Abstract
Background: Pharmacogenomics (PGx) testing is increasingly used in clinical practice to optimize drug therapies. This study aims to understand the involvement of clinical pharmacists in PGx testing at tertiary hospitals in China and their self-assessed capacity to deliver such services. Methods: We developed a questionnaire exploring clinical pharmacists’ involvement and self-assessed level of capacity of performing PGx tests. A random sample was obtained from the Pharmaceutical Affairs Management Professional Committee of the Chinese Hospital Association. Results: A total of 1005 clinical pharmacists completed the survey. Of these, 996 (99.1%) had heard of PGx tests and 588 (59.0%) had been involved in PGx testing and related services. Some clinical pharmacists (28.9%) provided PGx services at the rate of “1–5 cases/year” while 21.9% of clinical pharmacists provided PGx services at the rate of “>30 cases/year”. Clinical pharmacists most frequently provided PGx testing for cardiovascular diseases. “Consult relevant guidelines/literature” (90.1%) was the most frequently used method to familiarize oneself with PGx testing. About 60% of the pharmacists considered themselves to have poor or fair capacity to provide PGx testing and related services. Conclusions: More than half of the pharmacists had been involved in PGx testing and related services. However, pharmacists generally had little confidence in their knowledge level of and capacity to provide PGx-related services.
1. Introduction
Pharmacogenomics (PGx) derives from pharmacogenetics and studies variations of DNA and RNA characteristics related to drug response [1]. It can explain individual differences in pharmacokinetics and pharmacodynamics and guide personalized medicine in clinical practice to improve drug efficacy and safety. Clopidogrel is a good example that supports the importance of PGx in improving drug efficacy. As the CYP2C19 genotype affects the formation of clopidogrel active metabolites, people with a moderate and low metabolism of CYP2C19 who are treated with clopidogrel experience reduced platelet inhibition and increased risks of major adverse cardiovascular and cerebrovascular events [2]. PGx can also help determine dose adjustments, as seen in estimating the starting dose of warfarin based on the different genotypes of CYP2C9*2, CYP2C9*3, and VKORC1-1639G>A. [3] Moreover, PGx testing can help avoid adverse drug reactions, such as severe cutaneous adverse reactions (SCAR) due to allele mutations in HLA-B*58:01 during allopurinol treatment [4], as well as the Stevens–Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) induced by carbamazepine due to HLA-B*15:02 mutations [5]. Although PGx has many advantages in personalized therapy and enhancing patient outcomes, it has not yet been used extensively in clinical practice partly due to its cost. However, many studies have shown that PGx-guided treatment can be a cost-effective and even a cost-cutting strategy [6,7,8].
By 2020, several international consortia and organizations, including the Pharmacogenomics Knowledge Base (PharmGKB), the Pharmacogenomics Research Network, Clinical Pharmacogenomics Implementation Consortium, European Pharmacogenomics Implementation Consortium, and the Dutch Pharmacogenomics Working Group, have collectively provided a comprehensive evidence base of PGx (encompassing clinical guidelines, drug labels, potentially clinically actionable gene-drug associations, and genotype-phenotype relationships) to help practitioners understand how PGx test results should be used to optimize drug therapies [9]. So far, PharmGKB has collected 168 clinical guideline annotations, 183 pathways, and 810 drug label annotations [10]. In China, the Division of Pharmacogenomics of the Chinese Pharmacological Society was established in 2011, indicating that the research and use of PGx and personalized medicine in China have entered a new era. Since 2014, China’s Food and Drugs Administration and the Health and Family Planning Commission has launched a range of policies around gene sequencing. On 31 July 2015, the Personalized Medical Testing Technology Expert Committee under China’s Health and Family Planning Commission established the “Interim Guidelines of Detection Techniques for Drug Metabolizing Enzymes and Acting Target Genes” and “ the Interim Guidelines of Detection Techniques of Individualized Antineoplastic Therapies”. The committee also put forward that by 2030, China’s investments in precision medicine will reach CNY 60 billion (USD ~8.96 billion), hoping that precision medicine will bring further changes to PGx testing in China through increased funding [11].
Despite growing evidence supporting the feasibility of PGx testing in clinical practice, the use of PGx testing in China remains limited. PGx testing for many drugs is limited to use in laboratories and has not been implemented into routine clinical practice. International research indicated that while most pharmacists remained optimistic about the potential of PGx to advance clinical care, their experience with PGx testing was limited. According to a study in Australia, five pharmacists (23.8%) claimed that they had never heard of one of the following terms: personalized medicine, PGx, or PGx tests [12]. A survey [13] in the U.S. indicated that approximately 34% of the respondents (n = 24) had performed PGx tests in their practices. Another report in the Netherlands [14] showed that only 98 respondents (14.7%) reported ordering or recommending a PGx test in the last six months. In Thailand and Malaysia, only 7.3% and 5.8% of respondents had ordered or recommended PGx testing in the past [15,16]. In China, Guo and colleagues [17] studied respondents’ (including physicians, pharmacists, and researchers) knowledge of, attitudes towards, and barriers to PGx testing. They found that over 50% of pharmacists recognized the importance of PGx testing (to their clinical practice) and most respondents affirmed that PGx should be utilized in clinical practice to enhance patient care. However, there is a lack of research on how personalized care and PGx testing are currently carried out at medical institutions and the role of clinical pharmacists in them in China.
China has a hierarchical, three-tier health care system. Tertiary hospitals provide medical care for patients with acute and complicated diseases, while clinical pharmacy services are also mainly provided in tertiary hospitals [18]. Thus, our study explores current practices of clinical pharmacists at tertiary medical institutions across China. We seek to understand the role of clinical pharmacists in PGx testing and personalized treatment services at these institutions and their self-assessed level of knowledge and capacity related to PGx testing. Our study should provide a reference for implementing PGx testing into routine clinical practice.
2. Materials and Methods
2.1. Questionnaire Development
We developed a questionnaire encompassing questions about clinical pharmacists’ characteristics, personal experience, and self-assessed level of knowledge and capacity related to PGx testing. According to current clinical practice in China and similar research on PGx testing in other countries [19,20,21,22,23,24,25], we developed a preliminary questionnaire and invited four PGx experts to review initial items, provide feedback, and suggest necessary changes. The survey was then piloted among 20 pharmacists not included in the study sample to establish the reliability of the questionnaire. The final survey contained 38 questions and was estimated to take 10–15 min to complete (Appendix A). In this paper, we report findings from 28 questions of the survey focusing on the implementation of PGx tests among clinical pharmacists and competencies related to PGx testing of clinical pharmacists. The remaining 10 questions related to the knowledge and attitude of clinical pharmacists will be presented in another article. Questions were divided into five sections: (1) respondent characteristics; (2) the current practice of PGx testing and related services at the respondent’s practicing hospital; (3) personal experience with PGx testing; (4) sources of information about PGx testing; and (5) self-assessed competencies related to PGx testing. The self-assessed competencies were measured on an integer scale of ten, and the proportion of PGx testing and related services in pharmacists’ daily workload was measured on a percentile scale. If the respondent selected “Volunteer to participate in the study”, any following questions had to be answered. In this paper, we report findings from the survey focusing on the practice experiences with PGx testing and related services and self-assessed competencies towards PGx testing.
2.2. Data collection and Population
The sample was obtained from the Pharmaceutical Affairs Management Professional Committee of the Chinese Hospital Association. A random sample of clinical pharmacists meeting the following criteria in the working group of the clinical pharmacist training platform was invited for the survey: (1) practicing in the capital cities and sub-provincial cities of each province, in the capital cities of the five autonomous regions or the four municipalities directly under the Central Government; and (2) working in tertiary hospitals. The first invitation was sent on October 14, 2021, and the questionnaire was then sent to all clinical pharmacists who indicated interest in participating in the study by an online survey platform named “Wenjuanxing” (www.wjx.cn) (accessed on 26 July 2022) for data collection questionnaire surveys. The study was approved by the Institutional Review Boards at Peking University, Beijing, China (IRB 2021100). Respondents who completed the survey received 10 yuan as compensation for their time.
2.3. Data Analysis
The analysis was based on survey data from questionnaires collected by Wenjuanxing. The self-assessed competencies score was categorized into four levels as described in a previous study [15]: excellent (score > 8 points), good (score 7–8 points), fair (score 5–6 points), and poor (score < 5 points). Descriptive statistics and categorical variables were summarized. Ordinal logistic regression was used to analyze the interaction item of pharmacists’ involvement in PGx testing and related services and their self-assessed capacity to perform PGx testing. Factors associated with the involvement in PGx testing and the self-assessed capacity were analyzed using the multivariate logistic regression analysis. The results of the multivariate analysis were reported as odds ratios (ORs) with 95% confidence intervals (CIs) and p-values. All statistical tests were two-sided, and p < 0.05 was considered statistically significant. All statistical analyses were conducted with STATA version 15.1.
3. Results
3.1. Respondent Characteristics
A total of 1005 clinical pharmacists completed the survey. Of these, 996 (99.1%) had heard of PGx tests while nine (0.9%) had not. 588 (59.0%) had been involved in PGx testing and related services while 408 (41.0%) had not. The following analysis was based on the respondents who had heard about PGx tests (n = 996, 99.1%) and who had been involved in PGx testing and related services (n = 588, 59.0%) before the survey. The characteristics of the studied groups are shown in Table 1 and Figure 1.

Table 1.
Characteristics of study respondents.
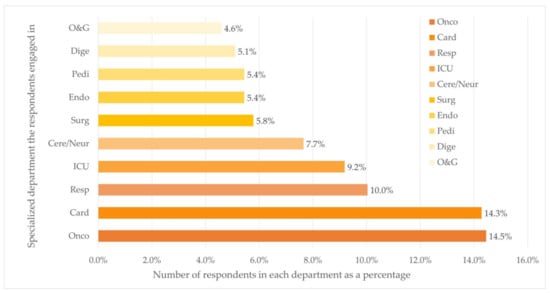
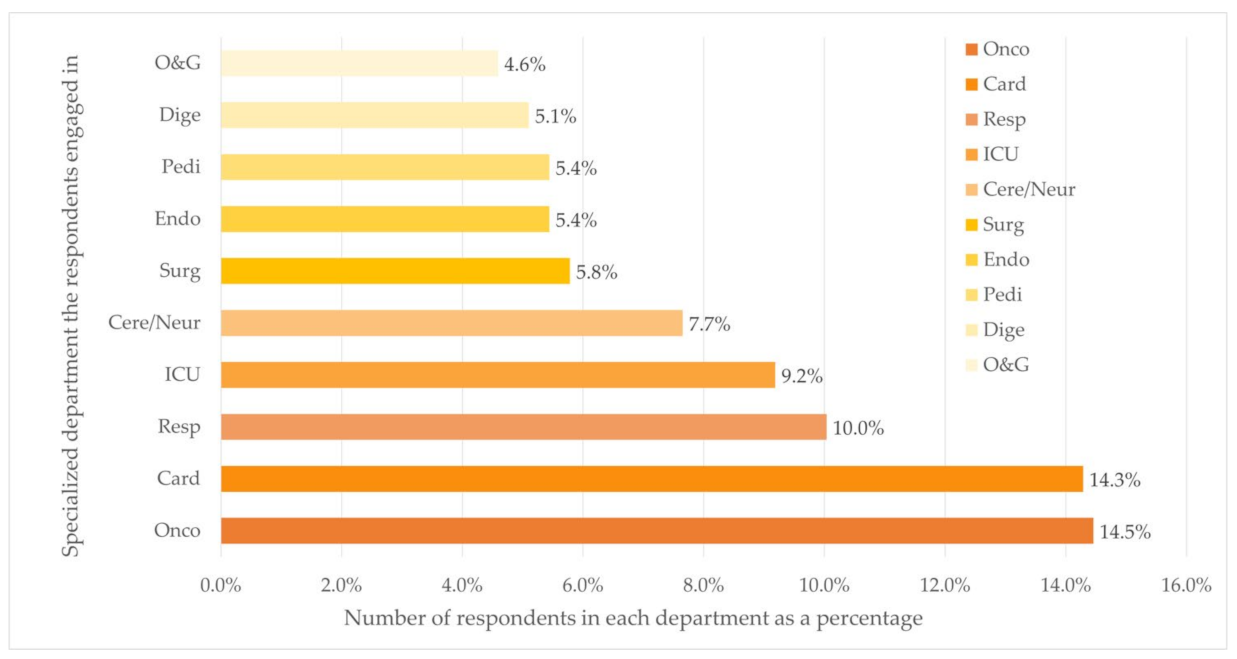
Figure 1.
Distribution of department (Top ten) (n = 588). Department: Oncology (Onco), Cardiovascular (Card), Respiratory (Resp), Cerebrovascular/Neurology (Cere/Neur), Surgery (Surg), Endocrine (Endo), Pediatrics (Pedi), Digestion (Dige), Obstetrics and Gynecology (O&G).
Of the 588 respondents, 27.0% (n = 159) were male and more than half aged less than 35 years (n = 353, 60.0%). The majority (n = 439, 74.7%) had a higher qualification level of master’s degree or above, and 8.0% (n = 47) had studied abroad. Most participants had 5–10 years (n = 227, 38.6%) or >10 years (n = 247, 42.0%) of clinical experience while the most common professional title was the middle-level pharmacist (n = 370, 62.9%). When it comes to the attitude toward new technologies, 479 respondents (81.5%) were neutral and chose “aware of the need to change and very comfortable adopting new technologies and adopt new technologies before the average person but need to see evidence of success before adopting”, while 78 (13.3%) held positive attitudes towards new technologies.
The majority of the respondents worked at university-affiliated hospitals (n = 384, 65.3%). Of all places where respondents practiced medical care, 22.1% (n = 130), 33.7% (n = 198), and 44.2% (n = 260) located in cities with low, middle, and high level of economic status, respectively. Over half of the respondents (n = 327, 55.6%) worked in five departments, namely the departments of Oncology (n = 85, 14.5%), Cardiology (n = 84, 14.3%), Respiratory Diseases (n = 59, 10.0%), ICU (n = 54, 9.2%), and Cerebrovascular Diseases and Neurology (n = 45, 7.7%).
3.2. Pharmacogenomics Tests and Related Services Provided in Hospitals
The availability of PGx testing and related services in the hospitals of the 588 respondents who had been involved in PGx testing and related services and 408 respondents who had not been involved in PGx testing and related services is shown in Table 2, respectively.

Table 2.
The implementation of pharmacogenomics tests in hospitals (n = 996).
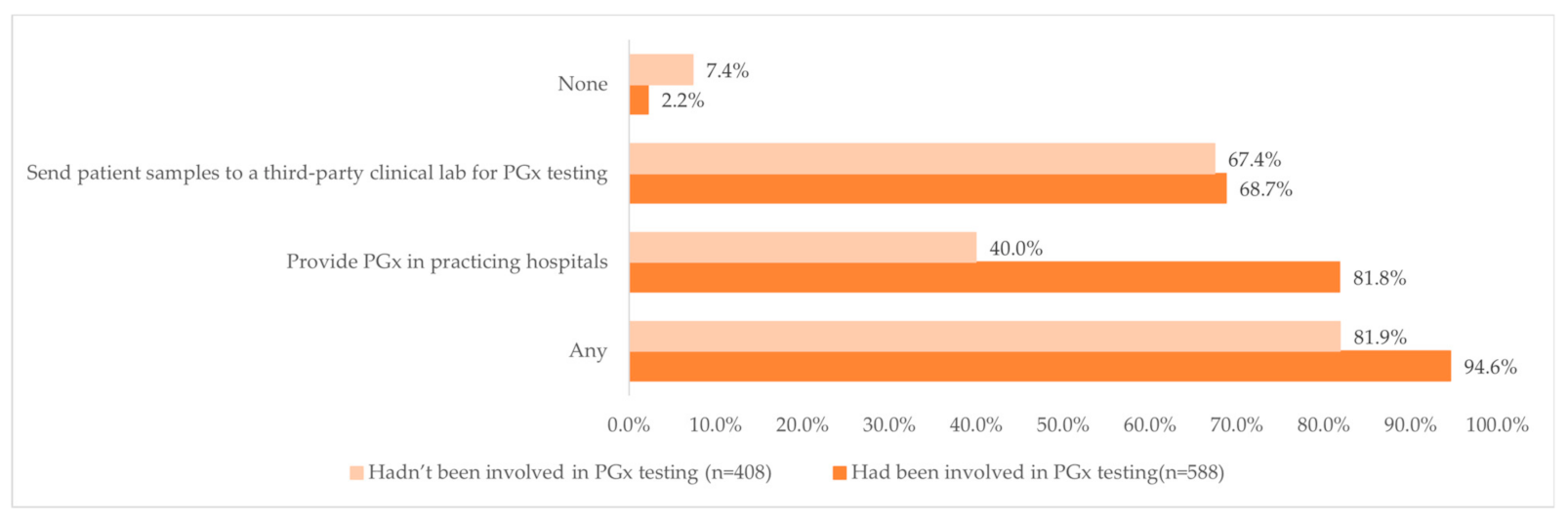
Overall, 94.6% of those who had been involved in PGx testing and 81.9% of those who had not been involved in PGx testing indicated that the hospitals where they worked in either provided PGx tests or sent patient samples to a third-party clinical lab (Figure 2). Interestingly, almost four-fifths of the respondents (81.8%, n = 481) who had been involved in PGx testing reported that the hospitals where they worked in provided PGx test, while less than half of the respondents (40.0%, n = 163) who had not been involved in PGx testing reported that the hospitals where they practiced at provided PGx tests.
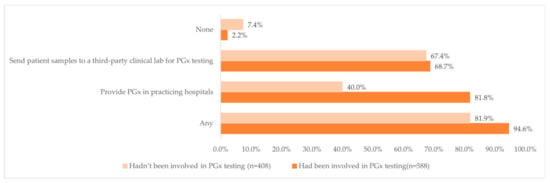
Figure 2.
Distribution of the availability of PGx testing at the pharmacists’ work hospital and the clinical pharmacists’ involvement in PGx testing.
Regarding the reason for providing PGx testing in their working hospitals, most respondents who had been involved in PGx testing identified that the primary reasons for PGx testing were “For clinical diagnosis, treatment, and research purpose” (45.1%, n = 265) and “Just for clinical diagnosis and treatment purpose” (35.7%, n = 210). Meanwhile, among the hospitals that provided PGx tests, the tests were provided mostly in the Pharmacy Department (either alone or with other departments; 66.9%, n = 322). Furthermore, 374 (63.6%) of respondents who had been involved in PGx testing suggested that PGx testing was provided by clinical pharmacists as an integral part of personalized pharmacy services compared to only 17.2% of respondents who had not been involved in PGx testing indicating that PGx testing was provided by a clinical pharmacist.
The multivariate analysis for clinical pharmacists’ experience with PGx testing indicated that the clinical pharmacists with the following variables were more likely to involve in PGx testing and related services: male (OR: 1.14; 95%CI: 1.02–1.28; p = 0.02), with higher degrees (master’s degree vs. doctoral degree, OR: 1.38 vs. 1.42; 95%CI: 1.20–1.57 vs. 1.17–1.73; p = 0.00 vs. 0.00), with a higher professional title (middle level pharmacist vs. associate chief or chief clinical pharmacist, OR: 1.33 vs. 1.37; 95%CI: 1.06–1.67 vs. 1.04–1.79; p = 0.01 vs. 0.02), practicing province with higher GDP rank (middle vs. high, OR: 1.16 vs. 1.20; 95%CI: 1.00–1.34 vs. 1.04–1.38; p = 0.04 vs. 0.01), and holds a positive attitude towards new technologies (OR: 0.02; 95%CI:1.04–1.82; p = 0.02).
3.3. Clinical Pharmacists’ Experience with Pharmacogenomics Tests
The top three main content of work for clinical pharmacists who are involved in PGx tests were “Explained results to the patient” (30.9%), “Advised physicians on drug selection, dosage, and monitoring based on the results of PGx testing” (29.7%), and “Explained the results to the doctor” (29.1%).
On average, PGx testing and related services accounted for 16.5% of respondents’ daily workload, with 74.8% (n = 440) of respondents indicating that these services accounted for only 0–20% of the daily workload, while a minority of participants (4.4%) indicated that these services accounted for over 60% of the daily workload. More than half of respondents who had any personal experience with PGx testing and related services (52.4%, n = 308) reported having been consulted by patients about PGx testing results, and 231 (39.3%) and 49 (8.3%) said that no patients had consulted them or that they did not remember.
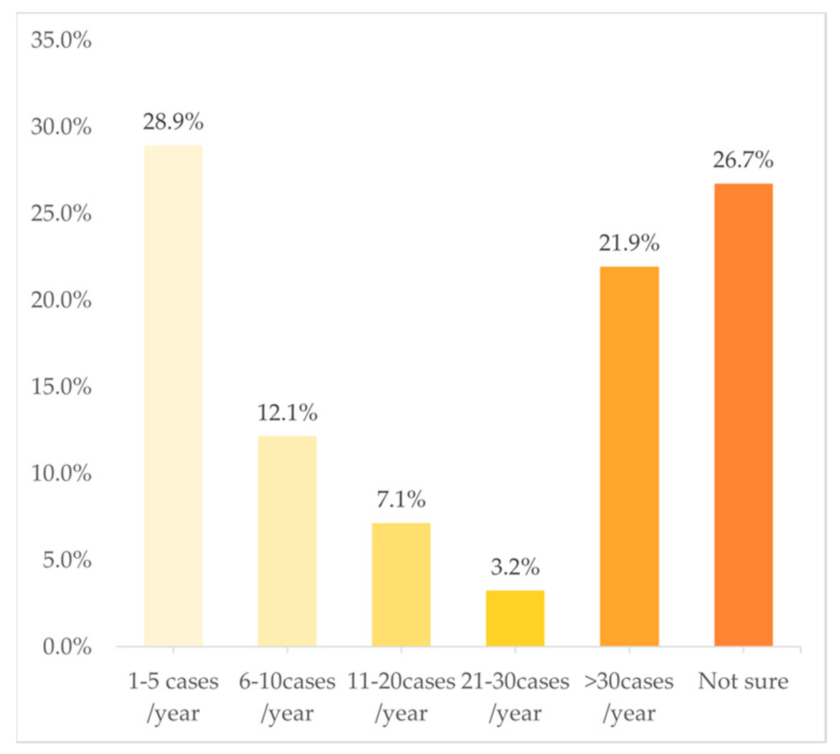
In terms of the frequency of cases in PGx testing and related services, Figure 3 shows a U-shaped trend, with 28.9% of the clinical pharmacists choosing “1–5 cases/year” and 21.9% choosing “>30 cases/year”. However, just 12.1%, 7.1%, and 3.2% chose “6–10 cases/year”, “11–20 cases/year”, and “21–30 cases/year”, respectively. The multivariate analysis indicated that the following variables were independently associated with a higher frequency of involvement in PGx testing and related services: doctoral degree (OR: 1.17; 95%CI: 1.03–1.34; p = 0.02) and practicing in a province with a middle GDP rank (OR: 1.12; 95%CI: 1.02–1.23; p = 0.02).
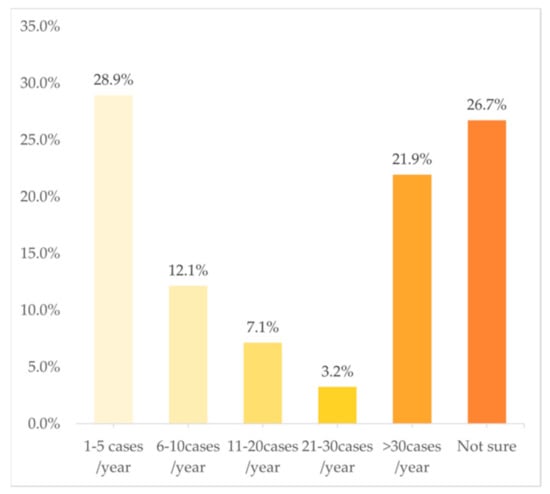
Figure 3.
Frequency of work involved in PGx tests (n = 588).
Among the top ten department with the largest number of clinical pharmacists who had been involved in PGx testing and related services, more than 40% provided PGx testing and related services less than once a month on average (<10 cases/year), except for the pharmacists of the Department of Cardiology and Obstetrics and Gynecology. However, respondents who claimed to provide PGx testing and related services more than 30 times per year were mostly from the Department of Obstetrics and Gynecology (33.3%, n = 9) (Table S1).
The following section asked which drugs the respondents supplied PGx testing and related services for the most frequently; respondents can select multiple drugs based on their experiences. As for providing individual PGx testing, the top five individual PGx tests in our study (Table 3) were for clopidogrel (CYP2C19) (57.7%, n = 339), warfarin (CYP2C9, VKORC1, CYP4F2) (49.7%, n = 292), voriconazole (CYP2C19) (24.3%, n = 143), carbamazepine and phenytoin (HLA-B) (21.3%, n = 125), and tacrolimus (CYP3A5) (19.2%, n = 113). After classifying the diseases treated by these drugs, respondents suggested that PGx testing was mostly provided for cardiovascular diseases (n = 433, 73.6%). Clinical pharmacists in the Department of Cardiology (n = 81) were mostly involved in PGx tests for cardiovascular diseases, followed by Respiratory (Resp) (n = 48), and Oncology (Onco) (n = 41) (Figure S1).

Table 3.
Experience in individual PGx tests and multiple-gene PGx panel tests.
As for providing multiple-gene PGx panel testing, the top three PGx panels (Table 3) were for commonly used clinical drug testing kits (26.9%, n = 158), cardiovascular disease common drugs test kit (25.5%, n = 150), and cancer drug gene testing kit (23.5%, n = 138). After classifying the diseases treated by these panels, participants were most involved in those for cardiovascular diseases (n = 273, 46.4%) too. Few respondents (5.4%, n = 32) had never been involved in delivering multiple-gene PGx panel testing. Clinical pharmacists in the Department of Cardiology (Card) (n = 68) were mostly involved in PGx tests for cardiovascular diseases, followed by Cerebrovascular Diseases and Neurology (Cere/Neur) (n = 32), and Respiratory (Resp) (n = 29) (Figure S2).
3.4. Resources of Information about PGx Testing
When respondents were interpreting test results or assisting doctors to make clinical treatment decisions according to test results, the majority would “Consult relevant professional guidelines/literature reports” (90.1%, n = 530), followed by “Drug labels” (65.5%, n = 385), and “Consult medical professional software or APP” (58.8%, n = 346).
3.5. Self-Assessment of the Competencies Related to Pharmacogenomics Testing
When it comes to self-assessing PGx testing-related abilities, among the total group (n = 996), more than 60% of the pharmacists considered themselves to have poor or fair PGx-related abilities (score 0–6; 10 being highest confidence). Clinical pharmacists were most confident in identifying which PGx tests were available at their practicing healthcare facility (mean 5.12 ± 3.09), while they were the least confident in suggesting which drugs need PGx testing (mean 4.79 ± 2.61). Only a small proportion of the pharmacists believed that they were fully equipped (scored > 8) in terms of knowledge related to PGx testing and related services, whether it was to identify PGx tests at their practicing facility (17.5%, n = 174), to interpret PGx testing results to physicians and/or patients (11.1%, n = 111), to advise physicians on treatment decisions (drug selection, dosage, and monitoring) according to PGx test results (10.7%, n = 107), or to recommend PGx tests to physicians and/or patients (12.3%, n = 122). Only 84 pharmacists (8.4%) claimed fully equipped to evaluate which drugs require PGx testing. Ordinal logistic regression analysis indicates strong positive associations between the pharmacist‘s 5-item self-assessed level of competency related to PGx testing and whether they had been involved in PGx testing (Table S2). Moreover, the analysis of experiences (1–10 cases/year, 10–30 cases/year, etc.) and competency shows that respondents who underwent PGx testing more frequently (>30 cases/year) rated their self-competence higher (ORs > 2.80 for each self-competency assessment item) (Table S3).
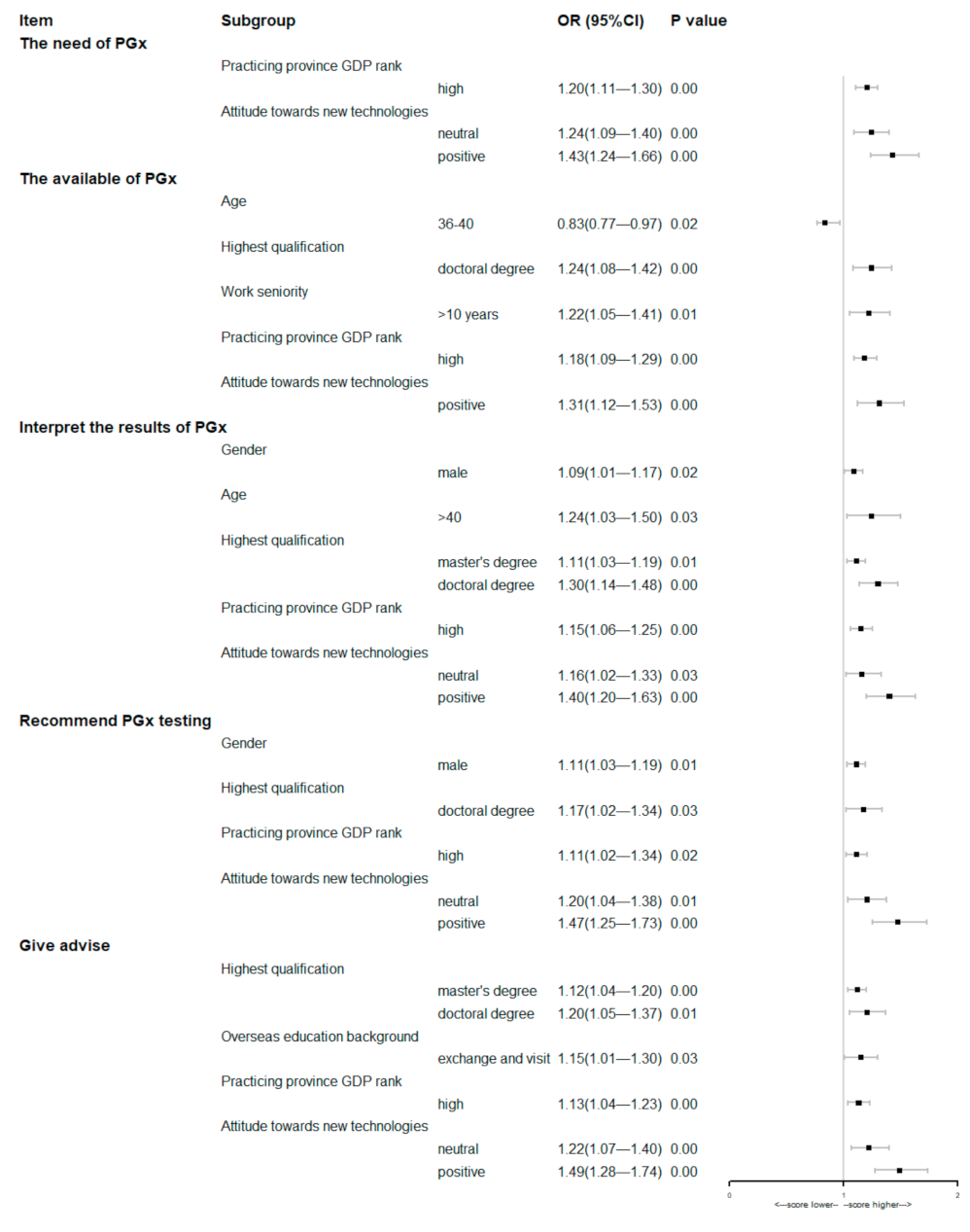
The results of the multivariate analysis of the baseline characteristics of respondents involving these five items were showed in the Figure 4. The multivariate analysis suggested that pharmacists practicing in provinces with a high GDP rank or who hold positive attitude towards new technologies were more likely to score himself/herself higher on every item of competency.
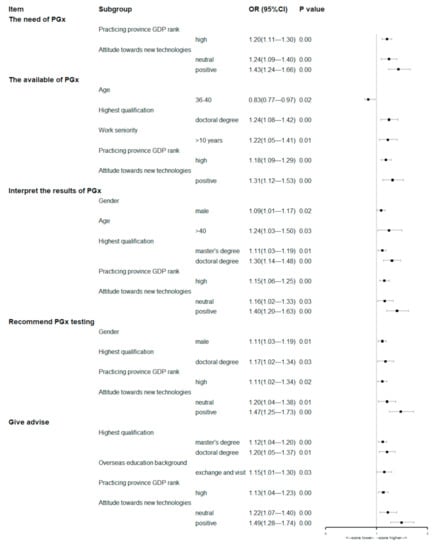
Figure 4.
Forest plots of multivariate analyses of respondents’ baseline characteristics associated with the self-assessment of the competencies related to PGx testing (the first option listed in Table 1 was used as the control group, except for gender).
4. Discussion
Our study aims to understand the involvement of clinical pharmacists in PGx testing and related services at tertiary hospitals in China and their self-assessed capacity to deliver such services. The questionnaire was designed based on past literature and current PGx testing in China. We found that most tertiary medical institutions in China are currently providing PGx testing services, and more than half of clinical pharmacists had been involved in PGx testing and related services. However, regardless of previous experience with PGx testing, pharmacists had little confidence in their knowledge level of and capacity to provide PGx-related services.
The poll was completed by 1005 clinical pharmacists. Of these, 996 (99.1%) had heard of PGx testing, with 588 (59.0%) having been involved in PGx testing and related services, while 408 (41.0%) had not been. Overall, 94.6% (556/558) of those who had been involved in PGx testing and 81.9% (334/408) of those who had not, indicated that the hospitals where they worked in either provided PGx tests or sent patient samples to a third-party clinical lab. Among those who had been involved in PGx service, 28.9% had been involved in 1–5 cases/year, while 21.9% had been involved in more than >30 cases/year. Cardiovascular diseases (n = 433, 73.6%), rheumatic immune diseases (n = 172, 29.3%), infectious diseases (n = 151, 25.7%), and oncology therapy (n = 141, 24.0%) are the most involved categories for PGx testing. For approaches to attain information to interpret the test results or assist doctors to make clinical decisions, the vast majority chose “Consult relevant professional guidelines/literature reports” (90.1%, n = 530), followed by “Drug labels” (65.5%, n = 385) and “Consult medical professional software or APP” (58.8%, n = 346). About 60% (344/588) of the pharmacists considered themselves to have poor or fair capacity (i.e., scored 0–6 on the self-assessment) to provide PGx services.
For the accessibility of PGx testing in hospitals, interestingly, respondents who had been involved in PGx service were significantly more likely to work in hospitals that provided PGx tests (81.9% vs. 40.0%). Chinese clinical pharmacists are more likely to participate in PGx testing when their practicing hospitals provide PGx testing. This is different to the model in the United States, where clinical pharmacists are more likely to actively participate in services such as interpreting the results of the test and adjusting the dose of drugs after sending the PGx test to a third-party testing platform and are less influenced by whether PGx tests are available at the hospitals where they work. Our findings are higher than those of a Thailand-based investigation, where 30.9% of the respondents reported that PGx testing was available at their hospital [15]. This might reflect that PGx testing is considerably more accessible at medical institutions in China. As mentioned above, relevant measures were taken to promote access to and use of PGx in China, including the establishment of Division of Pharmacogenomics in 2011, the launch of gene-sequencing-related policies in 2014, and the formulation of the “Interim Guidelines of Detection Techniques for Drug Metabolizing Enzymes and Acting Target Genes” and “the Interim Guidelines of Detection Techniques of Individualized Antineoplastic Therapies” in 2015. Healthcare professionals thus have favorable conditions to perform relevant PGx tests as needed. However, we can see that in 40% of the cases, although the affiliated hospitals offered PGx testing, the respondents were not involved in it. The reason for this may be the limitation of their knowledge and the fact that PGx testing-related service is handled by non-clinical pharmacist healthcare providers in the institution, such as physicians and lab staff.
Our study participants had a higher level of engagement (59.0% of our respondents had been involved in PGx services) compared to other countries (Australia, US, Thailand, Malaysia, Kuwait, and European) where only 5.8% to 34.3% [12,13,14,15,16,24,26] of respondents had been involved in PGx services. The reason for this could be that our respondents are clinical pharmacists from tertiary medical institutions, whereas the data from other countries could be healthcare professionals, community pharmacists, outpatient pharmacists, or even preceptors in school of pharmacy. Clinical pharmacists are a subgroup of hospital pharmacists who have a higher level of training in hospitals and have more opportunities to provide clinical pharmaceutical care than general pharmacists who work in pharmacies, general hospitals, or even schools of pharmacy. Moreover, compared to the frequency of practicing PGx in Japan (25.7%, ≥1/month), 32.3% of our respondents provided PGx services at a rate of more than 10 cases per year. We can reasonably infer that at Chinese medical institutions, PGx testing and relative services have been gradually integrated into routine clinical practice and thus the practice of clinical pharmacies and pharmacists over the years. The study also found that pharmacists in Department of Obstetrics and Gynecology performed PGx testing most frequently, likely because pregnancy is a special physiological condition that requires more individual selection of drugs and doses in order to exclude drugs that may cause harm to the mother or child.
Clinical pharmacists most frequently provided PGx testing services for cardiovascular diseases, which is consistent with the priority given to cardiovascular diseases by current international precision medicine and pharmacogenomics. Notably, our study found that the clinical pharmacists who specialize in one department (for example, the oncology department) do PGx testing for other conditions (Respiratory, CVD). In China, specialist clinical pharmacists are not only trained regarding medication problems of their particular specialty but also drug-related problems of the most commonly used drugs, so from that perspective, they are also more generalists.
Furthermore, the top five individual PGx tests in our study were similar to the results of an international study [27] but different from the results of a study in Japan [28], which suggested that the most frequently used PGx tests were those for irinotecan (96.8%), tacrolimus (86.3%), warfarin (83.9%), azathioprine (76.3%), clopidogrel (74.9%), and carbamazepine (72.4%). The possible reason for this is that the frequency of performing various PGx tests in different countries differed by the frequency of genetic mutations in the local population and reimbursement policies by health insurance schemes. For example, the most compelling PGx evidence comes from non-Caucasian populations from South-East Asia, HLA-related testing is less frequently performed in North America where the Asian population is a minority. Meanwhile, the PGx test for HLA-B*15: 02 has been included in the Universal Health Coverage (UHC) scheme in Thailand and a higher utilization of PGx testing for HLA-B*15: 02 was observed in these hospitals. In China, since 15 June 2019, the comprehensive reform of medical consumption linkage and the new policy concerning insurance coverage of pathological tests were implemented in Beijing. These policy changes may incentivize other provinces to incorporate more PGx tests into medical insurance schemes and thus promote the uptake of PGx tests.
Turning now to approaches to obtaining information related to PGx testing, our respondents most frequently turned to relevant professional guidelines/literature reports (90.1%), drug labels (65.5%), and professional medical software or APPs (58.8%) for information concerning PGx tests and the interpretation of results, which is consistent with the situation in other countries [13,14,26,28], in general, local guidelines, academic journals, and package inserts are recognized as the most useful sources for learning about PGx testing. China has made considerable endeavors to develop local guidelines on PGx testing and related services to guide future practice PGx tests and clinical decisions that could benefit from PGx testing. Specifically, in the 2016 “Precision Medicine Research” program in China, the “Pharmacogenomics and The Comprehensive Evaluation System for Accurate Drug Use of Chinese People” project led by Professor Cui Yi-min at Peking University First Hospital contributed to establishing the evidence base related to PGx testing in China [29].
Developing local guidelines and promoting the capacity of health professionals to provide PGx services are crucial for enhancing the level of pharmacists’ knowledge about PGx testing and their willingness to provide PGx testing and related services. Gauging from the 5-item self-assessed level of competency in this study, most clinical pharmacists were not confident in their capacity to provide PGx services while clinical pharmacists who had been involved PGx testing generally scored themselves higher on the assessed competencies. This is consistent with previous findings in a study from the United States that preceptors who had used PGx in their practice self-assessed their level of PGx knowledge significantly higher than non-PGx users’ self-assessed knowledge (respective mean values, 2.7 ± 0.8 vs. 1.6 ± 0.7, p < 0.001) [13]. Furthermore, in an interaction item analysis of experience and competency ratings for PGx testing, respondents providing PGx testing services more than 30 times per year reported greater confidence in their abilities, so there is reason to believe that clinical pharmacists in China have confidence in providing PGx testing and related services if they are involved enough with the process.
Pharmacist capacity to provide PGx services also varied across countries and by evaluation proxies. For example, our study showed that only 8.43% respondents felt fully competent to identify which drugs needed PGx tests (score > 8), while this proportion ranged from 42.9% in Saudi Arabia [30], 23.5% in Kuwait [24], and 30.8% in Japan [28] to more than half (58.6%) in Europe [26]. Our study respondents were the least confident in the capacity to provide PGx services (score > 8, 8.4–17.5%) across these studies, which can be attributable to several aspects. Firstly, there are more than 200 guidelines on PGx testing in the world, and they have marked the recommended levels of whether some drugs need to be tested for PGx. In addition, the Clinical Pharmacogenomics Implementation Consortium (CPIC) [31] has developed 26 guidelines, which involve 139 genes-drugs information pairs, including 65 gene–drugs that are PGx high-risk. Meanwhile, the FDA has provided specific information regarding therapeutic management for 81 gene–drug pairs, for which there is sufficient supportive scientific evidence [32]. However, very few guidelines related to PGx testing exist in China. Most of these guidelines focus on testing techniques but lack information on whether to recommend PGx testing for a certain drug. There are only 12 drugs in the Chinese package inserts, with limited information on drug–gene relationship and recommendations on PGx testing. The insufficient information provided for the pharmacogenomics aspect of drugs and patient health in China is one of the reasons why pharmacists are not confident in their capacity to provide PGx testing and related services. Secondly, there is a lack of professional training and courses on PGx testing and related services for pharmacy students and in the continuing education of pharmacists. A study [24] showed that the lack of education or training was the biggest obstacle to implementing PGx testing in clinical practice. Therefore, the addition of PGx information to undergraduate courses in schools of pharmacy will improve the knowledge of PGx among pharmacy students. Regular training and seminars for clinical pharmacists at medical institutions will also facilitate the effective use and service provision of PGx testing by clinical pharmacists and help increase pharmacists’ self-efficacy. Last but not least, we defined pharmacists’ confidence as scoring greater than 8 on the self-assessed competencies, while other studies often defined it as a binary or categorical variable. Hence, the lack of confidence of clinical pharmacists in our study as compared with results from other studies may partly stem from methodological factors. However, if we lower the threshold score 8 to 6, still only 25.5% of pharmacists were deemed confident in their capacity to determine which drugs need PGx testing.
Our study has some limitations. First, our respondents were all from tertiary medical institutions in China, therefore, the basic knowledge and academic background of clinical pharmacists in this study are overall higher than average pharmacists. The clinical pharmacists in this study were more likely to participate in PGx testing and related services than pharmacists practicing at medical institutions of other tiers (i.e., primary care facilities and secondary hospitals) in China. Thus, our results do not reflect the involvement of clinical pharmacists in PGx testing at medical institutions of other tiers or the overall Chinese health system. Second, clinical pharmacists who had heard of or been involved in PGx testing and related services before the survey were more likely to participate in our study; thus, the level of engagement of clinical pharmacists in PGx testing observed in our study may be higher than the actual level. Third, the number of clinical pharmacists included in our sample is relatively small, which is likely related to the current low overall number of clinical pharmacists in China and the need to develop clinical pharmacist talent teams. According to our search of the literature on the distribution of clinical pharmacists by department in China, we were unable to find any relevant data, demonstrating that there is still little research in this field in China. As a result, we are unsure whether the distribution of clinical pharmacists in different departments in this study sample is representative, so we will refine this survey and conduct more in-depth studies in this question in the future. Fourth, our study data stemmed from questionnaires and not from field studies, subjects may provide more favorable answers to fit a more socially accepted view [33,34], leading to over-estimations in our results. Finally, the types of individuals- and multiple-gene PGx panel tests involved in this study questionnaire were not exhaustive, and there may be cases where drugs that are frequently tested for PGx were not listed in the questionnaire.
5. Conclusions
This study found that many tertiary medical institutions in China are currently providing PGx testing, and more than half of the clinical pharmacist respondents have engaged in PGx testing and related services. However, many were not confident in their knowledge and capacity to provide PGx tests and PGx-related services. Our study results highlight the need to design targeted multifaceted interventions to facilitate the uptake of PGx testing in China. The next step should strive to enhance the confidence of pharmacists to carry out PGx testing and related services.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm12081267/s1, Table S1: Distribution of frequency of PGx tests and department (top ten); Table S2: Self-assessment of the competencies related to PGx testing involved personal practice; Table S3: Self-assessment of the competencies related to PGx testing involved the personal practice of frequency of PGx tests (n = 588); Figure S1: Distribution of experience in individual PGx tests (multiple choices) and department (top ten); Figure S2: Distribution of experience in multiple-gene PGx panel tests (multiple choices) and department (top ten).
Author Contributions
Conceptualization, X.N. and C.Y.L.; methodology, X.N. and C.Y.L.; software, X.H. and T.J.; validation, X.H., X.N. and C.Y.L.; formal analysis, X.H.; investigation, T.J., X.Z., C.W., Y.Z. and J.C.; resources, X.N., X.G. and L.S.; data curation, X.H.; writing—original draft preparation, X.H.; writing—review and editing, X.H., X.N. and C.Y.L.; visualization, X.H. and X.N.; supervision, X.N., C.Y.L., X.G. and L.S.; project administration, Y.Z., X.N., X.G. and L.S.; funding acquisition, X.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China to Xiaoyan Nie (grant no.81803497).
Institutional Review Board Statement
The study was approved by the Institutional Review Boards at Peking University, Beijing, China (IRB 2021100).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to all pharmacists who responded to this survey; the Pharmaceutical Affairs Management Professional Committee of the Chinese Hospital Association for supporting the collection of samples and Jiancun Zhen for data resources; and Sicong Li, Yuxuan Zhao, Yuchun Cai, and CongXiao Han for data collection. Special thanks to Huang Qianyu Li for language correction.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Questionnaire for Clinical applications and Attitudes Towards Pharmacogenomics Tests.
Dear clinical pharmacist:
We are the Pharmacogenomics Research Team of Peking University. We sincerely hope to understand your views on the clinical application of pharmacogenomic testing and would like to invite you to participate in this questionnaire survey. The survey results do not contain any of your identity information, and the survey results will be kept strictly confidential and used only for academic research and not for any commercial purpose.
Please answer according to your actual situation and attitude. If you have any questions about the requirements of the questionnaire, please feel free to send to PKUPharmageno@163.com. Thank you for your support and cooperation.
(It will take you about 10 min to complete this questionnaire.)
0. Do you agree to participate in this survey.
○YES
○NO (end)
Part 1: Basic information
1. What is your gender? ○Male ○Female
2. What is your age? ○≤25 ○26–30 ○31–35 ○36–40 ○41–45 ○46–50 ○51–55 ○56–60 ○61–65 ○>65
3. What is your degree and major at each level?
○ Lower than Bachelor degree ○Bachelor degree ○Master’s degree ○Doctoral degree
4. Have you ever studied abroad?
○ No (please skip to question 6) ○ Yes (for a degree) ○ Yes (for an exchange study)
5. Where did you study abroad?
○UK ○UA ○Japan ○Australia ○Canada ○Others: _________________ *
6. Which province/autonomous region/municipality do you practice in?
○Gansu (GS) ○Hainan (HaiN) ○Heilongjiang (HLJ) ○Jilin (JL)
○ Neimenggu (NMG) ○ Ningxia (NX) ○ Qinghai (QH) ○ Shanxi (S1X)
○Tianjin (TJ) ○Xinjiang (XJ) ○Xizang (XZ) ○Anhui (AH) ○Beijing (BJ)
○Chongqing (CQ) ○Guangxi (GX) ○Guizhou (GZ) ○Hebei (HeB)○Jiangxi (JX)
○Liaoning (LN) ○Shaanxi (S3X) ○Yunan (YN) ○Fujian (FJ) ○Guangdong (GD)
○Henan (HeN) ○Hubei (HuB) ○Hunan (HuN) ○Jiangsu (JS) ○Shandong (SD)
○Shanghai (SH) ○Sichuan (SC) ○Zhejiang (ZJ)
7. The name of your medical institution is [fill in the blank] _________________________
8. How many years have you worked? ○Still in training ○<1 year ○1 year–5 years
○5 year–10 years ○10 year–30 years ○>30 year
9. The level of the hospital you work for ○Tertiary hospitals ○Secondary hospital
○First hospital ○ I don’t know
10. The relationship between your hospital and medical college is
○ Affiliated Hospital ○ Teaching hospital
○ Neither affiliated nor teaching hospital○ Other_________________
11. What is your technical title?
○Assistant pharmacist ○Pharmacist ○Pharmacist-in-charge
○Deputy chief pharmacist ○Chief pharmacist ○Others: _________________
12. Are you a clinical pharmacist? ○ YES ○ NO
13. Your department is
○Cardiovascular Dep ○Cerebrovascular and Neurology Dep ○Anti-infectives
○Oncology Dep ○Psychiatry Dep ○Digestion Dept ○Respiratory Dept
○Hematology Dept ○Nephrology Dept ○Endocrine Dept ○Rheumatology Dept
○Dermatology Dept ○ICU ○Emergency ○Obstetrics and Gynecology Dept
○Pediatrics Dept ○Pain ○Others *
14. For new surgical approaches, new tests, and new drugs (Collectively referred to as “new technologies”) that emerge in clinical practice, which of the following description suits you best:
○Want to be the first person to try an innovation.
○Aware of the need to change and very comfortable adopting new technologies
○Adopt new technologies before the average person, but need to see evidence of success before adopting.
○Skeptical of change, only adopt an innovation after it has been tried by the majority
Part two: practical experience of drug-gene testing
Genetic testing is a kind of medical test that can predict, diagnose a disease or evaluate drug response. It has the following forms:
1. Diagnostic test: a test designed to diagnose or predict disease risk. For example, genetic testing for Marfan’s syndrome or Huntington’s disease.
2. Disease susceptibility test: a test with the aim of predicting the risk of future disease by detecting whether there exist genetic variations associated with the disease. For example: detection of AGT, ATR, apoE, CYP11B2, and LPL genes associated with an increased risk of coronary heart disease
3. Pharmacogenomic (PGx) testing: a test with the aim of detecting drug metabolism or target genes, so as to find out the causes of adverse drug reactions or poor drug efficacy and guide the selection of therapeutic drugs and their dosages. For example, CYP2C9 and VKORC1 genotypes can be detected to guide the application of warfarin, CYP2C19 genotypes can be detected to guide the application of clopidogrel, and CYP2D6, ADRB1, GRK4 genotypes can be detected to guide the application of metoprolol.
15. Have you ever heard the term ‘pharmacogenomic testing’ before you took this survey? ○YES ○NO
16. Does your medical institution provide PGx testing? ○YES ○NO ○I don’t know.
17. What is the purpose of providing PGx testing at your medical institution? [multiple choice] *
○ For clinical diagnosis and treatment items ○ For research items ○Others ○I don’t know
18. The department that offers PGx testing in your medical institution is [multiple choice] * ○Pharmacy ○Clinical laboratory ○Pathology department
○Others: _________________ ○I don’t know
19. Does your medical institution send patient samples to an out-of-hospital facility for PGx testing? ○YES ○NO ○I don’t know.
20. Does your medical institution have a dedicated person to provide individualized pharmaceutical care based on PGx testing results.
○YES, clinical pharmacists. ○YES, others. ○NO ○I don’t know.
21. Have you ever been involved in any PGx testing-related services? [multiple choice] *
○Yes, lab work ○Yes, I have explained the results to the patient.
○Yes, I have explained the results to the doctor.
○Yes, I have recommended PGx testing to patients
○Yes, I have recommended PGx testing to doctors
○Yes, I have advised physicians on drug selection, dosage and monitoring based on the results of PGx testing ○None (please skip to question 29)
22. What is the proportion of pharmacogenomic testing-related services in your daily work (including pharmaceutical outpatient service, drug consultation, working hours inwards, pharmacies, etc.) _______________ [Enter the number from 0 to 100]
23. Have any patients come to you for consultation with the results of PGx tests tested?
○YES ○NO ○Uncertain
24. On average, how often do you participate in PGx testing related services per year?
○1–5 cases/year ○6–10 cases/year ○11–20 cases/year ○21–30 cases/year
○>30 cases/year ○Uncertain
25. Which individual PGx tests have you participated in in your clinical practice?
○Antihypertensive drugs—ACE I/D polymorphism test; ADRB1 polymorphism was detected ○Losartan—CYP2C9*3 polymorphism detection
○Nitroglycerin—ALDH2*2 polymorphism detection ○Voriconazole—CYP2C19*2 and *3 polymorphism detection
○Warfarin-CYP2C9*3 polymorphism; Vkorc1-1639 G > A polymorphism was detected. Detection of CYP4F2*3 polymorphism
○Coumarin anticoagulants (acetamino-coumarin, phenylpropylcoumarin)—CYP4F2*3 polymorphism Detection
○Clopidogrel—CYP2C19*2 and *3 polymorphism detection
○Simvastatin, cerivastatin—SLCO1B1 521T>C polymorphism detection
○Pravastatin-APOE polymorphism ○Celecoxib—CYP2C9*3 polymorphism detection
○Tacrolimus—CYP3A5*3 polymorphism detection
○Chloroquine-G6PD gene polymorphism detection ○Dapsone—G6PD gene polymorphism was detected ○Irinotecan—UGT1A1 polymorphism detection
○Isoniazid—Slow NAT1/NAT2 genotype detection ○Polymorphisms of peginterferon α-2a, peginterferon α-2b and ribavirin—IFNL3 were detected
○Abacavir—HLA-B allele test ○Labliase—G6PD gene polymorphism detection
○Capecitabine—DPYD*2A allele test ○Tegafur—DPYD*2A allele test
○5-FU—Mismatch repair protein deficiency (dMMR) detection; Microsatellite instability (MSI) detection; DPYD*2A allele detection
○Allopurinol—HLA-B allele test ○Platinum—ERCC1 mRNA expression was detected. TPMT polymorphism detection
○Anthracycline—TOP2A gene abnormality (gene amplification or gene deletion) test
○Tamoxifen—CYP2D6*10 polymorphism detection
○Trastuzumab—HER2 gene test ○Gemcitabine—RRM1 mRNA expression was detected
○Antipsychotics—ANKK1 RS1800497 polymorphism Detection
○Amitriptyline—CYP2C19*2 and *3 polymorphism detection; Detection of CYP2D6*10 polymorphism
○Carbamazepine, phenytoin—HLA-B allele detection
○Others
26. Which multiple-gene PGx panel tests have you participated in in your clinical practice [multiple choice] *
○Commonly used clinical drug testing kits
○Commonly used clinical medicines for children
○Cardiovascular disease common drugs test kit
○Anti-arrhythmic drug test kit ○Antithrombotic drug test kit
○Antihypertensive drug test kit ○Hypoglycemic drug test kit
○Antihyperlipidemic drugs test kit ○Anti-gout drug test kit
○Anti-hyperthyroidism drug test kit ○Anti-infective drug test kit
○Cancer drug gene testing kit ○Immunosuppressant test kit
○Rheumatic diseases common medicine test kit ○Antiepileptic drug test kit
○Anxiety and depression drug test kit ○Schizophrenia drug test kit
27. When you interpret PGx test results or assist doctors to make clinical treatment decisions according to the results, what kind of resources do you usually use to obtain information about pharmacogenomics testing? [multiple choice] *
○Drug labels ○Consult relevant professional guidelines/literature reports
○Consult medical professional software or APP(medication assistant/doctor station, etc.)
○Enquire PharmGKB and other genomics knowledge base
○Promotional materials from genetic testing companies
○Popular search engines such as Baidu ○Consult your supervisor
○Consult experienced doctors ○Consult the person who provides the PGx test report
28. Your self-assessment of the competence related to PGx testing is: [Enter the number from 0–10]
(1) Evaluate which drugs need PGx testing__________
(2) Determine which PGx tests are available at your health care facility__________
(3) Interpret the results of PGx testing to the patient/physician__________
(4) Recommend PGx testing to a doctor or patient__________
(5) Advise physicians on drug selection, dosage and monitoring__________
Part three: Views and attitudes towards PGx testing
29. How much do you know about PGx testing
○Poor ○Fair ○Average ○Good ○Excellent
30. To what extent do you know about the following pharmacogenomic-related guides, monographs, and databases
(1) Clinical Pharmacogenomic Implementation Consortium (CPIC) guidelines
○Poor ○Fair ○Average ○Good ○Excellent
(2) Pharmacogenomics knowledge base (Pharm GKB)
○Poor ○Fair ○Average ○Good ○Excellent
(3)Clinical Genomic Resources (ClinGen)
○Poor ○Fair ○Average ○Good ○Excellent
(4)Resources such as domestic guidelines or expert consensus
○Poor ○Fair ○Average ○Good ○Excellent
31. Where have you obtained/learned information about PGx testing [multiple choice] *
○In the drug labels ○In the undergraduate courses ○In the postgraduate courses
○In the guidelines, consensus, standard clinical paths for disease diagnosis and treatment
○Residency training or training period
○In the professional guidelines, monographs, or databases
○Specialized training in genomics ○In the academic conferences
○In the discussion of colleagues others: _________________
32. Which is true about the role of pharmacogenomic testing in your opinion?
(1) It can help to explain most of the drug reactions
○Disagree ○Somewhat disagree ○Neutral○Somewhat agree ○Agree
(2) It can help to identify drug interactions
○Disagree ○Somewhat disagree ○Neutral○Somewhat agree ○Agree
(3) It can help to choose drugs and optimize drug dosing
○Disagree ○Somewhat disagree ○Neutral○Somewhat agree ○Agree
(4) It can help to improve efficacy and reduce the incidence of adverse reactions
○Disagree ○Somewhat disagree ○Neutral○Somewhat agree ○Agree
(5) it can effectively help to decrease pharmacotherapeutic costs
○Disagree ○Somewhat disagree ○Neutral○Somewhat agree ○Agree
33.For patients in different areas of disease treatment, what is your opinion: [Enter the number from 0 to 100]
(1) Targeted Oncology Therapy____________
(2) Pain Treatment____________
(3) The field of psychiatry and neurology____________
(4) The field of cardiovascular disease____________
(5) The field of infectious diseases____________
(6) The field of rheumatic immune diseases____________
(7) Other fields____________
34. Which of the following categories of patients do you think will benefit most from pharmacogenomic testing?
○All patients ○Patients with numerous comorbidities
○Patients whose pharmacogenomic testing can guide the determination of the initial dose
○Patients who use the test result to help to predict that s/he will have a poor response to a medication
○Patients who use the test result to help to predict that s/he will experience side effects from a medication
○I do not think pharmacogenomic testing is beneficial to patients.
35. Which do you think are the barriers to the development of pharmacogenomic testing [multiple choice] *
○Pharmacogenomic testing does not influence clinical decision making
○Lack of knowledge ○Lack of relevant professionals
○Lack of hospitals or institutions to provide testing ○No guidelines, consensus, etc.
○High cost of testing ○Insurance does not cover PGx testing ○others
36. In your opinion, the responsibilities of pharmacists in PGx testing include [multiple choice] *
○Participate in testing works ○Interpretation of PGx test results for physicians and patients
○Make recommendations to physicians on drug selection, dosage and monitoring based on the results of PGx testing
○Recommend PGx testing to physicians or patients ○Others ○None
37. If there is special training related to pharmacogenomics, are you willing to participate in it? ○Yes ○No ○Not sure
38. The type of training do you want [multiple choice] *
○Expert lectures ○Academic conferences ○Short-term intensive training
○Spread the training over several weeks ○Online video courses ○Others
References
- ICH. Definitions for Genomic Biomarkers, Pharmacogenomics, Pharmacogenetics, Genomic Data and Sample Coding Categories; ICH: Geneva, Switzerland, 2007. [Google Scholar]
- Pereira, N.L.; Rihal, C.S.; So, D.Y.F.; Rosenberg, Y.; Lennon, R.J.; Mathew, V.; Goodman, S.G.; Weinshilboum, R.M.; Wang, L.; Baudhuin, L.M.; et al. Clopidogrel Pharmacogenetics. Circ. Cardiovasc. Interv. 2019, 12, e007811. [Google Scholar] [CrossRef]
- Kaye, J.B.; Schultz, L.E.; Steiner, H.E.; Kittles, R.A.; Cavallari, L.H.; Karnes, J.H. Warfarin Pharmacogenomics in Diverse Populations. Pharmacotherapy 2017, 37, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Dean, L.; Kane, M. Allopurinol Therapy and HLA-B*58:01 Genotype. In Medical Genetics Summaries; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kane, M.S., Kattman, B.L., Malheiro, A.J., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Wang, Q.; Sun, S.; Xie, M.; Zhao, K.; Li, X.; Zhao, Z. Association between the HLA-B alleles and carbamazepine-induced SJS/TEN: A meta-analysis. Epilepsy. Res. 2017, 135, 19–28. [Google Scholar] [CrossRef]
- Wong, W.B.; Carlson, J.J.; Thariani, R.; Veenstra, D.L. Cost effectiveness of pharmacogenomics: A critical and systematic review. Pharmacoeconomics 2010, 28, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Berm, E.J.; Looff, M.; Wilffert, B.; Boersma, C.; Annemans, L.; Vegter, S.; Boven, J.F.; Postma, M.J. Economic Evaluations of Pharmacogenetic and Pharmacogenomic Screening Tests: A Systematic Review. Second Update of the Literature. PLoS ONE 2016, 11, e0146262. [Google Scholar] [CrossRef] [PubMed]
- Verbelen, M.; Weale, M.E.; Lewis, C.M. Cost-effectiveness of pharmacogenetic-guided treatment: Are we there yet? Pharm. J. 2017, 17, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Caudle, K.E.; Gammal, R.S.; Whirl-Carrillo, M.; Hoffman, J.M.; Relling, M.V.; Klein, T.E. Evidence and resources to implement pharmacogenetic knowledge for precision medicine. Am. J. Health-Syst. Pharm. 2016, 73, 1977–1985. [Google Scholar] [CrossRef][Green Version]
- The Pharmacogenomics Knowledge Base (PharmGKB). Available online: https://www.pharmgkb.org/ (accessed on 18 January 2022).
- Cai, L. Medical Revolution under Precision Medicine. Available online: https://news.medlive.cn/all/info-news/show-80790_97.html (accessed on 1 June 2022).
- Dias, M.M.; Ward, H.M.; Sorich, M.J.; McKinnon, R.A. Exploration of the perceptions, barriers and drivers of pharmacogenomics practice among hospital pharmacists in Adelaide, South Australia. Pharm. J. 2014, 14, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Huston, S.; Zdanowicz, M.; Fetterman, J. Pharmacogenomics in advanced pharmacy practice experiences. Curr. Pharm. Teach. Learn. 2010, 2, 196–203. [Google Scholar] [CrossRef]
- Bank, P.C.; Swen, J.J.; Guchelaar, H.-J. A nationwide survey of pharmacists’ perception of pharmacogenetics in the context of a clinical decision support system containing pharmacogenetics dosing recommendations. Pharmacogenomics 2017, 18, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Karuna, N.; Tragulpiankit, P.; Mahasirimongkol, S.; Chumnumwat, S. Knowledge, attitude, and practice towards pharmacogenomics among hospital pharmacists in Thailand. Pharm. Genom. 2020, 30, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Bannur, Z.; Bahaman, S.; Salleh, M.Z.; Teh, L.K. Pharmacogenomics Based Practice in Malaysia: The Attitude, Knowledge and Adoption by the Healthcare Professionals. IIUM Med. J. 2014, 13. [Google Scholar] [CrossRef]
- Guo, C.; Hu, B.; Guo, C.; Meng, X.; Kuang, Y.; Huang, L.; Wang, D.; Xu, K.; Zhao, Y.; Yang, G.; et al. A Survey of Pharmacogenomics Testing Among Physicians, Pharmacists, and Researchers From China. Front. Pharmacol. 2021, 12, 682020. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yao, D.; Zhou, H.; Xi, X.; Wang, Y.; Yao, W. Association of hospital pharmacy-related knowledge and skills with occupational stress of clinical pharmacists in tertiary hospitals of China. J. Am. Pharm. Assoc. 2021, 61, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Muflih, S.; Al-Husein, B.A.; Karasneh, R.; Alzoubi, K.H. Physicians’ Attitudes and Ethical Obligations to Pharmacogenetic Testing. J. Multidiscip. Healthc. 2020, 13, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Bonter, K.; Desjardins, C.; Currier, N.; Pun, J.; Ashbury, F.D. Personalised medicine in Canada: A survey of adoption and practice in oncology, cardiology and family medicine. BMJ Open 2011, 1, e000110. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, S.; Haynes, K.; Zayac, C.; Sprague, J.E.; Bernhardt, B.; Pyeritz, R. Community pharmacists’ attitudes towards clinical utility and ethical implications of pharmacogenetic testing. Per. Med. 2013, 10, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Fargher, E.A.; Eddy, C.; Newman, W.; Qasim, F.; Tricker, K.; Elliott, R.A.; Payne, K. Patients’ and healthcare professionals’ views on pharmacogenetic testing and its future delivery in the NHS. Pharmacogenomics 2007, 8, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- de Denus, S.; Letarte, N.; Hurlimann, T.; Lambert, J.P.; Lavoie, A.; Robb, L.; Sheehan, N.L.; Turgeon, J.; Vadnais, B. An evaluation of pharmacists’ expectations towards pharmacogenomics. Pharmacogenomics 2013, 14, 165–175. [Google Scholar] [CrossRef]
- Albassam, A.; Alshammari, S.; Ouda, G.; Koshy, S.; Awad, A. Knowledge, perceptions and confidence of physicians and pharmacists towards pharmacogenetics practice in Kuwait. PLoS ONE 2018, 13, e0203033. [Google Scholar] [CrossRef] [PubMed]
- McCullough, K.B.; Formea, C.M.; Berg, K.D.; Burzynski, J.A.; Cunningham, J.L.; Ou, N.N.; Rudis, M.I.; Stollings, J.L.; Nicholson, W.T. Assessment of the pharmacogenomics educational needs of pharmacists (Chinese version). Am. J. Pharm. Educ. 2011, 75, 51. [Google Scholar] [CrossRef]
- Just, K.S.; Steffens, M.; Swen, J.J.; Patrinos, G.P.; Guchelaar, H.J.; Stingl, J.C. Medical education in pharmacogenomics-results from a survey on pharmacogenetic knowledge in healthcare professionals within the European pharmacogenomics clinical implementation project Ubiquitous Pharmacogenomics (U-PGx). Eur. J. Clin. Pharmacol. 2017, 73, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Abou Diwan, E.; Zeitoun, R.I.; Abou Haidar, L.; Cascorbi, I.; Khoueiry Zgheib, N. Implementation and obstacles of pharmacogenetics in clinical practice: An international survey. Br. J. Clin. Pharmacol. 2019, 85, 2076–2088. [Google Scholar] [CrossRef]
- Tsuji, D.; Saito, Y.; Mushiroda, T.; Miura, M.; Hira, D.; Terada, T. Results of a nationwide survey of Japanese pharmacists regarding the application of pharmacogenomic testing in precision medicine. J. Clin. Pharm. Ther. 2021, 46, 649–657. [Google Scholar] [CrossRef]
- Xiang, Q.; Fu, D. A team led by Yimin Cui is Working on Pharmacogenomics and a Comprehensive Evaluative System for the Precise Use of Drugs in China. Available online: https://bynews.bjmu.edu.cn/zhxw/2016n/185555.htm (accessed on 1 June 2022).
- Bagher, A.M.; Neamatallah, T.; Balto, G.; Almikhy, L.; Almutairi, S.S.; Abushal, M.O.; Baghlaf, K.; Bagher, S.M. Knowledge, perception, and confidence of hospital pharmacists toward pharmacogenetics in Jeddah, Kingdom of Saudi Arabia. Saudi Pharm. J. 2021, 29, 53–58. [Google Scholar] [CrossRef]
- (CPIC) C.P.I.C. Genes-Drugs. Available online: https://cpicpgx.org/genes-drugs/ (accessed on 1 June 2022).
- Food and Drug Administration, F. Table of Pharmacogenetic Associations. Available online: https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations (accessed on 27 June 2022).
- Fan, Y.; Zhang, X.; Yan, J. Analysis of Subjective Reasons for Distortion of Questionnaire Results by Subjects. Human Resour. Manag. 2011, 156–159. [Google Scholar]
- Li, X. An Introduction to Self-Presentation in Daily Life. In Proceedings of the 2011 Guizhou China Annual Social Science Academic Conference, Guiyang, China, 11 November–22 December 2011; pp. 156–159. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).