genet. 2015, 56, 205–210. [Google Scholar] [CrossRef]
- Cho, S.Y.; Sohn, Y.B.; Jin, D.K. An overview of Korean patients with mucopolysaccharidosis and collaboration through the Asia Pacific MPS Network. Intractable Rare Dis. Res. 2014, 3, 79–86. [Google Scholar] [CrossRef]
- Wasserstein, M.P.; Caggana, M.; Bailey, S.M.; Desnick, R.J.; Edelmann, L.; Estrella, L.; Holzman, I.; Kelly, N.R.; Kornreich, R.; Kupchik, S.G.; et al. The New York pilot newborn screening program for lysosomal storage diseases: Report of the First 65,000 Infants. Genet. Med. 2019, 21, 631–640. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book, 3rd ed.; University of Oregon Press: Eugene, OR, USA, 1995. [Google Scholar]
- Chuang, C.K.; Lin, H.Y.; Wang, T.J.; Tsai, C.C.; Liu, H.Y.; Lin, S.P. A modified liquid chromatography/tandem mass spectrometry method for predominant disaccharide units of urinary glycosaminoglycans in patients with mucopolysaccharidoses. Orphanet J. Rare Dis. 2014, 9, 135. [Google Scholar] [CrossRef][Green Version]
- Lin, C.Y.; He, J.Y.; Zeng, C.W.; Loo, M.R.; Chang, W.Y.; Zhang, P.H.; Tsai, H.J. microRNA-206 modulates an Rtn4a/Cxcr4a/Thbs3a axis in newly forming somites to maintain and stabilize the somite boundary formation of zebrafish embryos. Open Biol. 2017, 7, 170009. [Google Scholar] [CrossRef]
- Chamoles, N.A.; Blanco, M.B.; Gaggioli, D.; Casentini, C. Hurler-like phenotype- enzymatic diagnosis in dried blood spots on filter paper. Clin. Chem. 2001, 47, 2098–2102. [Google Scholar] [CrossRef]
- Lin, S.P.; Lin, H.Y.; Wang, T.J.; Chang, C.Y.; Lin, C.H.; Huang, S.F.; Tsai, C.C.; Liu, H.L.; Keutzer, J.; Chuang, C.K. A pilot newborn screening program for Mucopolysaccharidosis type I in Taiwan. Orphanet J. Rare Dis. 2013, 8, 147. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lee, H.C.; Chen, H.C.; Hsieh, C.C.; Tsai, H.J. Normal function of Myf5 during gastrulation is required for pharyngeal arch cartilage development in zebrafish embryos. Zebrafish 2013, 10, 486–499. [Google Scholar] [CrossRef]
- Lin, C.Y.; Zhang, P.H.; Chen, Y.J.; Wu, C.L.; Tsai, H.J. Conditional Overexpression of rtn4al in Muscle of Adult Zebrafish Displays Defects Similar to Human Amyotrophic Lateral Sclerosis. Mar. Biotechnol. 2019, 21, 52–64. [Google Scholar] [CrossRef]
- Bie, H.; Yin, J.; He, X.; Kermode, A.R.; Goddard-Borger, E.D.; Withers, S.G.; James, M.N. Insights into mucopolysaccharidosis I from the structure and action of alpha-L-iduronidase. Nat. Chem. Biol. 2013, 9, 739–745. [Google Scholar] [CrossRef]
- Maita, N.; Tsukimura, T.; Taniguchi, T.; Saito, S.; Ohno, K.; Taniguchi, H.; Sakuraba, H. Human alpha-L-iduronidase uses its own N-glycan as a substrate-binding and catalytic module. Proc. Natl. Acad. Sci. USA 2013, 110, 14628–14633. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chuang, C.K.; Liu, S.C.; Lin, S.P. Awareness of attenuated mucopolysaccharidoses in a pediatric orthopedic clinic. Pediatr. Neonatol. 2019, 60, 100–101. [Google Scholar] [CrossRef]
- Lee, I.J.; Hwang, S.H.; Jeon, B.H.; Song, S.M.; Kim, J.S.; Paik, K.H.; Kwon, E.K.; Jin, D.K. Mutational analysis of the alpha-L-iduronidase gene in 10 unrelated Korean type I mucopolysaccharidosis patients: Identification of four novel mutations. Clin. Genet. 2004, 66, 575–576. [Google Scholar] [CrossRef]
- Lee-Chen, G.J.; Lin, S.P.; Tang, Y.F.; Chin, Y.W. Mucopolysaccharidosis type I: Characterization of novel mutations affecting alpha-L-iduronidase activity. Clin. Genet. 1999, 56, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Habicher, J.; Haitina, T.; Eriksson, I.; Holmborn, K.; Dierker, T.; Ahlberg, P.E.; Ledin, J. Chondroitin/dermatan sulfate modification enzymes in zebrafish development. PLoS ONE 2015, 10, e0121957. [Google Scholar]
- Filipek-Gorniok, B.; Holmborn, K.; Haitina, T.; Habicher, J.; Oliveira, M.B.; Hellgren, C.; Eriksson, I.; Kjellen, L.; Kreuger, J.; Ledin, J. Expression of chondroitin/dermatan sulfate glycosyltransferases during early zebrafish development. Dev. Dyn. 2013, 242, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Philipse, A.; Vrij, A. The Donnan equilibrium: I. On the thermodynamic foundation of the Donnan equation of state. J. Phys. Condens. Matter 2011, 23, 194106. [Google Scholar] [CrossRef]

| Mutants | Forward Primers (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| z-idua-L346R | CTACACTCTGCGGAGCAATGATAACG | CGTTATCATTGCTCC-GCAGAGTGTAG |
| z-idua-T364M | CCAGCGCATGCTCACCGC | GCGGTGAGCATGCGC-TGG |
| z-idua-E398-deletion | CACTGTTAGGCACTC-AGGTGCAG | CTGCACCTGAGTGCC-TAACAGTG |
| z-idua-E540-frameshift | GCTCAGTCTGGGGGA-AATGCCC | GGGCATTTCCCCCAG-ACTGAGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Lin, H.-Y.; Chuang, C.-K.; Zhang, P.-H.; Tu, Y.-R.; Lin, S.-P.; Tsai, H.-J. Quantification of Idua Enzymatic Activity Combined with Observation of Phenotypic Change in Zebrafish Embryos Provide a Preliminary Assessment of Mutated idua Correlated with Mucopolysaccharidosis Type I. J. Pers. Med. 2022, 12, 1199. https://doi.org/10.3390/jpm12081199
Lin C-Y, Lin H-Y, Chuang C-K, Zhang P-H, Tu Y-R, Lin S-P, Tsai H-J. Quantification of Idua Enzymatic Activity Combined with Observation of Phenotypic Change in Zebrafish Embryos Provide a Preliminary Assessment of Mutated idua Correlated with Mucopolysaccharidosis Type I. Journal of Personalized Medicine. 2022; 12(8):1199. https://doi.org/10.3390/jpm12081199
Chicago/Turabian StyleLin, Cheng-Yung, Hsiang-Yu Lin, Chih-Kuang Chuang, Po-Hsiang Zhang, Yuan-Rong Tu, Shuan-Pei Lin, and Huai-Jen Tsai. 2022. "Quantification of Idua Enzymatic Activity Combined with Observation of Phenotypic Change in Zebrafish Embryos Provide a Preliminary Assessment of Mutated idua Correlated with Mucopolysaccharidosis Type I" Journal of Personalized Medicine 12, no. 8: 1199. https://doi.org/10.3390/jpm12081199
APA StyleLin, C.-Y., Lin, H.-Y., Chuang, C.-K., Zhang, P.-H., Tu, Y.-R., Lin, S.-P., & Tsai, H.-J. (2022). Quantification of Idua Enzymatic Activity Combined with Observation of Phenotypic Change in Zebrafish Embryos Provide a Preliminary Assessment of Mutated idua Correlated with Mucopolysaccharidosis Type I. Journal of Personalized Medicine, 12(8), 1199. https://doi.org/10.3390/jpm12081199








