Longitudinal Association of Universal Screening and Treatment for Major Depressive Disorder with Survival in Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. MDD Intervention
2.3. Primary Cancer Type
2.4. Mortality
2.5. Statistical Methods
3. Results
3.1. Demographics
3.2. Survival Analyses
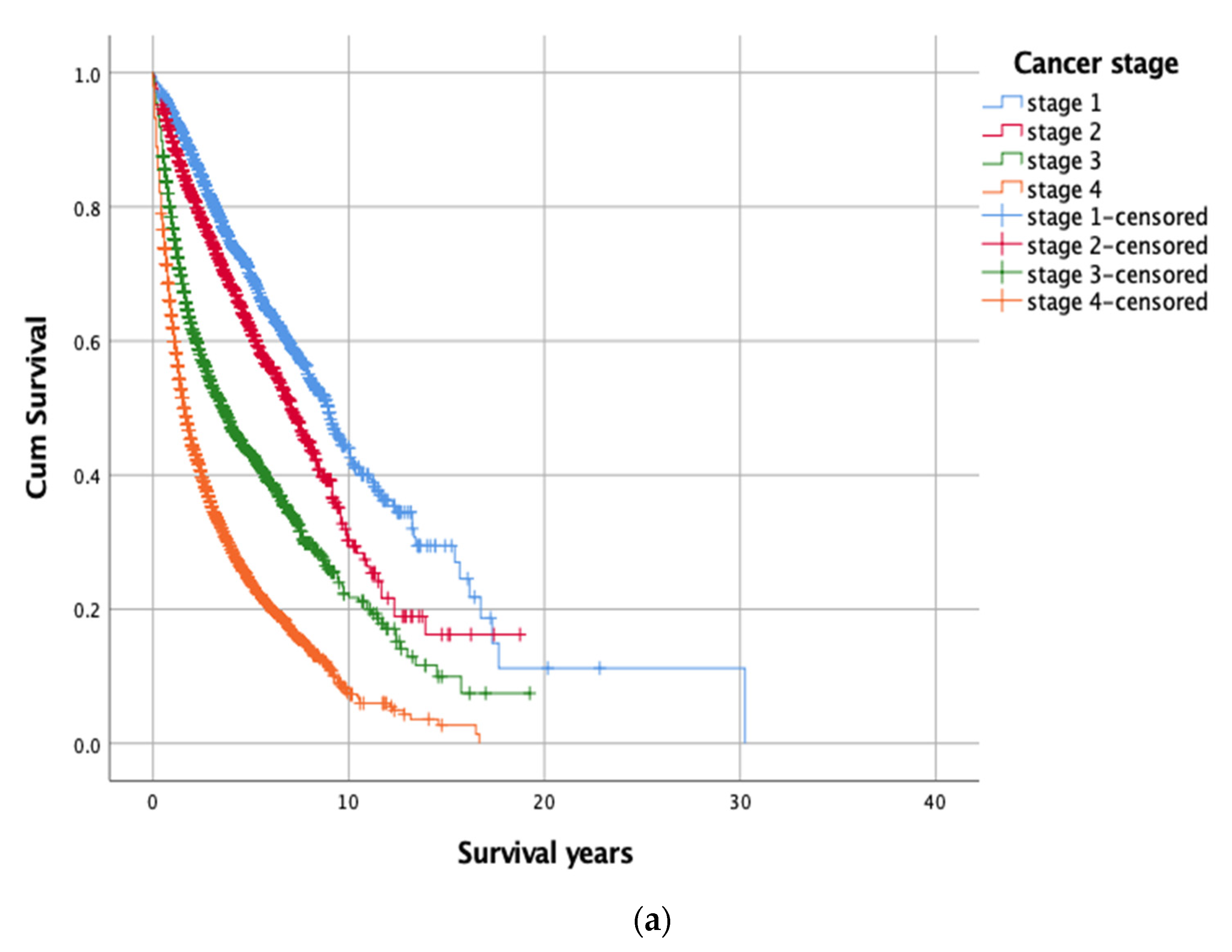
3.2.1. Kaplan-Meier’s Analysis
3.2.2. Cox Proportional Hazards Model
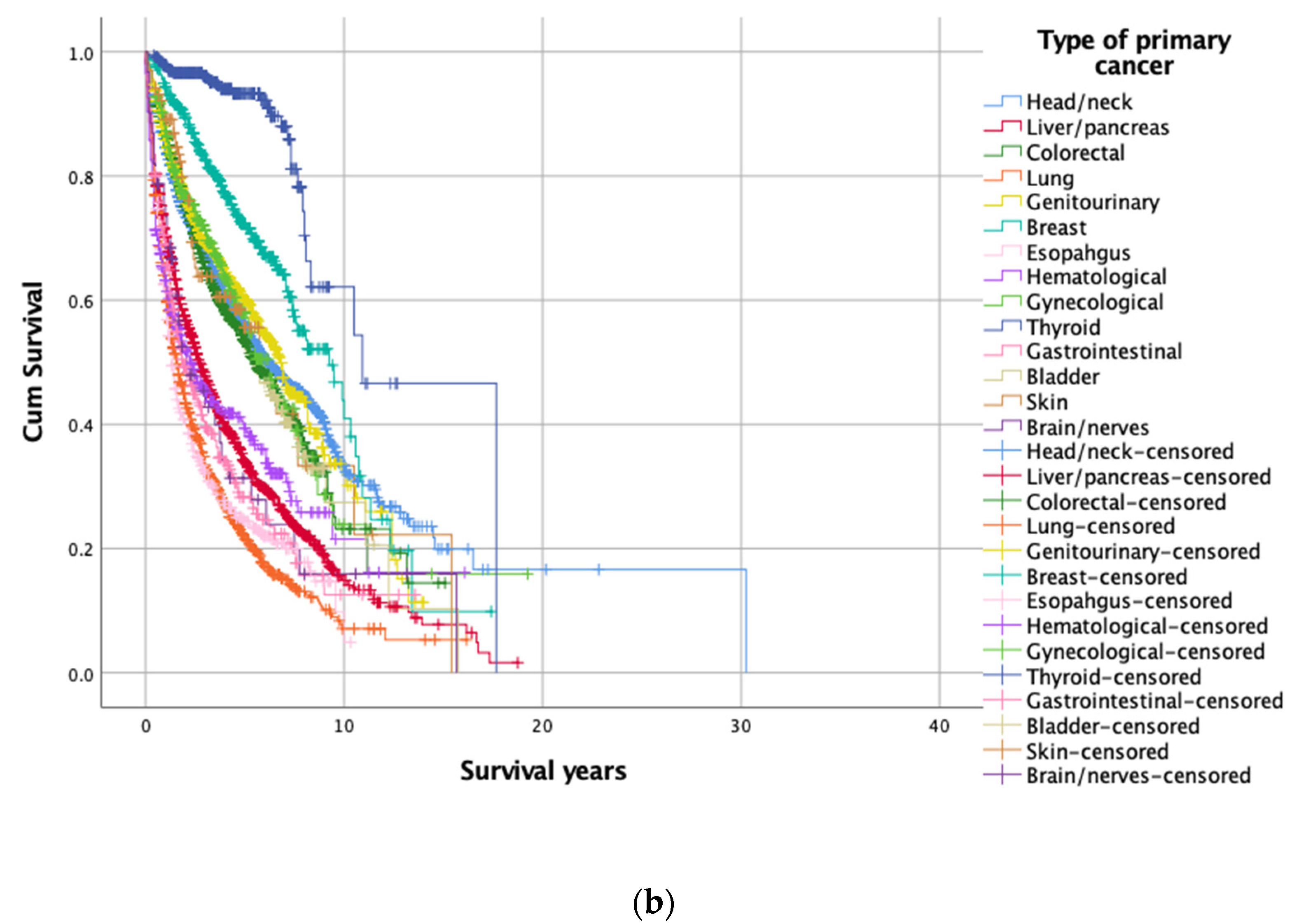
3.3. Subgroup Analysis of 14 Primary Cancer Types
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.; Carioli, G.; Bertuccio, P.; Malvezzi, M.; Pastorino, U.; Boffetta, P.; Negri, E.; Bosetti, C.; La Vecchia, C. Progress in cancer mortality, incidence, and survival: A global overview. Eur. J. Cancer Prev. 2020, 29, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Kheirouri, S.; Alizadeh, M. Prognostic Potential of the Preoperative Controlling Nutritional Status (CONUT) Score in Predicting Survival of Patients with Cancer: A Systematic Review. Adv. Nutr. 2021, 12, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Van der Elst, S.; Bardash, Y.; Wotman, M.; Kraus, D.; Tham, T. The prognostic impact of depression or depressive symptoms on patients with head and neck cancer: A systematic review and meta-analysis. Head Neck 2021, 43, 3608–3617. [Google Scholar] [CrossRef]
- Wang, X.; Wang, N.; Zhong, L.; Wang, S.; Zheng, Y.; Yang, B.; Zhang, J.; Lin, Y.; Wang, Z. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: A systematic review and meta-analysis of 282,203 patients. Mol. Psychiatry 2020, 25, 3186–3197. [Google Scholar] [CrossRef]
- Hu, S.; Li, L.; Wu, X.; Liu, Z.; Fu, A. Post-surgery anxiety and depression in prostate cancer patients: Prevalence, longitudinal progression, and their correlations with survival profiles during a 3-year follow-up. Ir. J. Med. Sci. 2021, 190, 1363–1372. [Google Scholar] [CrossRef]
- McFarland, D.C.; Miller, A.H.; Nelson, C. A Longitudinal Analysis of Inflammation and Depression in Patients With Metastatic Lung Cancer: Associations With Survival. Biol. Res. Nurs. 2021, 23, 301–310. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, H. The longitudinal changes of anxiety and depression, their related risk factors and prognostic value in colorectal cancer survivors: A 36-month follow-up study. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101511. [Google Scholar] [CrossRef]
- Caruso, R.; GiuliaNanni, M.; Riba, M.B.; Sabato, S.; Grassi, L. Depressive Spectrum Disorders in Cancer: Diagnostic Issues and Intervention. A Critical Review. Curr. Psychiatry Rep. 2017, 19, 33. [Google Scholar] [CrossRef] [Green Version]
- Trudel-Fitzgerald, C.; Tworoger, S.S.; Zhang, X.; Giovannucci, E.L.; Meyerhardt, J.A.; Kubzansky, L.D. Anxiety, Depression, and Colorectal Cancer Survival: Results from Two Prospective Cohorts. J. Clin. Med. 2020, 9, 3174. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, L.; Eeltink, C.M.; Visser, O.; Janssen, J.; Maaskant, J.M. Prevalence and associated factors of medication non-adherence in hematological-oncological patients in their home situation. BMC Cancer 2017, 17, 739. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, B.; Hyphantis, T.N.; Valpione, S.; Perini, G.; Maes, M.; Morris, G.; Kubera, M.; Kohler, C.A.; Fernandes, B.S.; Stubbs, B.; et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat Rev. 2017, 52, 58–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.M.; Ma, L.L.; Li, C.; Cao, B.; Jiang, Y.; Han, L.; Xu, R.; Lin, J.; Zhang, D. The molecular mechanism of chronic stress affecting the occurrence and development of breast cancer and potential drug therapy. Transl. Oncol. 2022, 15, 101281. [Google Scholar] [CrossRef]
- Mampay, M.; Flint, M.S.; Sheridan, G.K. Tumour brain: Pretreatment cognitive and affective disorders caused by peripheral cancers. Br. J. Pharm. 2021, 178, 3977–3996. [Google Scholar] [CrossRef]
- Walker, J.; Mulick, A.; Magill, N.; Symeonides, S.; Gourley, C.; Burke, K.; Belot, A.; Quartagno, M.; van Niekerk, M.; Toynbee, M.; et al. Major Depression and Survival in People with Cancer. Psychosom. Med. 2021, 83, 410–416. [Google Scholar] [CrossRef]
- McFarland, D.C.; Saracino, R.M.; Miller, A.H.; Breitbart, W.; Rosenfeld, B.; Nelson, C. Prognostic implications of depression and inflammation in patients with metastatic lung cancer. Future Oncol. 2021, 17, 183–196. [Google Scholar] [CrossRef]
- Walker, J.; Hansen, C.H.; Martin, P.; Symeonides, S.; Ramessur, R.; Murray, G.; Sharpe, M. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: A cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry 2014, 1, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Butow, P.; Price, M.A.; Shaw, J.M.; Turner, J.; Clayton, J.M.; Grimison, P.; Rankin, N.; Kirsten, L. Clinical pathway for the screening, assessment and management of anxiety and depression in adult cancer patients: Australian guidelines. Psychooncology 2015, 24, 987–1001. [Google Scholar] [CrossRef]
- Andersen, B.L.; Rowland, J.H.; Somerfield, M.R. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: An american society of clinical oncology guideline adaptation. J. Oncol. Pract. 2015, 11, 133–134. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Tong, G.; Feng, R.; Chai, J.; Cheng, J.; Wang, D. Psychosocial and Behavioral Interventions and Cancer Patient Survival Again: Hints of an Adjusted Meta-Analysis. Integr Cancer 2014, 13, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.W.; Popovic, M.; Agarwal, A.; Milakovic, M.; Fu, T.S.; McDonald, R.; Fu, G.; Lam, M.; Chow, R.; Cheon, S.; et al. The impact of psychosocial intervention on survival in cancer: A meta-analysis. Ann. Palliat Med. 2016, 5, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Oh, P.J.; Shin, S.R.; Ahn, H.S.; Kim, H.J. Meta-analysis of psychosocial interventions on survival time in patients with cancer. Psychol Health 2016, 31, 396–419. [Google Scholar] [CrossRef] [PubMed]
- Mulick, A.; Walker, J.; Puntis, S.; Burke, K.; Symeonides, S.; Gourley, C.; Wanat, M.; Frost, C.; Sharpe, M. Does depression treatment improve the survival of depressed patients with cancer? A long-term follow-up of participants in the SMaRT Oncology-2 and 3 trials. Lancet Psychiatry 2018, 5, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Tu, C.H.; Hsu, M.C.; Chi, S.C.; Lin, H.Y.; Yen, Y.C. Routine depression screening and diagnosing strategy for cancer inpatients. Psychooncology 2014, 23, 1057–1067. [Google Scholar] [CrossRef]
- Lee, Y.; Yang, M.J.; Lai, T.J.; Chiu, N.M.; Chau, T.T. Development of the Taiwanese Depression Questionnaire. Chang. Gung. Med. J. 2000, 23, 688–694. [Google Scholar]
- Panjwani, A.A.; Li, M. Recent trends in the management of depression in persons with cancer. Curr. Opin. Psychiatry 2021, 34, 448–459. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Rizvi, M.A.; Fatima, M.; Mondal, A.C. Pathophysiological implications of neuroinflammation mediated HPA axis dysregulation in the prognosis of cancer and depression. Mol. Cell Endocrinol. 2021, 520, 111093. [Google Scholar] [CrossRef]
- Peng, Y.N.; Huang, M.L.; Kao, C.H. Prevalence of Depression and Anxiety in Colorectal Cancer Patients: A Literature Review. Int. J. Environ. Res. Public Health 2019, 16, 411. [Google Scholar] [CrossRef] [Green Version]


| Characteristics | Number (%) | Mean (SD) |
|---|---|---|
| Male | 7062 (67.0) | |
| Age at diagnosis of cancer (year) | 10,534 (100) | 61.1 (13.0) |
| Age at death (year) | 5394 (51.2) | 65.0 (13.0) |
| Age at screening MDD (year) | 10,534 (100) | 61.9 (12.9) |
| Survival Time (Year) | Number (%) | Censored (%) | Mean (SD) | p ** |
|---|---|---|---|---|
| Overall | 10,534 (100) | 5140 (48.8) | 7.00 (0.29) | |
| Cancer stage 1 | 2859 (27.1) | 1981 (69.3) | 10.84 (0.75) | <0.001 |
| Cancer stage 2 | 1719 (16.3) | 1088 (63.3) | 8.01 (0.33) | |
| Cancer stage 3 | 2390 (22.7) | 1104 (46.2) | 5.86 (0.24) | |
| Cancer stage 4 | 3566 (33.9) | 967 (27.1) | 3.39 (0.10) | |
| MDD intervention group 1 | 254 (2.4) | 122 (48.0) | 7.67 (0.58) | <0.001 |
| MDD intervention group 2 | 437 (4.1) | 244 (55.8) | 8.14 (0.40) | |
| MDD intervention group 3 | 882 (8.4) | 148 (16.8) | 4.16 (0.33) | |
| MDD intervention group 4 | 8961 (85.1) | 4626 (51.6) | 6.42 (0.13) | |
| Head/neck (C00–13, C30–32, C77) | 2314 (22.0) | 1312 (56.7) | 9.85 (0.69) | <0.001 |
| Liver/pancreas (C22–C35) | 1780 (16.9) | 596 (33.5) | 4.73 (0.17) | |
| Colorectal (C18–21) | 1365 (13.0) | 787 (57.7) | 6.59 (0.28) | |
| Lung (C33–38, C45) | 1301 (12.4) | 362 (27.8) | 3.33 (0.17) | |
| Genitourinary (C60–68) | 1085 (10.3) | 677 (62.4) | 6.94 (0.24) | |
| Breast (C50) | 518 (4.9) | 363 (70.1) | 8.58 (0.50) | |
| Esophagus (C15) | 498 (4.7) | 133 (26.7) | 3.16 (0.17) | |
| Hematological (C7A, C81–94) | 373 (3.5) | 155 (41.6) | 5.19 (0.49) | |
| Gynecological (C51–58) | 358 (3.4) | 113 (31.6) | 7.33 (0.79) | |
| Thyroid (C73) | 337 (3.2) | 305 (90.5) | 12.4 (0.98) | |
| Gastrointestinal (C16–17) | 290 (2.8) | 100 (34.5) | 3.96 (0.35) | |
| Bladder (C67) | 152 (1.4) | 54 (35.5) | 6.50 (0.55) | |
| Skin (C43–44) | 102 (1.0) | 62 (60.8) | 7.02 (1.00) | |
| Brain/nerves (C70–72, C79) | 61 (0.5) | 21 (34.4) | 4.63 (0.85) |
| Characteristics | HR | 95% CI | p | ||
|---|---|---|---|---|---|
| Male (vs. female) | 1.179 | 1.103 | 1.261 | <0.001 | |
| Age at the diagnosis of cancer (per year) | 1.212 | 1.193 | 1.231 | <0.001 | |
| Age at screening of MDD (per year) | 0.843 | 0.830 | 0.856 | <0.001 | |
| Cancer stage | 1 | 0.197 | 0.181 | 0.214 | <0.001 |
| 2 | 0.295 | 0.270 | 0.323 | <0.001 | |
| 3 | 0.508 | 0.474 | 0.546 | <0.001 | |
| 4 | reference | - | - | - | |
| MDD intervention group ** | 1 | 1.061 | 0.889 | 1.267 | 0.512 |
| 2 | 0.702 | 0.607 | 0.812 | <0.001 | |
| 3 | 1.829 | 1.687 | 1.984 | <0.001 | |
| 4 | reference | - | - | - | |
| Head/neck | 0.496 | 0.361 | 0.681 | <0.001 | |
| Liver/pancreas | 1.630 | 1.186 | 2.239 | 0.003 | |
| Colorectal | 0.552 | 0.400 | 0.763 | <0.001 | |
| Lung | 1.061 | 0.772 | 1.458 | 0.714 | |
| Genitourinary | 0.485 | 0.349 | 0.673 | <0.001 | |
| Breast | 0.512 | 0.360 | 0.728 | <0.001 | |
| Esophagus | 1.482 | 1.066 | 2.061 | 0.019 | |
| Hematological | 0.970 | 0.692 | 1.361 | 0.861 | |
| Thyroid | 0.858 | 0.602 | 1.223 | 0.396 | |
| Gynecological | 0.179 | 0.112 | 0.286 | <0.001 | |
| Gastrointestinal | 1.268 | 0.900 | 1.786 | 0.175 | |
| Bladder | 1.002 | 0.691 | 1.453 | 0.991 | |
| Skin | 0.664 | 0.427 | 1.033 | 0.069 | |
| Brain/nerves | reference | - | - | - | |
| Head/Neck | HR | 95% CI | p | Hematological | HR | 95% CI | p | ||||
| Male (vs. female) | 1.092 | 0.873 | 1.092 | 0.443 | Male (vs. female) | 0.957 | 0.729 | 1.255 | 0.748 | ||
| Age ** | 1.114 | 1.080 | 1.149 | <0.001 | Age | 1.307 | 1.184 | 1.443 | <0.001 | ||
| Screening age *** | 0.916 | 0.889 | 0.945 | <0.001 | Screening age | 0.798 | 0.724 | 0.881 | <0.001 | ||
| Group **** | 1 | 1.008 | 0.640 | 1.588 | 0.971 | Group | 1 | 0.943 | 0.437 | 2.038 | 0.882 |
| 2 | 0.806 | 0.597 | 1.090 | 0.162 | 2 | 0.436 | 0.212 | 0.895 | 0.024 | ||
| 3 | 1.976 | 1.687 | 2.314 | <0.001 | 3 | 1.928 | 1.319 | 2.819 | 0.001 | ||
| 4 | reference | - | - | - | 4 | reference | - | - | - | ||
| Liver/Pancreas | HR | 95% CI | p | Gynecological | HR | 95% CI | p | ||||
| Male (vs. female) | 1.078 | 0.948 | 1.226 | 0.254 | Male (vs. female) | - | - | - | |||
| Age | 1.327 | 1.282 | 1.374 | <0.001 | Age | 1.114 | 1.014 | 1.223 | 0.024 | ||
| Screening age | 0.764 | 0.738 | 0.791 | <0.001 | Screening age | 0.913 | 0.833 | 1.001 | 0.052 | ||
| Group | 1 | 0.889 | 0.610 | 1.296 | 0.540 | Group | 1 | 0.208 | 0.024 | 1.771 | 0.151 |
| 2 | 0.674 | 0.459 | 0.990 | 0.044 | 2 | 0.838 | 0.303 | 2.320 | 0.734 | ||
| 3 | 1.448 | 1.176 | 1.783 | <0.001 | 3 | 2.835 | 1.745 | 4.605 | <0.001 | ||
| 4 | reference | - | - | - | 4 | reference | - | - | - | ||
| Colorectal | HR | 95% CI | p | Thyroid | HR | 95% CI | p | ||||
| Male (vs. female) | 1.085 | 0.913 | 1.289 | 0.356 | Male (vs. female) | 2.346 | 1.096 | 5.025 | 0.028 | ||
| Age | 1.149 | 1.095 | 1.206 | <0.001 | Age | 1.207 | 1.015 | 1.436 | 0.034 | ||
| Screening age | 0.893 | 0.851 | 0.937 | <0.001 | Screening age | 0.892 | 0.753 | 1.058 | 0.190 | ||
| Group | 1 | 1.008 | 0.610 | 1.664 | 0.976 | Group | 1 | 2.721 | 0.517 | 14.33 | 0.238 |
| 2 | 0.704 | 0.449 | 1.105 | 0.127 | 2 | 0.539 | 0.070 | 4.139 | 0.552 | ||
| 3 | 1.646 | 1.256 | 2.157 | <0.001 | 3 | 12.142 | 3.279 | 44.96 | <0.001 | ||
| 4 | reference | - | - | - | 4 | reference | - | - | - | ||
| Lung | HR | 95% CI | p | Gastrointestinal | HR | 95% CI | p | ||||
| Male (vs. female) | 1.565 | 1.363 | 1.797 | <0.001 | Male (vs. female) | 1.071 | 0.791 | 1.451 | 0.656 | ||
| Age | 1.377 | 1.300 | 1.459 | <0.001 | Age | 1.166 | 1.051 | 1.294 | 0.004 | ||
| Screening age | 0.739 | 0.698 | 0.783 | <0.001 | Screening age | 0.877 | 0.791 | 0.973 | 0.013 | ||
| Group | 1 | 0.823 | 0.492 | 1.377 | 0.458 | Group | 1 | 0.582 | 0.140 | 2.426 | 0.457 |
| 2 | 0.631 | 0.437 | 0.911 | 0.014 | 2 | 0.559 | 0.197 | 1.587 | 0.275 | ||
| 3 | 1.662 | 1.361 | 2.031 | <0.001 | 3 | 1.557 | 0.997 | 2.430 | 0.051 | ||
| 4 | reference | - | - | - | 4 | reference | - | - | - | ||
| Genitourinary | HR | 95% CI | p | Bladder | HR | 95% CI | p | ||||
| Male (vs. female) | 0.608 | 0.473 | 0.781 | <0.001 | Male (vs. female) | 0.916 | 0.583 | 1.439 | 0.705 | ||
| Age | 1.201 | 1.134 | 1.273 | <0.001 | Age | 1.332 | 1.191 | 1.490 | <0.001 | ||
| Screening age | 0.861 | 0.813 | 0.912 | <0.001 | Screening age | 0.774 | 0.693 | 0.865 | <0.001 | ||
| Group | 1 | 1.265 | 0.706 | 2.265 | 0.429 | Group | 1 | 1.943 | 0.247 | 15.29 | 0.528 |
| 2 | 0.719 | 0.473 | 1.093 | 0.123 | 2 | 1.285 | 0.509 | 3.243 | 0.595 | ||
| 3 | 2.946 | 2.072 | 4.188 | <0.001 | 3 | 1.665 | 0.778 | 3.564 | 0.189 | ||
| 4 | reference | - | - | - | 4 | reference | - | - | - | ||
| Breast | HR | 95% CI | p | Skin | HR | 95% CI | p | ||||
| Male (vs. female) | <0.001 | <0.001 | <0.001 | 0.969 | Male (vs. female) | 1.225 | 0.623 | 2.447 | 0.566 | ||
| Age | 1.121 | 1.043 | 1.204 | 0.002 | Age | 1.446 | 1.102 | 1.897 | 0.008 | ||
| Screening age | 0.915 | 0.851 | 0.983 | 0.015 | Screening age | 0.707 | 0.538 | 0.929 | 0.013 | ||
| Group | 1 | 1.276 | 0.620 | 2.627 | 0.509 | Group | 1 | 11.21 | 2.215 | 56.72 | 0.003 |
| 2 | 1.334 | 0.688 | 2.584 | 0.394 | 2 | 0.756 | 0.098 | 5.838 | 0.789 | ||
| 3 | 2.208 | 1.353 | 3.601 | 0.002 | 3 | 2.734 | 0.655 | 11.41 | 0.168 | ||
| 4 | reference | - | - | - | 4 | reference | - | - | - | ||
| Esophagus | HR | 95% CI | p | Brain/Nerves | HR | 95% CI | p | ||||
| Male (vs. female) | 1.461 | 0.847 | 2.519 | 0.172 | Male (vs. female) | 1.517 | 0.682 | 3.373 | 0.307 | ||
| Age | 1.243 | 1.138 | 1.357 | <0.001 | Age | 2.023 | 1.432 | 2.858 | <0.001 | ||
| Screening age | 0.810 | 0.741 | 0.885 | <0.001 | Screening age | 0.502 | 0.356 | 0.708 | <0.001 | ||
| Group | 1 | 1.073 | 0.546 | 2.110 | 0.838 | Group | 1 | 2.346 | 0.700 | 7.861 | 0.167 |
| 2 | 0.463 | 0.235 | 0.912 | 0.026 | 2 | 0.260 | 0.048 | 1.397 | 0.116 | ||
| 3 | 1.479 | 1.119 | 1.955 | 0.006 | 3 | 3.292 | 1.033 | 10.49 | 0.044 | ||
| 4 | reference | - | - | - | 4 | reference | - | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, Y.-C.; Huang, C.-Y.; Chan, H.-W.; Wang, Y.-Y.; Changchien, T.-C.; Wang, D.-W.; Lin, P.-C.; Chang, T.-T.; Chiu, Y.-W. Longitudinal Association of Universal Screening and Treatment for Major Depressive Disorder with Survival in Cancer Patients. J. Pers. Med. 2022, 12, 1213. https://doi.org/10.3390/jpm12081213
Yen Y-C, Huang C-Y, Chan H-W, Wang Y-Y, Changchien T-C, Wang D-W, Lin P-C, Chang T-T, Chiu Y-W. Longitudinal Association of Universal Screening and Treatment for Major Depressive Disorder with Survival in Cancer Patients. Journal of Personalized Medicine. 2022; 12(8):1213. https://doi.org/10.3390/jpm12081213
Chicago/Turabian StyleYen, Yung-Chieh, Chin-Yu Huang, Hsue-Wei Chan, You-Yu Wang, Te-Chang Changchien, Deng-Wu Wang, Po-Chun Lin, Ting-Ting Chang, and Yu-Wen Chiu. 2022. "Longitudinal Association of Universal Screening and Treatment for Major Depressive Disorder with Survival in Cancer Patients" Journal of Personalized Medicine 12, no. 8: 1213. https://doi.org/10.3390/jpm12081213
APA StyleYen, Y.-C., Huang, C.-Y., Chan, H.-W., Wang, Y.-Y., Changchien, T.-C., Wang, D.-W., Lin, P.-C., Chang, T.-T., & Chiu, Y.-W. (2022). Longitudinal Association of Universal Screening and Treatment for Major Depressive Disorder with Survival in Cancer Patients. Journal of Personalized Medicine, 12(8), 1213. https://doi.org/10.3390/jpm12081213






