A Machine-Learning-Algorithm-Based Prediction Model for Psychotic Symptoms in Patients with Depressive Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Overviews and Participants
2.2. Demographic Characteristics, Psychotic Symptoms, and Depressive Symptom Profiles
2.3. Variable Profiles for the Prediction Model of Concurrent Psychotic Symptoms
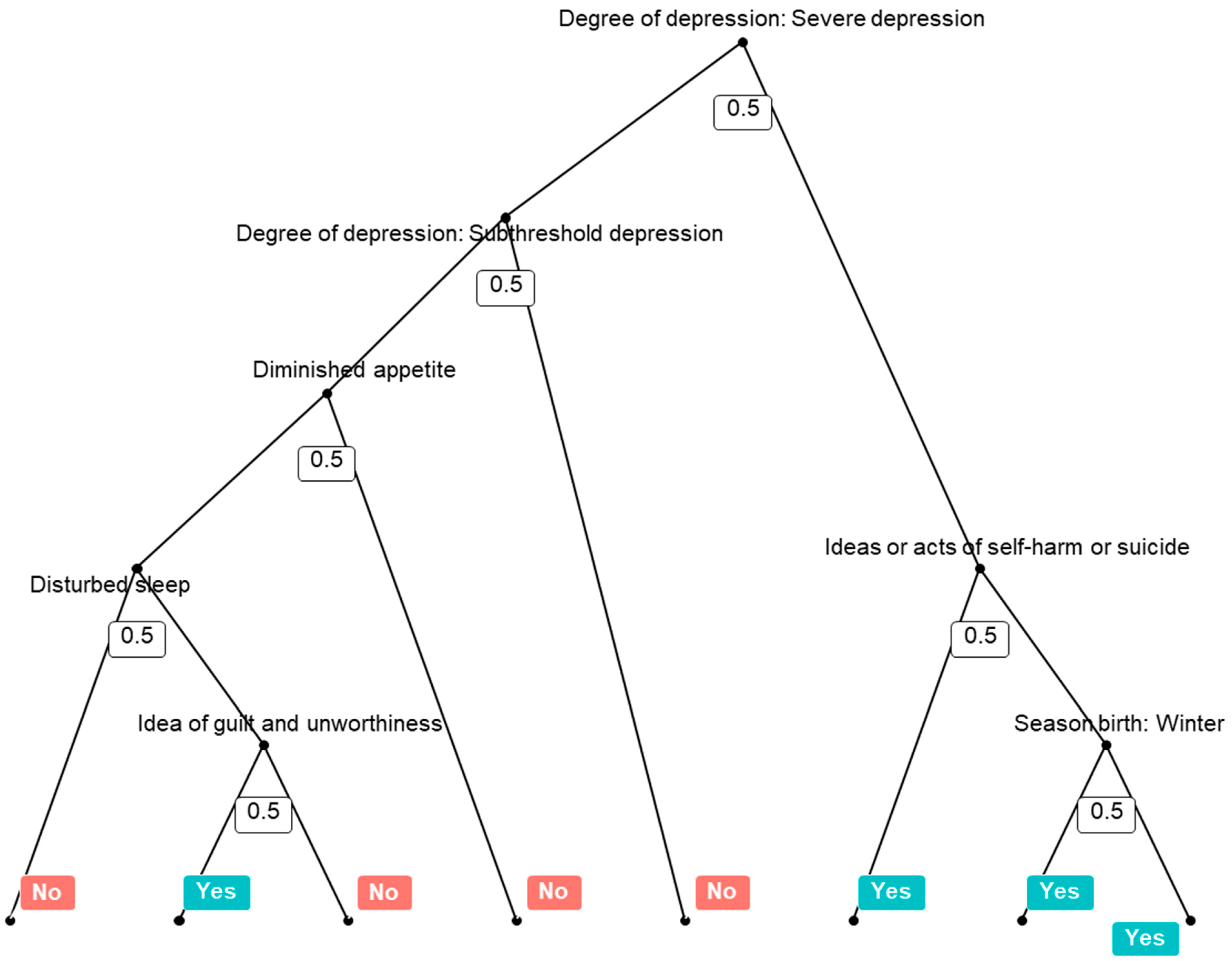
2.4. Data Processing and Machine Learning
3. Results
3.1. Participant Characteristics
3.2. Prediction Model Performance for Concurrent Psychotic Symptoms and Its Variable Importance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.-C. Neurogenesis and antidepressant action. Cell Tissue Res. 2019, 377, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; Schatzberg, A.F. Prevalence of Depressive Episodes with Psychotic Features in the General Population. Am. J. Psychiatry 2002, 159, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Horwath, E.; Weissman, M.M. The validity of major depressive disorder with psychotic features based on a community study. Arch. Gen. Psychiatry 1991, 48, 1075–1081. [Google Scholar] [CrossRef]
- Coryell, W.; Pfohl, B.; Zimmerman, M. The Clinical and Neuroendocrine Features of Psychotic Depression. J. Nerv. Ment. Dis. 1984, 172, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, S.D.; Meyers, B.S.; Flint, A.J.; Mulsant, B.H.; Whyte, E.M.; Ulbricht, C.M.; Bech, P.; Rothschild, A.J.; STOP-PD Study Group. Measuring psychotic depression. Acta Psychiatr. Scand. 2013, 129, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Østergaard, S.D.; Meyers, B.S.; Flint, A.J.; Mulsant, B.H.; Whyte, E.M.; Ulbricht, C.M.; Bech, P.; Rothschild, A.J.; on behalf of the STOP-PD Study Group. Measuring treatment response in psychotic depression: The Psychotic Depression Assessment Scale (PDAS) takes both depressive and psychotic symptoms into account. J. Affect. Disord. 2014, 160, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Schatzberg, A.F.; Rothschild, A.J. Psychotic (delusional) major depression: Should it be included as a distinct syndrome in DSM-IV? Am. J. Psychiatry 1992, 149, 733–745. [Google Scholar] [CrossRef]
- Østergaard, S.D.; Rothschild, A.J.; Uggerby, P.; Munk-Jørgensen, P.; Bech, P.; Mors, O. Considerations on the ICD-11 Classification of Psychotic Depression. Psychother. Psychosom. 2012, 81, 135–144. [Google Scholar] [CrossRef]
- Stergaard, S.D.; Bille, J.; Søltoft, H.; Lauge, N.; Bech, P. The validity of the severity-psychosis hypothesis in depression. J. Affect. Disord. 2012, 140, 48–56. [Google Scholar] [CrossRef]
- Park, S.-C.; Lee, H.-Y.; Sakong, J.-K.; Jun, T.-Y.; Lee, M.-S.; Kim, J.-M.; Kim, J.-B.; Yim, H.-W.; Park, Y.C. Distinctive Clinical Correlates of Psychotic Major Depression: The CRESCEND Study. Psychiatry Investig. 2014, 11, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-C.; Choi, J.; Kim, J.-M.; Jun, T.-Y.; Lee, M.-S.; Kim, J.-B.; Yim, H.-W.; Park, Y.C. Is the Psychotic Depression Assessment Scale a useful diagnostic tool? The CRESCEND study. J. Affect. Disord. 2014, 166, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Østergaard, S.D.; Choi, J.; Kim, J.-M.; Jun, T.-Y.; Lee, M.-S.; Kim, J.-B.; Yim, H.-W.; Park, Y.C. Is the BPRS-5 subscale of the psychotic depression assessment scale a reliable screening tool for psychotic depression? Results from the CRESCEND Study. J. Affect. Disord. 2015, 174, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Jang, E.Y.; Kim, J.-M.; Jun, T.-Y.; Lee, M.-S.; Kim, J.-B.; Yim, H.-W.; Park, Y.C. Clinical Validation of the Psychotic Depression Assessment Scale, Hamilton Depression Rating Scale-6, and Brief Psychiatric Rating Scale-5: Results from the Clinical Research Center for Depression Study. Psychiatry Investig. 2017, 14, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Glassman, A.H.; Roose, S.P. Delusional depression: A distinct clinical entity? Arch. Gen. Psychiatry 1981, 138, 831–833. [Google Scholar] [CrossRef]
- Lykouras, E.; Malliaras, D.; Christodoulou, G.N.; Papakostas, Y.; Voulgari, A.; Tzonou, A.; Stefanis, C. Delusional depression: Phenomenology and response to treatment. A prospective study. Acta Psychiatr. Scand. 1986, 73, 324–329. [Google Scholar] [CrossRef]
- Frances, A.; Brown, R.P.; Kocsis, J.H.; Mann, J.J. Psychotic depression: A separate entity? Am. J. Psychiatry 1981, 138, 831–833. [Google Scholar] [CrossRef]
- Zaninotto, L.; Guglielmo, R.; Calati, R.; Ioime, L.; Camardese, G.; Janiri, L.; Bria, P.; Serretti, A. Cognitive markers of psychotic unipolar depression: A meta-analytic study. J. Affect. Disord. 2015, 174, 580–588. [Google Scholar] [CrossRef]
- Hill, S.K.; Keshavan, M.S.; Thase, M.E.; Sweeney, J.A. Neuropsychological Dysfunction in Antipsychotic-Naive First-Episode Unipolar Psychotic Depression. Am. J. Psychiatry 2004, 161, 996–1003. [Google Scholar] [CrossRef]
- Coryell, W.; Endicott, J.; Keller, M. The importance of psychotic features to major depression: Course and outcome during a 2-year follow-up. Acta Psychiatr. Scand. 1987, 75, 78–85. [Google Scholar] [CrossRef]
- Østergaard, S.D.; Straszek, S.; Petrides, G.; Skadhede, S.; Jensen, S.O.W.; Nielsen, J.; Munk-Jørgensen, P. Risk factors for conversion from unipolar psychotic depression to bipolar disorder. Bipolar Disord. 2014, 16, 180–189. [Google Scholar] [CrossRef]
- Østergaard, S.D.; Bertelsen, A.; Nielsen, J.; Mors, O.; Petrides, G. The association between psychotic mania, psychotic depression and mixed affective episodes among 14,529 patients with bipolar disorder. J. Affect. Disord. 2013, 147, 44–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leckman, J.F.; Weissman, M.; Caruso, K.A.; Merikangas, K.R.; Pauls, D.L.; Prusoff, B.A.; Kidd, K.K. Subtypes of depression. Family study perspective. Arch. Gen. Psychiatry 1984, 41, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.H.; Khan, A.; Orr, W.W., Jr. Delusional depression, Phenomenology, neuroendocrine and tricyclic antidepressant response. J. Affect. Disord. 1984, 6, 297–306. [Google Scholar] [CrossRef]
- Østergaard, S.D.; Waltoft, B.L.; Mortensen, P.B.; Mors, O. Environmental and familial risk factors for psychotic and non-psychotic severe depression. J. Affect. Disord. 2013, 147, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Vythilingam, M.; Chen, J.; Bremner, J.D.; Mazure, C.M.; Maciejewski, P.K.; Nelson, J.C. Psychotic Depression and Mortality. Am. J. Psychiatry 2003, 160, 574–576. [Google Scholar] [CrossRef]
- Zalpuri, I.; Rothschild, A. Does psychosis increase the risk of suicide in patients with major depression? A systemic review. J. Affect. Disord. 2016, 98, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Anton, R.F. Urinary free cortisol in psychotic depression. Biol. Psychiatry 1987, 22, 24–34. [Google Scholar] [CrossRef]
- Nelson, J.C.; Davis, J.M. DST Studies in Psychotic Depression: A Meta-Analysis. Am. J. Psychiatry 1997, 154, 1497–1503. [Google Scholar] [CrossRef]
- Rothschild, A.J.; Schatzberg, A.F.; Rosenbaum, A.H.; Stahl, J.B.; Cole, J.O. The Dexamethasone Suppression Test as a Discriminator among Subtypes of Psychotic Patients. Br. J. Psychiatry 1982, 141, 471–474. [Google Scholar] [CrossRef]
- Schatzberg, A.F.; Rothschild, A.J.; Stahl, J.B.; Bond, T.C.; Rosenbaum, A.H.; Lofgren, S.B.; MacLaughlin, R.A.; Sullivan, M.A.; Cole, J.O. The dexamethasone suppression test: Identification of subtypes of depression. Am. J. Psychiatry 1983, 140, 88–91. [Google Scholar] [CrossRef]
- Kim, D.K.; Kim, B.L.; Sohn, S.E.; Lim, S.W.; Na, D.G.; Paik, C.H.; Krishnan, K.R.R.; Carroll, B.J. Candidate neuroanatomic substrates of psychosis in old-aged depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 1999, 23, 793–807. [Google Scholar] [PubMed]
- Salokangas, R.K.R.; Cannon, T.; van Erp, T.; Ilonen, T.; Taiminen, T.; Karlsson, H.; Lauerma, H.; Leinonen, K.M.; Wallenius, E.; Kaljonen, A.; et al. Structural magnetic resonance imaging in patients with first-episode schizophrenia, psychotic and severe non-psychotic depression and healthy controls: Results of the schizophrenia and affective psychoses (SAP) project. Br. J. Psychiatry 2002, 43, S58–S65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, S.; Baldwin, R.C.; Jackson, A.; Burns, A. The differentiation of DSM-III-R psychotic depression in later life from nonpsychotic depression: Comparisons of brain changes measured by multispectral analysis of magnetic resonance brain images, neuropsychological findings, and clinical features. Biol. Psychiatry 1999, 45, 193–204. [Google Scholar] [CrossRef]
- Cubells, J.F.; Price, L.H.; Meyers, B.A.; Anderson, G.M.; Zabetian, C.P.; Alexopoulos, G.S.; Nelson, J.C.; Sanacora, G.; Kirwin, P.; Carpenter, L.; et al. Genotype-controlled analysis of plasma dopamine beta-hydroxylase activity in psychotic unipolar major depression. Biol. Psychiatry 2002, 51, 358–364. [Google Scholar] [CrossRef]
- Meyers, B.S.; Alexopoulos, G.S.; Kakuma, T.; Tirumalasetti, F.; Gabriele, M.; Alpert, S.; Bowden, C.; Meltzer, H.Y. Decreased dopamine beta-hyrodoxylase activity in unipolar geriatirc delusional depression. Biol. Psychiatry 1999, 45, 448–452. [Google Scholar] [CrossRef]
- Leadholm, A.K.; Rothschild, A.J.; Nolen, W.A.; Bech, P.; Munk-Jørgensen, P.; Østergaard, S.D. The treatment of psychotic depression: Is there consensus among guidelines and psychiatrists? J. Affect. Disord. 2013, 145, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Wijkstra, J.; Lijmer, J.; Burger, H.; Cipriani, A.; Geddes, J.; Nolen, W.A. Pharmacological treatment for psychotic depression. Cochrane Database Syst. Rev. 2015, 7, CD004044. [Google Scholar] [CrossRef] [Green Version]
- Won, E.; Park, S.-C.; Han, K.-M.; Sung, S.-H.; Lee, H.-Y.; Paik, J.-W.; Jeon, H.J.; Lee, M.-S.; Shim, S.-H.; Ko, Y.-H.; et al. Evidence-Based, Pharmacological Treatment Guideline for Depression in Korea, Revised Edition. J. Korean Med Sci. 2014, 29, 468–484. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.S.; Song, H.R.; Bin Lee, H.; Park, Y.-M.; Hong, J.-W.; Kim, W.; Wang, H.-R.; Lim, E.-S.; Jeong, J.-H.; Jon, D.-I.; et al. The Korean medication algorithm for depressive disorder: Second revision. J. Affect. Disord. 2014, 167, 312–321. [Google Scholar] [CrossRef]
- Block, T.S.; Kushner, H.; Kalin, N.; Nelson, C.; Belanoff, J.; Schatzberg, A. Combined Analysis of Mifepristone for Psychotic Depression: Plasma Levels Associated with Clinical Response. Biol. Psychiatry 2018, 84, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Arns, M.; van Dijk, H.; Luykx, J.J.; van Wingen, G.; Olbrich, S. Stratified psychiatry: Tomorrow’s precision psychiatry? Eur. Neuropsychopharmacol. 2022, 55, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Shinfuku, N.; Maramis, M.M.; Lee, M.-S.; Park, Y.C. Adjunctive Antipsychotic Prescriptions for Outpatients with Depressive Disorders in Asia: The Research on Asian Psychotropic Prescription Patterns for Antidepressants (REAP-AD) Study. Am. J. Psychiatry 2015, 172, 684–685. [Google Scholar] [CrossRef]
- Park, S.-C.; Lee, M.S.; Shinfuku, N.; Sartorius, N.; Park, Y.C. Gender differences in depressive symptom profiles and patterns of psychotropic drug usage in Asian patients with depression: Findings from the Research on Asian Psychotropic Prescription Patterns for Antidepressants (REAP-AD) study. Aust. N. Z. J. Psychiatry 2015, 49, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jang, E.Y.; Xiang, Y.; Kanba, S.; Kato, T.A.; Chong, M.; Lin, S.; Yang, S.; Avasthi, A.; Grover, S.; et al. Network analysis of the depressive symptom profiles in Asian patients with depressive disorders: Findings from the Research on Asian Psychotropic Prescription Patterns for Antidepressants (REAP-AD). Psychiatry Clin. Neurosci. 2020, 74, 344–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. The ICD-10 Classification of Mental and Behavioral Disorders, Clinical Descriptions and Diagnostic Guidelines; World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- World Health Organization. Anatomical Therapeutic Chemical (ATC) Classification System. Available online: https://www.whocc.no/atc/structure_and_principles/ (accessed on 2 May 2022).
- Fountoulakis, K.N.; Iacovides, A.; Karamouzis, M.; Kaprinis, G.S.; Ierodiakonou, C. Season of birth, clinical manifestations and Dexamethasone Suppression Test in unipolar major depression. Ann. Gen. Psychiatry 2007, 6, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pjrek, E.; Winkler, D.; Heiden, A.; Praschak-Rieder, N.; Willeit, M.; Konstantinidis, A.; Stastny, J.; Kasper, S. Seasonality of birth in seasonal affective disorder. J. Clin. Psychiatry 2004, 65, 1389–1393. [Google Scholar] [CrossRef]
- National Institute for Health and Clinical Excellence. Depression in Adults: The Treatment and Management of Depression in Adults; National Institute for Health and Clinical Excellence: London, UK, 2009; p. 63. [Google Scholar]
- R core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- RStudio Team. RStudio: Intergrated Development for R.; RStudio, Inc.: Boston, MA, USA, 2012. [Google Scholar]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Breiman, L. Random Forest. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Erickson, J.; Abbott, K.; Susienka, L. Automatic address validation and health record review to identify homeless Social Security disability applicants. J. Biomed. Inform. 2018, 82, 41–46. [Google Scholar] [CrossRef]
- Zhang, L.; Fabbri, D.; Lasko, T.A.; Ehrenfeld, J.M.; Wanderer, J.P. A System for Automated Determination of Perioperative Patient Acuity. J. Med Syst. 2018, 42, 123. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning, 2nd ed.; Springer: New York, NY, USA, 2017; pp. 593–594. [Google Scholar]
- World Health Organization. International Classification of Diseases, 11th revision (ICD-11): The Global Standard for Diagnostic Health Information. Available online: https://icd.who.int/ct11/icd11_mms/en/release/ (accessed on 2 May 2022).
- Gournellis, R.; Oulis, P.; Rizos, E.; Chourdaki, E.; Gouzaris, A.; Lykouras, L. Clinical correlates of age of onset in psychotic depression. Arch. Gerontol. Geriatr. 2011, 52, 94–98. [Google Scholar] [CrossRef]
- Davies, G.; Welham, J.; Chant, D.; Torrey, E.F.; McGrath, J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr. Bull. 2003, 29, 587–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csajbók, Z.; Kagstrom, A.; Cermakova, P. Season of birth has no effect on symptoms of depression and anxiety in older adults. Sci. Rep. 2022, 12, 6823. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2000; Chapter 5; pp. 160–164. [Google Scholar]
- Zhou, R.; Yin, W.; Li, W.; Wang, Y.; Lu, J.; Li, Z.; Hu, X. Prediction Model for Infectious Disease Health Literacy Based on Synthetic Minority Oversampling Technique Algorithm. Comput. Math. Methods Med. 2022, 2022, 8498159. [Google Scholar] [CrossRef] [PubMed]


| Total (n = 1171) | Concurrent Psychotic Symptoms | Statistical Coefficient | p-Value | ||
|---|---|---|---|---|---|
| Presence (n = 16) | Absence (n = 1155) | ||||
| Country/SAR | χ2 = 22.852 | 0.007 | |||
| China, n (%) | 240 (20.5) | 4 (25.0) | 236 (20.4) | ||
| Hong Kong, n (%) | 38 (9.1) | 0 (0.0) | 28 (9.3) | ||
| Japan, n (%) | 142 (12.1) | 5 (31.3) | 137 (11.9) | ||
| Korea, n (%) | 173 (14.8) | 0 (0.0) | 183 (15.0) | ||
| Singapore, n (%) | 38 (3.2) | 0 (0.0) | 38 (3.3) | ||
| Taiwan, n (%) | 50 (4.3) | 0 (0.0) | 50 (3.3) | ||
| India, n (%) | 130 (11.1) | 6 (37.5) | 124 (10.7) | ||
| Malaysia, n (%) | 109 (9.3) | 0 (0.0) | 109 (9.4) | ||
| Thailand, n (%) | 144 (12.3) | 1 (6.3) | 143 (12.4) | ||
| Indonesia, n (%) | 107 (9.1) | 0 (0.0) | 107 (9.3) | ||
| Age | 48.4 (16.9) | 45.3 (17.7) | 48.4 (16.9) | t = −0.707 | 0.490 |
| Male, n (%) | 477 (40.7) | 7 (43.8) | 470 (40.7) | χ2 = 0.061 | 0.805 |
| Outpatient, n (%) | 843 (72.0) | 9 (56.3) | 834 (72.2) | χ2 = 1.993 | 0.158 |
| Season of birth † | χ2 = 5.853 | 0.119 | |||
| Spring, n (%) | 251 (23.6) | 1 (10.0) | 250 (23.7) | ||
| Summer, n (%) | 261 (24.6) | 4 (40.0) | 257 (24.4) | ||
| Autumn, n (%) | 251 (23.6) | 0 (0.0) | 251 (23.8) | ||
| Winter, n (%) | 300 (28.2) | 5 (50.0) | 295 (28.0) | ||
| Depressive symptom profiles | |||||
| Depressed mood, n (%) | 856 (73.1) | 16 (100.0) | 840 (98.1) | χ2 = 5.969 | 0.015 |
| Loss of interest and enjoyment, n (%) | 620 (52.9) | 13 (81.3) | 607 (52.6) | χ2 = 5.216 | 0.022 |
| Reduced energy and diminished activity, n (%) | 535 (45.7) | 12 (75.0) | 523 (45.3) | χ2 = 5.617 | 0.018 |
| Reduced concentration and attention, n (%) | 347 (29.6) | 5 (31.3) | 342 (29.6) | χ2 = 0.020 | 0.887 |
| Reduced self-esteem and self-confidence, n (%) | 268 (22.9) | 7 (43.8) | 261 (22.6) | χ2 = 4.001 | 0.045 |
| Ideas of guilt and unworthiness, n (%) | 185 (15.8) | 7 (43.8) | 178 (15.4) | χ2 = 9.527 | 0.002 |
| Psychomotor agitation or retardation, n (%) | 266 (22.7) | 8 (50.0) | 258 (22.3) | χ2 = 6.879 | 0.009 |
| Ideas or acts of self-harm or suicide, n (%) | 267 (22.8) | 6 (37.5) | 261 (22.6) | χ2 = 1.991 | 0.158 |
| Disturbed sleep, n (%) | 747 (63.8) | 15 (93.8) | 732 (63.4) | χ2 = 6.303 | 0.012 |
| Diminished appetite, n (%) | 383 (32.7) | 11 (68.8) | 372 (32.2) | χ2 = 9.575 | 0.002 |
| Degree of depression | χ2 = 21.104 | <0.0001 | |||
| Subthreshold, n (%) | 533 (45.5) | 1 (6.3) | 532 (46.1) | ||
| Mild, n (%) | 211 (18.0) | 3 (18.8) | 208 (18.0) | ||
| Moderate, n (%) | 296 (25.3) | 5 (31.3) | 291 (25.2) | ||
| Severe, n (%) | 131 (11.2) | 7 (43.8) | 124 (10.7) | ||
| Comorbid symptom profiles | |||||
| Anxiety symptoms, n (%) | 20 (1.7) | 0 (0.0) | 20 (1.7) | χ2 = 0.282 | 0.595 |
| Somatic symptoms, n (%) | 15 (1.3) | 0 (0.0) | 15 (1.3) | χ2 = 0.210 | 0.646 |
| Psychiatric comorbidity | |||||
| Anxiety and somatoform disorder (F4), n (%) | 87 (7.4) | 1 (6.3) | 86 (7.4) | χ2 = 0.033 | 0.856 |
| Substance use disorder (F1), n (%) | 20 (1.7) | 0 (0.0) | 20 (1.7) | χ2 = 0.282 | 0.595 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Ryu, J.i.; Lee, B.J.; Na, E.; Xiang, Y.-T.; Kanba, S.; Kato, T.A.; Chong, M.-Y.; Lin, S.-K.; Avasthi, A.; et al. A Machine-Learning-Algorithm-Based Prediction Model for Psychotic Symptoms in Patients with Depressive Disorder. J. Pers. Med. 2022, 12, 1218. https://doi.org/10.3390/jpm12081218
Kim K, Ryu Ji, Lee BJ, Na E, Xiang Y-T, Kanba S, Kato TA, Chong M-Y, Lin S-K, Avasthi A, et al. A Machine-Learning-Algorithm-Based Prediction Model for Psychotic Symptoms in Patients with Depressive Disorder. Journal of Personalized Medicine. 2022; 12(8):1218. https://doi.org/10.3390/jpm12081218
Chicago/Turabian StyleKim, Kiwon, Je il Ryu, Bong Ju Lee, Euihyeon Na, Yu-Tao Xiang, Shigenobu Kanba, Takahiro A. Kato, Mian-Yoon Chong, Shih-Ku Lin, Ajit Avasthi, and et al. 2022. "A Machine-Learning-Algorithm-Based Prediction Model for Psychotic Symptoms in Patients with Depressive Disorder" Journal of Personalized Medicine 12, no. 8: 1218. https://doi.org/10.3390/jpm12081218






