Towards Treatable Traits for Pulmonary Fibrosis
Abstract
:1. Introduction
2. Application of the ‘Treatable Traits’ Concept in Fibrotic ILD
2.1. Environmental Traits
2.2. Pulmonary Traits
2.3. Comorbidities
2.4. Functional Traits
2.5. Future Avenues for Detection and Treatment of Treatable Traits
2.6. Development of Novel Treatments within the Treatable Traits Framework
3. Discussion
- Create larger patient cohorts through international collaboration, and study markers for disease behaviour in non-IPF fibrotic ILD
- Investigate in which patients immunosuppressive therapy is effective in addition to antifibrotic therapy
- Develop targeted therapies for fibrotic ILD patients with genetic abnormalities
- Investigate whether response to targeted therapies can be predicted by serum, BAL, or exhaled air biomarkers
- Investigate the value of molecular classifiers based on gene expression in transbronchial lung biopsies for identifying treatable traits
- Investigate the value of machine learning on radiological imaging for identifying treatable traits
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agusti, A.; Bel, E.; Thomas, M.; Vogelmeier, C.; Brusselle, G.; Holgate, S.; Humbert, M.; Jones, P.; Gibson, P.G.; Vestbo, J.; et al. Treatable traits: Toward precision medicine of chronic airway diseases. Eur. Respir. J. 2016, 47, 410–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, V.M.; Fingleton, J.; Agusti, A.; Hiles, S.A.; Clark, V.L.; Holland, A.E.; Marks, G.B.; Bardin, P.P.; Beasley, R.; Pavord, I.D.; et al. Treatable traits: A new paradigm for 21st century management of chronic airway diseases: Treatable Traits Down Under International Workshop report. Eur. Respir. J. 2019, 53, 1802058. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A. Phenotypes and disease characterization in chronic obstructive pulmonary disease: Toward the extinction of phenotypes? Ann. Am. Thorac. Soc. 2013, 10, S125–S130. [Google Scholar] [CrossRef] [PubMed]
- Mikolasch, T.A.; Garthwaite, H.S.; Porter, J.C. Update in diagnosis and management of interstitial lung Disease. Clin. Med. J. R. Coll. Physicians Lond. 2017, 17, 146–153. [Google Scholar] [CrossRef]
- Wijsenbeek, M.; Cottin, V. Spectrum of Fibrotic Lung Diseases. N. Engl. J. Med. 2020, 8, 958–968. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef] [Green Version]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef]
- Maher, T.M.; Corte, T.J.; Fischer, A.; Kreuter, M.; Lederer, D.J.; Molina-Molina, M.; Axmann, J.; Kirchgaessler, K.U.; Samara, K.; Gilberg, F.; et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2020, 8, 147–157. [Google Scholar] [CrossRef]
- Behr, J.; Prasse, A.; Kreuter, M.; Johow, J.; Rabe, K.F.; Bonella, F.; Bonnet, R.; Grohe, C.; Held, M.; Wilkens, H.; et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 476–486. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Antoniou, K.M.; Bissell, B.D.; Bouros, D.; Buendia-Roldan, I.; Caro, F.; Crestani, B.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, E18–E47. [Google Scholar] [CrossRef]
- Wongkarnjana, A.; Scallan, C.; Kolb, M.R.J. Progressive fibrosing interstitial lung disease: Treatable traits and therapeutic strategies. Curr. Opin. Pulm. Med. 2020, 26, 436–442. [Google Scholar] [CrossRef]
- Boaventura, R.; Sibila, O.; Agusti, A.; Chalmers, J.D. Treatable traits in bronchiectasis. Eur. Respir. J. 2018, 52, 1801269. [Google Scholar] [CrossRef]
- Margaritopoulos, G.A.; Vasarmidi, E.; Jacob, J.; Wells, A.U.; Antoniou, K.M. Smoking and interstitial lung diseases. Eur. Respir. Rev. 2015, 24, 428–435. [Google Scholar] [CrossRef]
- Antoniou, K.M.; Hansell, D.M.; Rubens, M.B.; Marten, K.; Desai, S.R.; Siafakas, N.M.; Nicholson, A.G.; Du Bois, R.M.; Wells, A.U. Idiopathic Pulmonary Fibrosis: Outcome in Relation to Smoking Status. Am. J. Respir. Crit. Care Med. 2007, 177, 190–194. [Google Scholar] [CrossRef]
- Platenburg, M.G.J.P.; van der Vis, J.J.; Kazemier, K.M.; Grutters, J.C.; van Moorsel, C.H.M. The detrimental effect of quantity of smoking on survival in progressive fibrosing ILD. Respir. Med. 2022, 194, 106760. [Google Scholar] [CrossRef]
- Tazi, A.; De Margerie, C.; Naccache, J.M.; Fry, S.; Dominique, S.; Jouneau, S.; Lorillon, G.; Bugnet, E.; Chiron, R.; Wallaert, B.; et al. The natural history of adult pulmonary Langerhans cell histiocytosis: A prospective multicentre study. Orphanet J. Rare Dis. 2015, 10, 1–10. [Google Scholar] [CrossRef]
- De Sadeleer, L.J.; Hermans, F.; De Dycker, E.; Yserbyt, J.; Verschakelen, J.A.; Verbeken, E.K.; Verleden, G.M.; Wuyts, W.A. Effects of Corticosteroid Treatment and Antigen Avoidance in a Large Hypersensitivity Pneumonitis Cohort: A Single-Centre Cohort Study. J. Clin. Med. 2018, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Pérez, E.R.F.; Swigris, J.J.; Forssén, A.V.; Tourin, O.; Solomon, J.J.; Huie, T.J.; Olson, A.L.; Brown, K.K. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest 2013, 144, 1644–1651. [Google Scholar] [CrossRef] [Green Version]
- Gimenez, A.; Storrer, K.; Kuranishi, L.; Soares, M.R.; Ferreira, R.G.; Pereira, C.A.C. Change in FVC and survival in chronic fibrotic hypersensitivity pneumonitis. Thorax 2018, 73, 391–392. [Google Scholar] [CrossRef]
- Nishida, T.; Kawate, E.; Ishiguro, T.; Kanauchi, T.; Shimizu, Y.; Takayanagi, N. Antigen avoidance and outcome of nonfibrotic and fibrotic hypersensitivity pneumonitis. ERJ Open Res. 2021, 8, 00474–2021. [Google Scholar] [CrossRef]
- Barber, C.; Fishwick, D. Pneumoconiosis. Medicine 2020, 48, 417–421. [Google Scholar] [CrossRef]
- Ratwani, A.; Gupta, B.; Stephenson, B.W.; Mani, H.; Brown, A.W. The Spectrum of Drug-Induced Interstitial Lung Disease. Curr. Pulmonol. Rep. 2019, 8, 139–150. [Google Scholar] [CrossRef]
- Raghu, G.; Wilson, K.C.; Bargagli, E.; Bendstrup, E.; Chami, H.A.; Chua, A.T.; Chung, J.H.; Collins, B.F.; Corte, T.J.; Dalphin, J.C.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, E36–E69. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V. Significance of connective tissue diseases features in pulmonary fibrosis. Eur. Respir. Rev. 2013, 22, 273–280. [Google Scholar] [CrossRef]
- Salisbury, M.L.; Myers, J.L.; Belloli, E.A.; Kazerooni, E.A.; Martinez, F.J.; Flaherty, K.R. Diagnosis and treatment of fibrotic hypersensitivity pneumonia: Where we stand and where we need to go. Am. J. Respir. Crit. Care Med. 2017, 196, 690–699. [Google Scholar] [CrossRef]
- Graney, B.A.; Fischer, A. Interstitial pneumonia with autoimmune features. Ann. Am. Thorac. Soc. 2019, 16, 525–533. [Google Scholar] [CrossRef]
- Vacchi, C.; Sebastiani, M.; Cassone, G.; Cerri, S.; Della Casa, G.; Salvarani, C.; Manfredi, A. Therapeutic Options for the Treatment of Interstitial Lung Disease Related to Connective Tissue Diseases. A Narrative Review. J. Clin. Med. 2020, 9, 407. [Google Scholar] [CrossRef] [Green Version]
- George, P.M.; Spagnolo, P.; Kreuter, M.; Altinisik, G.; Bonifazi, M.; Martinez, F.J.; Molyneaux, P.L.; Renzoni, E.A.; Richeldi, L.; Tomassetti, S.; et al. Progressive fibrosing interstitial lung disease: Clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir. Med. 2020, 8, 925–934. [Google Scholar] [CrossRef]
- Horowitz, J.C.; Thannickal, V.J. Mechanisms for the resolution of organ fibrosis. Physiology 2019, 34, 43–55. [Google Scholar] [CrossRef]
- Ruffenach, G.; Hong, J.; Vaillancourt, M.; Medzikovic, L.; Eghbali, M. Pulmonary hypertension secondary to pulmonary fibrosis: Clinical data, histopathology and molecular insights. Respir. Res. 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Waxman, A.; Restrepo-Jaramillo, R.; Thenappan, T.; Ravichandran, A.; Engel, P.; Bajwa, A.; Allen, R.; Feldman, J.; Argula, R.; Smith, P.; et al. Inhaled Treprostinil in Pulmonary Hypertension Due to Interstitial Lung Disease. N. Engl. J. Med. 2021, 384, 325–334. [Google Scholar] [CrossRef]
- Galiè, N.; Channick, R.N.; Frantz, R.P.; Grünig, E.; Jing, Z.C.; Moiseeva, O.; Preston, I.R.; Pulido, T.; Safdar, Z.; Tamura, Y.; et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801889. [Google Scholar] [CrossRef]
- Schiza, S.; Mermigkis, C.; Margaritopoulos, G.A.; Daniil, Z.; Harari, S.; Poletti, V.; Renzoni, E.A.; Torre, O.; Visca, D.; Bouloukaki, I.; et al. Idiopathic pulmonary fibrosis and sleep disorders: No longer strangers in the night. Eur. Respir. Rev. 2015, 24, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Bosi, M.; Milioli, G.; Fanfulla, F.; Tomassetti, S.; Ryu, J.H.; Parrino, L.; Riccardi, S.; Melpignano, A.; Vaudano, A.E.; Ravaglia, C.; et al. OSA and Prolonged Oxygen Desaturation During Sleep are Strong Predictors of Poor Outcome in IPF. Lung 2017, 195, 643–651. [Google Scholar] [CrossRef]
- Mermigkis, C.; Bouloukaki, I.; Antoniou, K.; Papadogiannis, G.; Giannarakis, I.; Varouchakis, G.; Siafakas, N.; Schiza, S.E. Obstructive sleep apnea should be treated in patients with idiopathic pulmonary fibrosis. Sleep Breath. 2015, 19, 385–391. [Google Scholar] [CrossRef]
- Papadogiannis, G.; Bouloukaki, I.; Mermigkis, C.; Michelakis, S.; Ermidou, C.; Mauroudi, E.; Moniaki, V.; Tzanakis, N.; Antoniou, K.M.; Schiza, S.E. Patients with idiopathic pulmonary fibrosis with and without obstructive sleep apnea: Differences in clinical characteristics, clinical outcomes, and the effect of PAP treatment. J. Clin. Sleep Med. 2021, 17, 533–544. [Google Scholar] [CrossRef]
- Tse, L.A.; Yu, I.T.S.; Qiu, H.; Leung, C.C. Joint Effects of Smoking and Silicosis on Diseases to the Lungs. PLoS ONE 2014, 9, e104494. [Google Scholar] [CrossRef]
- Hung, C.L.; Su, P.L.; Ou, C.Y. Prognostic effect of tuberculosis on patients with occupational lung diseases: A 13-year observational study in a nationwide cohort. Medicine 2016, 95, e4748. [Google Scholar] [CrossRef]
- Novikova, L.; Ilkovich, Y.; Speranskaya, A. Tuberculosis in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 46, PA2046. [Google Scholar] [CrossRef]
- Lee, Y.H.; Cha, S.I.; Lim, J.K.; Yoo, S.S.; Lee, S.Y.; Lee, J.; Kim, C.H.; Park, J.Y. Clinical and radiological features of pulmonary tuberculosis in patients with idiopathic pulmonary fibrosis. Respir. Investig. 2019, 57, 544–551. [Google Scholar] [CrossRef]
- Furin, J.; Cox, H.; Pai, M. Tuberculosis. Lancet 2019, 393, 1642–1656. [Google Scholar] [CrossRef]
- Dowman, L.; Hill, C.J.; May, A.; Holland, A.E. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst. Rev. 2021, 2. [Google Scholar] [CrossRef]
- Visca, D.; Mori, L.; Tsipouri, V.; Fleming, S.; Firouzi, A.; Bonini, M.; Pavitt, M.J.; Alfieri, V.; Canu, S.; Bonifazi, M.; et al. Effect of ambulatory oxygen on quality of life for patients with fibrotic lung disease (AmbOx): A prospective, open-label, mixed-method, crossover randomised controlled trial. Lancet. Respir. Med. 2018, 6, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Holland, A.E.; Fiore, J.F.; Bell, E.C.; Goh, N.; Westall, G.; Symons, K.; Dowman, L.; Glaspole, I. Dyspnoea and comorbidity contribute to anxiety and depression in interstitial lung disease. Respirology 2014, 19, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Bajwah, S.; Ross, J.R.; Wells, A.U.; Mohammed, K.; Oyebode, C.; Birring, S.S.; Patel, A.S.; Koffman, J.; Higginson, I.J.; Riley, J. Palliative care for patients with advanced fibrotic lung disease: A randomised controlled phase II and feasibility trial of a community case conference intervention. Thorax 2015, 70, 830–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanania, A.N.; Mainwaring, W.; Ghebre, Y.T.; Hanania, N.A.; Ludwig, M. Radiation-Induced Lung Injury: Assessment and Management. Chest 2019, 156, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Trethewey, S.P.; Walters, G.I. The role of occupational and environmental exposures in the pathogenesis of idiopathic pulmonary fibrosis: A narrative literature review. Medicina 2018, 54, 108. [Google Scholar] [CrossRef] [Green Version]
- Johannson, K.A.; Vittinghoff, E.; Morisset, J.; Wolters, P.J.; Noth, E.M.; Balmes, J.R.; Collard, H.R. Air Pollution Exposure Is Associated With Lower Lung Function, but Not Changes in Lung Function, in Patients With Idiopathic Pulmonary Fibrosis. Chest 2018, 154, 119–125. [Google Scholar] [CrossRef]
- Skeoch, S.; Weatherley, N.; Swift, A.; Oldroyd, A.; Johns, C.; Hayton, C.; Giollo, A.; Wild, J.; Waterton, J.; Buch, M.; et al. Drug-Induced Interstitial Lung Disease: A Systematic Review. J. Clin. Med. 2018, 7, 356. [Google Scholar] [CrossRef] [Green Version]
- Chioma, O.S.; Drake, W.P. Focus: Infectious Diseases: Role of Microbial Agents in Pulmonary Fibrosis. Yale J. Biol. Med. 2017, 90, 219. [Google Scholar]
- O’Dwyer, D.N.; Ashley, S.L.; Gurczynski, S.J.; Xia, M.; Wilke, C.; Falkowski, N.R.; Norman, K.C.; Arnold, K.B.; Huffnagle, G.B.; Salisbury, M.L.; et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1127–1138. [Google Scholar] [CrossRef]
- Vasakova, M.; Selman, M.; Morell, F.; Sterclova, M.; Molina-Molina, M.; Raghu, G. Hypersensitivity pneumonitis: Current concepts of pathogenesis and potential targets for treatment. Am. J. Respir. Crit. Care Med. 2019, 200, 301–308. [Google Scholar] [CrossRef]
- Prasse, A.; Pechkovsky, D.V.; Toews, G.B.; Jungraithmayr, W.; Kollert, F.; Goldmann, T.; Vollmer, E.; Müller-Quernheim, J.; Zissel, G. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am. J. Respir. Crit. Care Med. 2006, 173, 781–792. [Google Scholar] [CrossRef] [Green Version]
- Wiertz, I.A.; Moll, S.A.; Seeliger, B.; Barlo, N.P.; van der Vis, J.J.; Korthagen, N.M.; Rijkers, G.T.; Ruven, H.J.T.; Grutters, J.C.; Prasse, A.; et al. Genetic Variation in CCL18 Gene Influences CCL18 Expression and Correlates with Survival in Idiopathic Pulmonary Fibrosis: Part A. J. Clin. Med. 2020, 9, 1940. [Google Scholar] [CrossRef]
- Caliskan, C.; Seeliger, B.; Jäger, B.; Fuge, J.; Welte, T.; Terwolbeck, O.; Freise, J.; van Moorsel, C.H.M.; Zhang, Y.; Prasse, A. Genetic Variation in CCL18 Gene Influences CCL18 Expression and Correlates with Survival in Idiopathic Pulmonary Fibrosis—Part B. J. Clin. Med. 2020, 9, 1993. [Google Scholar] [CrossRef]
- Hoffmann-Vold, A.M.; Tennøe, A.H.; Garen, T.; Midtvedt, Ø.; Abraityte, A.; Aaløkken, T.M.; Lund, M.B.; Brunborg, C.; Aukrust, P.; Ueland, T.; et al. High Level of Chemokine CCL18 Is Associated With Pulmonary Function Deterioration, Lung Fibrosis Progression, and Reduced Survival in Systemic Sclerosis. Chest 2016, 150, 299–306. [Google Scholar] [CrossRef]
- Bauer, Y.; Tedrow, J.; De Bernard, S.; Birker-Robaczewska, M.; Gibson, K.F.; Guardela, B.J.; Hess, P.; Klenk, A.; Lindell, K.O.; Poirey, S.; et al. A novel genomic signature with translational significance for human idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 52, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yella, J.; Chen, J.; McCormack, F.X.; Madala, S.K.; Jegga, A.G. Unsupervised gene expression analyses identify IPF-severity correlated signatures, associated genes and biomarkers. BMC Pulm. Med. 2017, 17, 133. [Google Scholar] [CrossRef] [Green Version]
- Ammar, R.; Sivakumar, P.; Jarai, G.; Thompson, J.R. A robust data-driven genomic signature for idiopathic pulmonary fibrosis with applications for translational model selection. PLoS ONE 2019, 14, e0215565. [Google Scholar] [CrossRef]
- Herazo-Maya, J.D.; Sun, J.; Molyneaux, P.L.; Li, Q.; Villalba, J.A.; Tzouvelekis, A.; Lynn, H.; Juan-Guardela, B.M.; Risquez, C.; Osorio, J.C.; et al. Validation of a 52-gene risk profile for outcome prediction in patients with idiopathic pulmonary fibrosis: An international, multicentre, cohort study. Lancet Respir. Med. 2017, 5, 857–868. [Google Scholar] [CrossRef]
- Luzina, I.G.; Salcedo, M.V.; Rojas-Peña, M.L.; Wyman, A.E.; Galvin, J.R.; Sachdeva, A.; Clerman, A.; Kim, J.; Franks, T.J.; Britt, E.J.; et al. Transcriptomic evidence of immune activation in macroscopically normal-appearing and scarred lung tissues in idiopathic pulmonary fibrosis. Cell. Immunol. 2018, 325, 1–13. [Google Scholar] [CrossRef]
- Lockstone, H.E.; Sanderson, S.; Kulakova, N.; Baban, D.; Leonard, A.; Kok, W.L.; McGowan, S.; McMichael, A.J.; Ho, L.P. Gene set analysis of lung samples provides insight into pathogenesis of progressive, fibrotic pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med. 2010, 181, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Heukels, P.; Van Hulst, J.A.C.; Van Nimwegen, M.; Boorsma, C.E.; Melgert, B.N.; Von Der Thusen, J.H.; Van Den Blink, B.; Hoek, R.A.S.; Miedema, J.R.; Neys, S.F.H.; et al. Enhanced Bruton’s tyrosine kinase in B-cells and autoreactive IgA in patients with idiopathic pulmonary fibrosis. Respir. Res. 2019, 20, 232. [Google Scholar] [CrossRef]
- Wallace, W.A.H.; Fitch, P.M.; Simpson, A.J.; Howie, S.E.M. Inflammation-associated remodelling and fibrosis in the lung—A process and an end point. Int. J. Exp. Pathol. 2007, 88, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luzina, I.G.; Todd, N.W.; Iacono, A.T.; Atamas, S.P. Roles of T lymphocytes in pulmonary fibrosis. J. Leukoc. Biol. 2008, 83, 237–244. [Google Scholar] [CrossRef]
- Desai, O.; Winkler, J.; Minasyan, M.; Herzog, E.L. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front. Med. 2018, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, J.; Grutters, J.C.; Arkema, E.V.; Saketkoo, L.A.; Moller, D.R.; Müller-Quernheim, J. Sarcoidosis. Nat. Rev. Dis. Primers 2019, 5, 45. [Google Scholar] [CrossRef]
- Wells, A.U.; Kokosi, M.; Karagiannis, K. Treatment strategies for idiopathic interstitial pneumonias. Curr. Opin. Pulm. Med. 2014, 20, 442–448. [Google Scholar] [CrossRef]
- Wells, A.U.; Flaherty, K.R.; Brown, K.K.; Inoue, Y.; Devaraj, A.; Richeldi, L.; Moua, T.; Crestani, B.; Wuyts, W.A.; Stowasser, S.; et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: A randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir. Med. 2020, 8, 453–460. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [Green Version]
- Behr, J.; Nathan, S.D.; Wuyts, W.A.; Mogulkoc Bishop, N.; Bouros, D.E.; Antoniou, K.; Guiot, J.; Kramer, M.R.; Kirchgaessler, K.U.; Bengus, M.; et al. Efficacy and safety of sildenafil added to pirfenidone in patients with advanced idiopathic pulmonary fibrosis and risk of pulmonary hypertension: A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 85–95. [Google Scholar] [CrossRef]
- Nathan, S.D.; Flaherty, K.R.; Glassberg, M.K.; Raghu, G.; Swigris, J.; Alvarez, R.; Ettinger, N.; Loyd, J.; Fernandes, P.; Gillies, H.; et al. A Randomized, Double-Blind, Placebo-Controlled Study of Pulsed, Inhaled Nitric Oxide in Subjects at Risk of Pulmonary Hypertension Associated With Pulmonary Fibrosis. Chest 2020, 158, 637–645. [Google Scholar] [CrossRef]
- Chung, M.J.; Goo, J.M.; Im, J.G. Pulmonary tuberculosis in patients with idiopathic pulmonary fibrosis. Eur. J. Radiol. 2004, 52, 175–179. [Google Scholar] [CrossRef]
- Holland, A.E.; Corte, T.; Chambers, D.C.; Palmer, A.J.; Ekström, M.P.; Glaspole, I.; Goh, N.S.L.; Hepworth, G.; Khor, Y.H.; Hoffman, M.; et al. Ambulatory oxygen for treatment of exertional hypoxaemia in pulmonary fibrosis (PFOX trial): A randomised controlled trial. BMJ Open 2020, 10, e040798. [Google Scholar] [CrossRef]
- Khor, Y.H.; Holland, A.E.; Goh, N.S.L.; Miller, B.R.; Vlahos, R.; Bozinovski, S.; Lahham, A.; Glaspole, I.; McDonald, C.F. Ambulatory Oxygen in Fibrotic Interstitial Lung Disease: A Pilot, Randomized, Triple-Blinded, Sham-Controlled Trial. Chest 2020, 158, 234–244. [Google Scholar] [CrossRef]
- Wijsenbeek, M.S.; Holland, A.E.; Swigris, J.J.; Renzoni, E.A. Comprehensive Supportive Care for Patients with Fibrosing Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2019, 200, 152–159. [Google Scholar] [CrossRef]
- Inchingolo, R.; Varone, F.; Sgalla, G.; Richeldi, L. Existing and emerging biomarkers for disease progression in idiopathic pulmonary fibrosis. Expert Rev. Respir. Med. 2019, 13, 39–51. [Google Scholar] [CrossRef]
- Hayton, C.; Terrington, D.; Wilson, A.M.; Chaudhuri, N.; Leonard, C.; Fowler, S.J. Breath biomarkers in idiopathic pulmonary fibrosis: A systematic review. Respir. Res. 2019, 20, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.J.; Weigt, S.S.; Belperio, J.A.; Brown, M.S.; Shi, Y.; Lai, J.H.; Goldin, J.G. Prediction of idiopathic pulmonary fibrosis progression using early quantitative changes on CT imaging for a short term of clinical 18–24-month follow-ups. Eur. Radiol. 2020, 30, 726–734. [Google Scholar] [CrossRef]
- Romei, C.; Tavanti, L.M.; Taliani, A.; De Liperi, A.; Karwoski, R.; Celi, A.; Palla, A.; Bartholmai, B.J.; Falaschi, F. Automated Computed Tomography analysis in the assessment of Idiopathic Pulmonary Fibrosis severity and progression. Eur. J. Radiol. 2020, 124, 108852. [Google Scholar] [CrossRef]
- Sverzellati, N.; Silva, M.; Seletti, V.; Galeone, C.; Palmucci, S.; Piciucchi, S.; Vancheri, C.; Poletti, V.; Tomassetti, S.; Karwoski, R.; et al. Stratification of long-term outcome in stable idiopathic pulmonary fibrosis by combining longitudinal computed tomography and forced vital capacity. Eur. Radiol. 2020, 30, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Bartholmai, B.J.; Rajagopalan, S.; Van Moorsel, C.H.M.; Van Es, H.W.; Van Beek, F.T.; Struik, M.H.L.; Kokosi, M.; Egashira, R.; Brun, A.L.; et al. Predicting outcomes in idiopathic pulmonary fibrosis using automated computed tomographic analysis. Am. J. Respir. Crit. Care Med. 2018, 198, 767–776. [Google Scholar] [CrossRef]
- Jacob, J.; Hirani, N.; Van Moorsel, C.H.M.; Rajagopalan, S.; Murchison, J.T.; Van Es, H.W.; Bartholmai, B.J.; Van Beek, F.T.; Struik, M.H.L.; Stewart, G.A.; et al. Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur. Respir. J. 2019, 53, 1800869. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Bartholmai, B.J.; Egashira, R.; Brun, A.L.; Rajagopalan, S.; Karwoski, R.; Kokosi, M.; Hansell, D.M.; Wells, A.U. Chronic hypersensitivity pneumonitis: Identification of key prognostic determinants using automated CT analysis. BMC Pulm. Med. 2017, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Wilton, K.M.; Ernste, F.C.; Kalra, S.; Crowson, C.S.; Rajagopalan, S.; Bartholmai, B.J. Novel Assessment of Interstitial Lung Disease Using the “Computer-Aided Lung Informatics for Pathology Evaluation and Rating” (CALIPER) Software System in Idiopathic Inflammatory Myopathies. Lung 2017, 195, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Ferrazza, A.M.; Gigante, A.; Gasperini, M.L.; Ammendola, R.M.; Paone, G.; Carbone, I.; Rosato, E. Assessment of interstitial lung disease in systemic sclerosis using the quantitative CT algorithm CALIPER. Clin. Rheumatol. 2020, 39, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Stainer, A.; Faverio, P.; Busnelli, S.; Catalano, M.; Della Zoppa, M.; Marruchella, A.; Pesci, A.; Luppi, F. Molecular Biomarkers in Idiopathic Pulmonary Fibrosis: State of the Art and Future Directions. Int. J. Mol. Sci. 2021, 22, 6255. [Google Scholar] [CrossRef]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.I.; Ren, Z.; et al. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef]
- Pankratz, D.G.; Choi, Y.; Imtiaz, U.; Fedorowicz, G.M.; Anderson, J.D.; Colby, T.V.; Myers, J.L.; Lynch, D.A.; Brown, K.K.; Flaherty, K.R.; et al. Usual interstitial pneumonia can be detected in transbronchial biopsies using machine learning. Ann. Am. Thorac. Soc. 2017, 14, 1646–1654. [Google Scholar] [CrossRef]
- Raghu, G.; Flaherty, K.R.; Lederer, D.J.; Lynch, D.A.; Colby, T.V.; Myers, J.L.; Groshong, S.D.; Larsen, B.T.; Chung, J.H.; Steele, M.P.; et al. Use of a molecular classifier to identify usual interstitial pneumonia in conventional transbronchial lung biopsy samples: A prospective validation study. Lancet Respir. Med. 2019, 7, 487–496. [Google Scholar] [CrossRef]
- Winterbottom, C.J.; Shah, R.J.; Patterson, K.C.; Kreider, M.E.; Panettieri, R.A.; Rivera-Lebron, B.; Miller, W.T.; Litzky, L.A.; Penning, T.M.; Heinlen, K.; et al. Exposure to Ambient Particulate Matter Is Associated With Accelerated Functional Decline in Idiopathic Pulmonary Fibrosis. Chest 2018, 153, 1221–1228. [Google Scholar] [CrossRef]
- Sesé, L.; Nunes, H.; Cottin, V.; Sanyal, S.; Didier, M.; Carton, Z.; Israel-Biet, D.; Crestani, B.; Cadranel, J.; Wallaert, B.; et al. Role of atmospheric pollution on the natural history of idiopathic pulmonary fibrosis. Thorax 2018, 73, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Dales, R.; Blanco-Vidal, C.; Cakmak, S. The Association Between Air Pollution and Hospitalization of Patients With Idiopathic Pulmonary Fibrosis in Chile: A Daily Time Series Analysis. Chest 2020, 158, 630–636. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Kim, S.Y.; Kim, O.J.; Song, J.W. Nitrogen dioxide increases the risk of mortality in idiopathic pulmonary fibrosis. Eur. Respir. J. 2021, 57, 2001877. [Google Scholar] [CrossRef]
- Johannson, K.A.; Vittinghoff, E.; Lee, K.; Balmes, J.R.; Ji, W.; Kaplan, G.G.; Kim, D.S.; Collard, H.R. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur. Respir. J. 2014, 43, 1124–1131. [Google Scholar] [CrossRef] [Green Version]
- Selman, M.; Pardo, A. Revealing the Pathogenic and Aging-related Mechanisms of the Enigmatic Idiopathic Pulmonary Fibrosis. An Integral Model. Am. J. Respir. Crit. Care Med. 2014, 189, 1161–1172. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Y.; Huang, H.; Dai, H.; Chen, J.; Tang, N. Progressive Pulmonary Fibrosis Is Caused by Elevated Mechanical Tension on Alveolar Stem Cells. Cell 2020, 180, 107–121. [Google Scholar] [CrossRef]
- Rush, B.; Wiskar, K.; Berger, L.; Griesdale, D. The use of mechanical ventilation in patients with idiopathic pulmonary fibrosis in the United States: A nationwide retrospective cohort analysis. Respir. Med. 2016, 111, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Mallick, S. Outcome of patients with idiopathic pulmonary fibrosis (IPF) ventilated in intensive care unit. Respir. Med. 2008, 102, 1355–1359. [Google Scholar] [CrossRef] [Green Version]
- Molyneaux, P.L.; Cox, M.J.; Wells, A.U.; Kim, H.C.; Ji, W.; Cookson, W.O.C.; Moffatt, M.F.; Kim, D.S.; Maher, T.M. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir. Res. 2017, 18, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Duckworth, A.; Longhurst, H.J.; Paxton, J.K.; Scotton, C.J. The Role of Herpes Viruses in Pulmonary Fibrosis. Front. Med. 2021, 8, 1137. [Google Scholar] [CrossRef]
- Invernizzi, R.; Barnett, J.; Rawal, B.; Nair, A.; Ghai, P.; Kingston, S.; Chua, F.; Wu, Z.; Wells, A.U.; Renzoni, E.R.; et al. Bacterial burden in the lower airways predicts disease progression in idiopathic pulmonary fibrosis and is independent of radiological disease extent. Eur. Respir. J. 2020, 55, 1901519. [Google Scholar] [CrossRef]
- Wilson, A.M.; Clark, A.B.; Cahn, T.; Chilvers, E.R.; Fraser, W.; Hammond, M.; Livermore, D.M.; Maher, T.M.; Parfrey, H.; Swart, A.M.; et al. Effect of Co-trimoxazole (Trimethoprim-Sulfamethoxazole) vs. Placebo on Death, Lung Transplant, or Hospital Admission in Patients With Moderate and Severe Idiopathic Pulmonary Fibrosis: The EME-TIPAC Randomized Clinical Trial. JAMA 2020, 324, 2282–2291. [Google Scholar] [CrossRef]
- Martinez, F.J.; Yow, E.; Flaherty, K.R.; Snyder, L.D.; Durheim, M.T.; Wisniewski, S.R.; Sciurba, F.C.; Raghu, G.; Brooks, M.M.; Kim, D.-Y.; et al. Effect of Antimicrobial Therapy on Respiratory Hospitalization or Death in Adults with Idiopathic Pulmonary Fibrosis: The CleanUP-IPF Randomized Clinical Trial. JAMA 2021, 325, 1841–1851. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, C.H.; Wang, C.Y.; Lai, C.C.; Chao, C.M.; Wei, Y.F. The effect of additional antimicrobial therapy on the outcomes of patients with idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Respir. Res. 2021, 22, 1–9. [Google Scholar] [CrossRef]
- van Moorsel, C.H.M.; van der Vis, J.J.; Grutters, J.C. Genetic disorders of the surfactant system: Focus on adult disease. Eur. Respir. Rev. 2021, 30, 200085. [Google Scholar] [CrossRef]
- Wang, Y.; Kuan, P.J.; Xing, C.; Cronkhite, J.T.; Torres, F.; Rosenblatt, R.L.; DiMaio, J.M.; Kinch, L.N.; Grishin, N.V.; Garcia, C.K. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am. J. Hum. Genet. 2009, 84, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Forstner, M.; Lin, S.; Yang, X.; Kinting, S.; Rothenaigner, I.; Schorpp, K.; Li, Y.; Hadian, K.; Griese, M. High-Content Screening Identifies Cyclosporin A as a Novel ABCA3-Specific Molecular Corrector. Am. J. Respir. Cell Mol. Biol. 2022, 66, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Cai, H.; Li, H.; Zhuang, Y.; Min, H.; Wen, Y.; Yang, J.; Gao, Q.; Shi, Y.; Yi, L. Association between telomere length and survival in patients with idiopathic pulmonary fibrosis. Respirology 2015, 20, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.D.; Lee, J.S.; Kozlitina, J.; Noth, I.; Devine, M.S.; Glazer, C.S.; Torres, F.; Kaza, V.; Girod, C.E.; Jones, K.D.; et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: An observational cohort study with independent validation. Lancet Respir. Med. 2014, 2, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Newton, C.A.; Oldham, J.M.; Ley, B.; Anand, V.; Adegunsoye, A.; Liu, G.; Batra, K.; Torrealba, J.; Kozlitina, J.; Glazer, C.; et al. Telomere length and genetic variant associations with interstitial lung disease progression and survival. Eur. Respir. J. 2019, 53, 1801641. [Google Scholar] [CrossRef]
- Newton, C.A.; Batra, K.; Torrealba, J.; Kozlitina, J.; Glazer, C.S.; Aravena, C.; Meyer, K.; Raghu, G.; Collard, H.R.; Garcia, C.K. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. Eur. Respir. J. 2016, 48, 1710–1720. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, T.W.; van Moorsel, C.H.M.; Borie, R.; Crestani, B. Pulmonary phenotypes associated with genetic variation in telomere-related genes. Curr. Opin. Pulm. Med. 2018, 24, 269–280. [Google Scholar] [CrossRef]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef] [Green Version]
- Townsley, D.M.; Dumitriu, B.; Liu, D.; Biancotto, A.; Weinstein, B.; Chen, C.; Hardy, N.; Mihalek, A.D.; Lingala, S.; Kim, Y.J.; et al. Danazol Treatment for Telomere Diseases. N. Engl. J. Med. 2016, 374, 1922–1931. [Google Scholar] [CrossRef]
- Povedano, J.M.; Martinez, P.; Serrano, R.; Tejera, Á.; Gómez-López, G.; Bobadilla, M.; Flores, J.M.; Bosch, F.; Blasco, M.A. Therapeutic effects of telomerase in mice with pulmonary fibrosis induced by damage to the lungs and short telomeres. Elife 2018, 7, e31299. [Google Scholar] [CrossRef]
- Peljto, A.L.; Zhang, Y.; Fingerlin, T.E.; Shwu-Fan, M.; Garcia, J.G.N.; Richards, T.J.; Silveira, L.J.; Lindell, K.O.; Steele, M.P.; Loyd, J.E.; et al. Association Between the MUC5B Promoter Polymorphism and Survival in Patients With Idiopathic Pulmonary Fibrosis. JAMA 2013, 309, 2232–2239. [Google Scholar] [CrossRef]
- Dudbridge, F.; Allen, R.J.; Sheehan, N.A.; Schmidt, A.F.; Lee, J.C.; Jenkins, R.G.; Wain, L.V.; Hingorani, A.D.; Patel, R.S. Adjustment for index event bias in genome-wide association studies of subsequent events. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- van der Vis, J.J.; Snetselaar, R.; Kazemier, K.M.; ten Klooster, L.; Grutters, J.C.; van Moorsel, C.H. Effect of Muc5b promoter polymorphism on disease predisposition and survival in idiopathic interstitial pneumonias. Respirology 2016, 21, 712–717. [Google Scholar] [CrossRef] [Green Version]
- Oldham, J.M.; Ma, S.F.; Martinez, F.J.; Anstrom, K.J.; Raghu, G.; Schwartz, D.A.; Valenzi, E.; Witt, L.; Lee, C.; Vij, R.; et al. TOLLIP, MUC5B, and the response to N-acetylcysteine among individuals with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2015, 192, 1475–1482. [Google Scholar] [CrossRef] [Green Version]
- Hancock, L.A.; Hennessy, C.E.; Solomon, G.M.; Dobrinskikh, E.; Estrella, A.; Hara, N.; Hill, D.B.; Kissner, W.J.; Markovetz, M.R.; Grove Villalon, D.E.; et al. Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Kolb, M.; Bondue, B.; Pesci, A.; Miyazaki, Y.; Song, J.W.; Bhatt, N.Y.; Huggins, J.T.; Oldham, J.M.; Padilla, M.L.; Roman, J.; et al. Acute exacerbations of progressive-fibrosing interstitial lung diseases. Eur. Respir. Rev. 2018, 27, 180071. [Google Scholar] [CrossRef]
- Hoffman, T.W.; van Moorsel, C.H.M.; Kazemier, K.M.; Biesma, D.H.; Grutters, J.C.; van Kessel, D.A. Humoral Immune Status in Relation to Outcomes in Patients with Idiopathic Pulmonary Fibrosis. Lung 2021, 199, 667–676. [Google Scholar] [CrossRef]
- Johannson, K.A.; Strambu, I.; Ravaglia, C.; Grutters, J.C.; Valenzuela, C.; Mogulkoc, N.; Luppi, F.; Richeldi, L.; Wells, A.U.; Vancheri, C.; et al. Antacid therapy in idiopathic pulmonary fibrosis: More questions than answers? Lancet Respir. Med. 2017, 5, 591–598. [Google Scholar] [CrossRef]
- Raghu, G.; Rochwerg, B.; Zhang, Y.; Garcia, C.A.; Azuma, A.; Behr, J.; Brozek, J.L.; Collard, H.R.; Cunningham, W.; Homma, S.; et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015, 192, e3–e19. [Google Scholar] [CrossRef]
- Tran, T.; Assayag, D.; Ernst, P.; Suissa, S. Effectiveness of Proton Pump Inhibitors in Idiopathic Pulmonary Fibrosis: A Population-Based Cohort Study. Chest 2021, 159, 673–682. [Google Scholar] [CrossRef]
- Raghu, G.; Pellegrini, C.A.; Yow, E.; Flaherty, K.R.; Meyer, K.; Noth, I.; Scholand, M.B.; Cello, J.; Ho, L.A.; Pipavath, S.; et al. Laparoscopic anti-reflux surgery for the treatment of idiopathic pulmonary fibrosis (WRAP-IPF): A multicentre, randomised, controlled phase 2 trial. Lancet Respir. Med. 2018, 6, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Glaspole, I.N.; Chapman, S.A.; Cooper, W.A.; Ellis, S.J.; Goh, N.S.; Hopkins, P.M.; Macansh, S.; Mahar, A.; Moodley, Y.P.; Paul, E.; et al. Health-related quality of life in idiopathic pulmonary fibrosis: Data from the Australian IPF Registry. Respirology 2017, 22, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Khor, Y.H.; Ng, Y.; Sweeney, D.; Ryerson, C.J. Nocturnal hypoxaemia in interstitial lung disease: A systematic review. Thorax 2021, 76, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.U.; Brown, K.K.; Flaherty, K.R.; Kolb, M.; Thannickal, V.J. What’s in a name? That which we call IPF, by any other name would act the same. Eur. Respir. J. 2018, 51, 1800692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
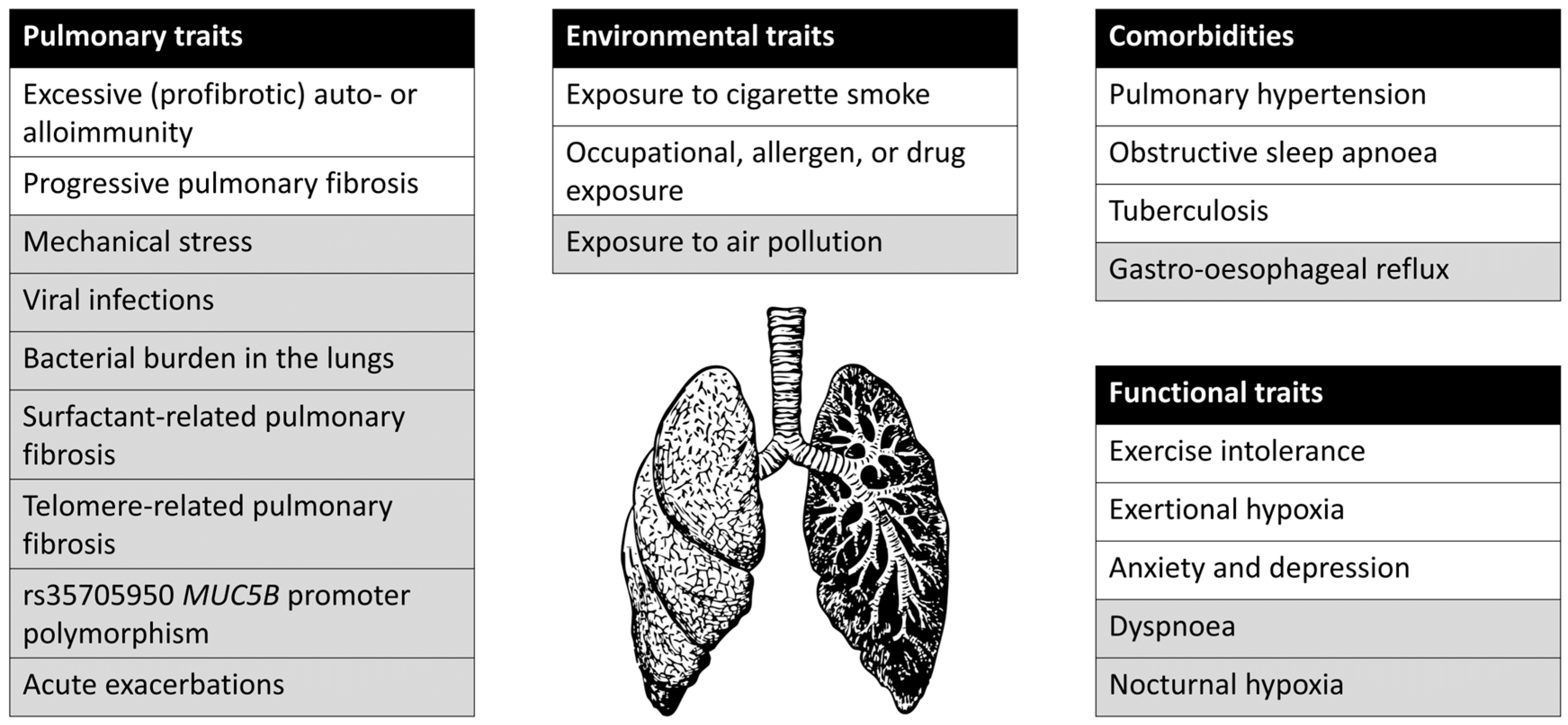
| Trait | Association with Outcomes | How to Detect | How to Intervene | Future Avenues for Detection and Treatment |
|---|---|---|---|---|
| Environmental | ||||
| Cigarette smoking | Probably associated with worse survival in patients with IPF and other progressive fibrotic ILD [13,14,15], associated with disease progression in PLCH [16] and other smoking-related ILD | Smoking history | Smoking cessation | - |
| Occupational, allergen, or drug exposures | Ongoing exposure is probably related to survival in fibrotic HP [17,18,19,20] and to disease progression in pneumoconiosis [21] and drug-induced ILD [22] | Occupational, exposure, and drug history, serum IgG testing targeting potential antigens for HP [23] | Avoid relevant exposures | Development and validation of exposure questionnaire |
| Pulmonary | ||||
| Excessive (profibrotic) auto- or alloimmunity | CTD-ILD or HP has more favorable prognosis than IPF [24,25] | Established diagnosis of CTD, established diagnosis of HP, features suggestive of auto-immune disease, but no formal CTD diagnosis (features consistent with IPAF in clinical, serological or morphological domain) [26] | Immunosuppressive drugs [25,27] | Determine whether combination therapy of immunosuppressive drugs and antifibrotic treatment is warranted for certain patients, determine whether patients with certain features consistent with IPAF benefit from immunosuppressive treatment; investigate whether other circulating auto-antibodies can be used as a marker to give immunosuppressive therapy |
| Progressive fibrosis | Associated with increased mortality [28] | Two out of three of: worsening respiratory symptoms, physiological evidence of disease progression (absolute decline in FVC ≥ 5% of predicted within 1 year of follow up or absolute decline in DLCOc ≥ 10% of predicted within 1 year), or radiographical evidence of disease progression (increased extent or severity of traction bronchiectasis or bronchiolectasis, or new ground-glass opacity with traction bronchiectasis, or new fine reticulation, or increased extent or increased coarseness of reticular abnormality, or new or increased honeycombing, or increased lobar volume loss) [10]. | Anti-fibrotic drugs [6,7,8,9], lung transplantation | Develop new radiological, histopathological, blood, BAL, or exhaled air biomarkers; develop novel strategies to replace fibrotic tissue with healthy tissue [29] |
| Comorbidities | ||||
| Pulmonary Hypertension | Associated with worse survival in patients with IPF [30] | Echocardiography, right heart catheterization | Consider inhaled treprostinil (associated with improved exercise capacity) [31]; consider PH-targeted therapy in patients with CTD-ILD and possible pulmonary arterial hypertension [32]; lung transplantation | Determine whether Treprostinil or inhaled nitric oxide leads to decreased mortality |
| Obstructive sleep apnea | Associated with decreased survival in patients with IPF [33,34] | Polysomnography | CPAP [35,36] | Determine how screening for obstructive sleep apnea can be implemented |
| Tuberculosis | Associated with decreased survival in patients with pneumoconiosis [37,38], might be associated with progression of IPF [39] | Can be suggested by CT-scan abnormalities [40], diagnosis by sputum or bronchial washing mycobacterial culture, molecular diagnostic tests [41] | Tuberculosis treatment depending on drug-sensitivity pattern | Determine if standard treatment regimens should be extended, determine if latent tuberculosis should be screened for |
| Functional | ||||
| Exercise intolerance | Reduced quality of life [42] | 6-min walking test | Pulmonary rehabilitation [42] | Further development of specific pulmonary rehabilitation programs |
| Exertional hypoxia | Reduced exercise tolerance | Exercise testing (transcutaneous oxygen saturation ≤88% on 6-min walking test) | Ambulatory oxygen suppletion [43] | Optimize oxygen-delivery system |
| Anxiety and depression | Reduced quality of life | Hospital Anxiety and Depression Scale [44] | Palliative care intervention including assessment, care plan, and community case conference [45] | Further development of interventions to treat anxiety and depression |
| Trait | Association with Outcomes | How to Detect | Potential Avenues for Treatment |
|---|---|---|---|
| Environmental | |||
| Air pollution | Associated with AE-IPF and progression of IPF [48,91,92,93,94,95] | Air quality monitoring, exposure history; no clear threshold for too much exposure | Possibly improve air quality, avoid exposure to bad quality air |
| Pulmonary | |||
| Mechanical stress | Continuous mechanical stress is hypothesized to contribute to disease progression in patients with IPF [96,97], mechanical ventilation of patients with IPF is associated with high mortality [98,99] | Not clear | Avoid mechanical ventilation, possibly develop novel methods to decrease mechanical tension on alveoli |
| Viral infections | Potential role of human herpes viruses as a co-factor in initiation and progression of IPF [100,101] | Viral PCR on bronchoalveolar lavage fluid | Possibly antiviral treatment; no randomized controlled trials have been done yet |
| Bacterial burden in lungs | Bacterial burden in lower airways associates with disease progression in patients with IPF [51,102] | 16s rRNA gene qPCR on bronchoalveolar lavage fluid | Possibly antibiotic treatment, vaccination; notably, treatment with cotrimoxazole or doxycycline had no effect on mortality or disease progression in patients with IPF [103,104,105] |
| Surfactant-related pulmonary fibrosis | Surfactant-related pulmonary fibrosis [106], higher risk of lung cancer in patients with SFTPA2 gene mutations [107] | Mutations in SFTPA1, SFTPA2, SFTPC, ABCA3, HPS, NKX2-1 | Development of novel treatments such as potentiators or gene-based therapy to correct surfactant processing [106], ABCA3 correction using cyclosporin A [108] |
| Telomere-related pulmonary fibrosis | Short leukocyte telomere length is associated with worse survival in patients with IPF or IPAF [109,110,111]; mutations in telomere-related genes are associated with a worse prognosis in patients with pulmonary fibrosis [112] | Telomere gene mutations, very short leukocyte telomere length [113] | Investigate anti-aging and telomere lengthening treatments such as dasatinib/quercetin [114], danazol [115], telomerase transfection [116] |
| rs35705950 MUC5B promoter polymorphism | Possibly associated with better survival in patients with IPF [117,118] and NSIP [119] | Genotyping of rs35705950 MUC5B promoter polymorphism | Investigate whether treatment with N-acetylcysteine [120], P-2 [119,121] or other mucolytics is effective |
| Acute exacerbation | Very poor prognosis in various types of ILD [122] | Further investigation of factors predicting acute exacerbation of pulmonary fibrosis such as lymphocytosis in bronchoalveolar lavage fluid [123]; investigate novel treatments | |
| Comorbidities | |||
| Gastro-esophageal reflux | Possibly associated with acute exacerbations or disease progression in patients with IPF [124] | 24-h pH monitoring, patient history | Antacid therapy might be helpful and was conditionally recommended in IPF treatment guidelines [125], however there are increasing signals that this is not effective [126], and it is no longer recommended in updated guidelines [10]; laparoscopic fundoplication was not found to affect disease progression or mortality in patients with IPF in a small randomized controlled trial [127] |
| Functional | |||
| Dyspnea | Reduced quality of life [128] | Clinical history | Investigate whether benzodiazepines and/or opioids can safely be used for symptom relief |
| Nocturnal hypoxia | Early mortality [129] | Polysomnography | Investigate efficacy of nocturnal oxygen suppletion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffman, T.W.; Grutters, J.C. Towards Treatable Traits for Pulmonary Fibrosis. J. Pers. Med. 2022, 12, 1275. https://doi.org/10.3390/jpm12081275
Hoffman TW, Grutters JC. Towards Treatable Traits for Pulmonary Fibrosis. Journal of Personalized Medicine. 2022; 12(8):1275. https://doi.org/10.3390/jpm12081275
Chicago/Turabian StyleHoffman, Thijs W., and Jan C. Grutters. 2022. "Towards Treatable Traits for Pulmonary Fibrosis" Journal of Personalized Medicine 12, no. 8: 1275. https://doi.org/10.3390/jpm12081275
APA StyleHoffman, T. W., & Grutters, J. C. (2022). Towards Treatable Traits for Pulmonary Fibrosis. Journal of Personalized Medicine, 12(8), 1275. https://doi.org/10.3390/jpm12081275






