Abstract
Escherichia coli releases outer membrane vesicles (OMVs) into the extracellular environment. OMVs, which contain the outer membrane protein, lipopolysaccharides (LPS), and genetic material, play an important role in immune response modulation. An isobaric tag for relative and absolute quantitation (iTRAQ) analysis was used to investigate OMV constituent proteins and their functions in burn trauma. OMV sizes ranged from 50 to 200 nm. Proteomics and Gene Ontology analysis revealed that ΔrfaC and ΔrfaG were likely involved in the upregulation of the structural constituent of ribosomes for the outer membrane and of proteins involved in protein binding and OMV synthesis. ΔrfaL was likely implicated in the downregulation of the structural constituent of the ribosome, translation, and cytosolic large ribosomal subunit. Kyoto Encyclopedia of Genes and Genomes analysis indicated that ΔrfaC and ΔrfaG downregulated ACP, ACEF, and ADHE genes; ΔrfaL upregulated ACP, ACEF, and ADHE genes. Heat map analysis demonstrated upregulation of galF, clpX, accA, fabB, and grpE and downregulation of pspA, ydiY, rpsT, and rpmB. These results suggest that RfaC, RfaG, and RfaL proteins were involved in outer membrane and LPS synthesis. Therefore, direct contact between wounds and LPS may lead to apoptosis, reduction in local cell proliferation, and delayed wound healing.
1. Introduction
Gram-negative bacteria release nanovesicles from their outer membrane into the extracellular milieu which are called outer membrane vesicles (OMVs) [1,2]. OMVs, which range from 20 to 200 nm in size, have a lipid-bilayered diameter and spherical proteolipids. They contain lipopolysaccharides (LPS), periplasmic proteins, outer membrane lipids, cytoplasmic proteins, DNA, RNA, outer membrane proteins, and other elements related to virulence [3,4,5,6,7]. OMVs play important roles in bacterial physiological and phthological aspects such as genetic material transfer, protein transport, nutrient acquisition, antibacterial activity, virulence factor delivery, interkingdom communication, immune response modulation, and neutralizing phage decoy activity [8,9,10,11,12,13,14]. As the major component of OMV, LPS contains lipid A, a core oligosaccharide, and the O-specific polysaccharide of the O-antigen (Figure 1b) [15]. LPS has been reported to be associated with infection. In burn injury and infection-related diseases, LPS of pathogens interact with membrane-bound or soluble CD14, lipopolysaccharide-binding protein (LBP), and Toll-like receptor 4 (TLR4) to initiate cellular production of pro-inflammatory cytokines, immune cell recruitment, and endotoxin clearance [15]. In Escherichia coli, the synthesis of LPS required rfa (also known as waa) operons that consist of many genes [16]. Three operons regulate the genes in the rfa locus. The first operon comprises rfaC (waaC), rfaD (or gmhD), rfaF (or waaF), and rfaL (or waaL) genes. Then, rfaB (or waaB), rfaG (or waaG), rfaI (or waaO), rfaJ (or warJ/waaJ), rfaK (or waaU), rfaP (or waaP), rfaQ (or waaQ), rfaS (or waaS), rfaY (or waaY), and rfaZ (or waaZ) are organized in the second operon. The remaining short kdtA operon consists of kdtA (or waaA) and kdtB (or coaD) [17,18,19,20]. In the LPS core biosynthesis pathway, rfaC has been proposed to play a crucial role in transferring heptose to the LPS core.
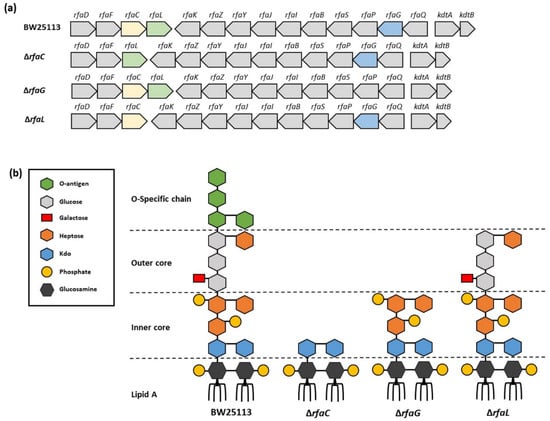
Figure 1.
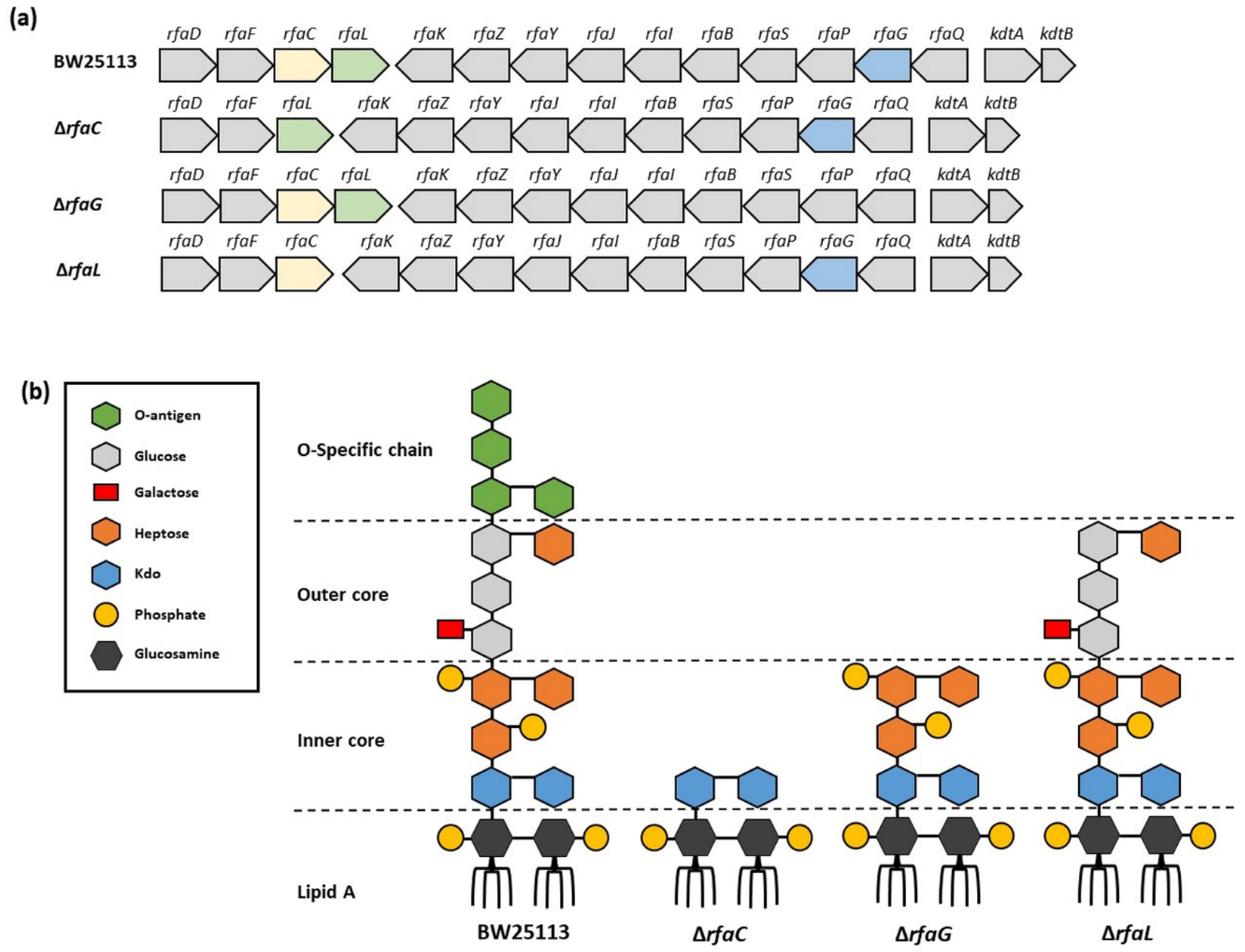
Lipopolysaccharide (LPS) architecture of OMVs. (a) OMV gene organization of E. coli BW25113, rfaC knockout (ΔrfaC), rfaG knockout (ΔrfaG), and rfaL knockout (ΔrfaL) strains, respectively; (b) LPS architecture of OMVs of E. coli BW25113, rfaC knockout (ΔrfaC), rfaG knockout (ΔrfaG), and rfaL knockout (ΔrfaL) strains, respectively. This figure is adapted from Pragnout et al. [17] and is licensed under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/, accessed on 28 December 2021).
Figure 1.
Lipopolysaccharide (LPS) architecture of OMVs. (a) OMV gene organization of E. coli BW25113, rfaC knockout (ΔrfaC), rfaG knockout (ΔrfaG), and rfaL knockout (ΔrfaL) strains, respectively; (b) LPS architecture of OMVs of E. coli BW25113, rfaC knockout (ΔrfaC), rfaG knockout (ΔrfaG), and rfaL knockout (ΔrfaL) strains, respectively. This figure is adapted from Pragnout et al. [17] and is licensed under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/, accessed on 28 December 2021).
The destruction of the LPS structure would influence the microbiological features of the bacteria and result in the alternation of the OMVs they released. Nakao et al. have reported that mutation in rfaC produced defective LPS in OMV but still maintained membrane integrity. E. coli with rfaC mutation generated OMV comparable to the wild type but produced more extracellular DNA (eDNA) in the culture, which is involved in initial attachment and biofilm formation as well as enhancing cell wall hydrophobicity [21,22]. Upon formation in the wound site, biofilms can have harmful effects including impaired epithelialization, granulation tissue formation, and reduced inflammatory response, which delays the healing process [23,24]. Several studies also mentioned that mutations in the rfa locus lead to severely truncated LPS and result in elevated antibiotic resistance, multidrug resistance, and resistance to several bacteriophages used in therapy for bacterial infections [25]. In burn injury patients, this phenomenon delays the wound healing process [26]. Another study reported that glycosyltransferase activity and O-antigen attachment to lipid A in E. coli was affected by mutations in the rfaL gene, thereby reducing biofilm formation [27]. Owing to the highly conserved inner-core composition of Gram-negative bacteria, the rfa gene may be a possible therapeutic target against infection.
Proteomic analysis of the entire set of proteins has been used to specify the composition of OMVs in several studies [1,15]. However, these studies remain limited, and only a few proteins have been recognized [6,7]. The main objective of this study was to elucidate the effect of LPS structure on OMV composition using the proteomics analysis. Based on the characteristics of the OMV genes above, we profiled rfaC, rfaG, and rfaL using quantitative proteomics to investigate E. coli outer membrane genes, and interpreted the effect of LPS structure on OMV composition. The novelty of this study is to provide proteomic data of the truncated RfaC, RfaG, and RfaL proteins in E. coli to support claims in previous publications that Rfa proteins could be a target for the treatment of the infected wounds of patients. An isobaric tag for relative and absolute quantitation (iTRAQ)-based proteomic analysis technique was employed to identify the expressed proteins between OMVs released by E. coli BW25113 and its mutant strains, including the rfaC-, rfaG-, and rfaL-defect strains. The functional classification of proteins and key pathways were analyzed by utilizing the Gene Ontology (GO) annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis, respectively. Our results provide essential information regarding the mechanism of the expressed genes and key pathways among proteins and E. coli strains.
2. Materials and Methods
2.1. Bacterial Strains, Cultures, and Growth Conditions
E. coli strain BW25113 and its mutants with deleted rfaC, rfaG, and rfaL genes were used (Figure 1, Table 1). All strains were purchased from Horizon Discovery (Cambridge, UK). The E. coli BW25113 strain is a K-12 derivative with a recA+ and hsdR genotype and the parent of the Keio collection of single-gene knockouts which has >100-fold higher transformation efficiency than the commonly used cloning E. coli hosts. The rfa genes in the mutant strains were deleted by using a one-step gene deletion method with the phage lambda Red recombinase. Bacteria were maintained and grown on agar at 37 °C with aeration.

Table 1.
Escherichia coli strains used in this study.
2.2. OMV Sample Preparation
OMVs were isolated from the late log-phase (16 h) culture of E. coli. In brief, 200 mL LB was inoculated with 2 mL overnight culture medium, incubated at 37 °C, and agitated by shaking at 200 rpm for 6 h. The cells were pelleted by centrifugation (10,000 rpm for 15 min) and the supernatant was filtered through a 0.22 μm membrane filter (JET BIOFIL, Guangzhou, China) to remove cells and cellular debris. The filtrate was subjected to ultracentrifugation (35,000 rpm) for 2 h at 4 °C using a Type 45 Ti rotor (Beckman, CA, USA). For washing the OMVs, the pellet was suspended in phosphate-buffered saline (PBS) and then ultracentrifuged (42,000 rpm) for 2 h at 4 °C using the same rotor. The pellet was finally resuspended in PBS and stored on ice. Three biological replicates of each experiment were performed in the following analysis.
2.3. Transmission Electron Microscopy (TEM)
Vesicles that were obtained were placed on a glow-discharged carbon-coated EM grid and allowed to rest briefly. They were then gently washed with deionized water twice and the grids were stained with 1% uranyl formate and dried at room temperature overnight. The grids were then observed using a transmission electron microscope (FEI Tecnai G2 F20 S-TWIN, FEI Company, Hillsboro, OR, USA) at 120 kV. TEM figures were visualized using the ImageJ software (National Institutes of Health, Bethesda, MD, USA) and analyzed using Student’s t-test. Diagrams were generated using the GraphPad Prism software (version 8.0, GraphPad software, San Diego, CA, USA) [28,29].
2.4. Nanoparticle Tracking Analysis (NTA) of OMVs
The quantities and sizes of OMVs were measured using the NanoSight NS500 nanoparticle tracking system (NTA) (Malvern Instruments Ltd., Malvern, Worcestershire, UK) supplied with a 488 nm blue laser and camera with a complementary metal-oxide semiconductor (CMOS) image sensor. Frozen OMV samples were thawed at room temperature and diluted 1/1000 in 50 mM HEPES buffer at pH 7.4 before analysis. Polystyrene beads (100 nm diameter) were used as the positive control and HEPES buffer alone served as the negative control. Samples were pumped into a NanoSight instrument using a syringe and set at the ‘20′ speed setting (in arbitrary units) on the NS500. The quantification was captured in five 60 s reads at room temperature (at approximately 23.9–25.2 °C), and the instrument was optimized at automatic setting (for ‘blur,’ minimum track length,’ and ‘minimum expected size’ setting), whereas viscosity was set to ‘water’ (0.883–0.911 cP). Camera level and focus for automated image setup were chosen for video enhancement, and the NTA software version 2.3 (Malvern Instruments Ltd., Malvern, Worcestershire, UK) was used to determine a total of 1498 frames per sample with a threshold of 5 (in arbitrary units). The data, including mean size (nm), mode size (nm), and concentration (particles/mL), were arranged, and an average of five reads was measured and plotted as particle size versus number of particles per Ml using Origin85 and the average size of OMVs was calculated using GraphPad Prism software [30].
2.5. Protein Extraction
The E. coli cultures were incubated at 37 °C for 16 h to acquire the total protein of OMVs. The cultures were centrifugated at 5000× g at 4 °C for 10 min, followed by protein extraction as previously described [24]. Trypsin digestion was then performed for 100 g of total cellular protein from each sample using Trypsin Gold (Promega, Madison, WI, USA) at a mass ratio of 30:1 trypsin-to-protein extract. Reactions were incubated at 37 °C for 16 h. Afterward, the digested proteins were dried using vacuum centrifugation [31].
2.6. iTRAQ Labeling and SCX Fractionation
First, 0.5 M triethylammonium bicarbonate (TEAB) was used to dissolve the dried peptide samples. Then, the peptide samples from E. coli cultures were labeled with iTRAQ reagents 114 and 116, respectively. The labeled proteins were incubated at room temperature for 2 h, then collected and dried using vacuum centrifugation. The protein labeled by iTRAQ was dissolved in 4 mL buffer A (25 mM NaH2PO4 in 25% ACN, pH 2.7) and separated using an LC-20AB HPLC pump system (Shimadzu, Kyoto, Japan) with Ultramex SCX column (4.6 mm × 250 mm, 5 µm, Phenomenex, Torrance, CA, USA). The sample was eluted by the linear gradient of buffer A for 10 min, 50–60% buffer B (25 mM NaH2PO4 and 1 M KCl in 25% ACN, pH 2.7) for 27 min, and the 60–100% buffer B for 1 min. Subsequently, the peptide was eluted every minute to 20 fractions at a flow rate of 1 mL/min and an absorbance wavelength of 214 nm. Finally, the protein fractions were desalted using Strata X C18 (Phenomenex, Torrance, CA, USA) and dried using vacuum centrifugation [32].
2.7. LC–MS/MS Analysis
The labeled protein fractions that were diluted in 40 µL 0.1% (v/v) trifluoroacetic acid were introduced to a nanoLC–MS/MS for analysis (Q Exactive mass spectrometer, Thermo Fisher Scientific, Waltham, MA, USA), performed in positive ion mode coupled with Easy nLC (Thermo Fisher Scientific, Waltham, MA, USA) for 60 min. MS data were obtained using a data-independent top 10 method. Furthermore, automatic gain control (ACG) target was set to 1e6 (1e6 = 1,000,000), the maximum inject time to 50 ms, and the duration of Dynamic exclusion was 60 s. Survey scans were obtained at a resolution of 70,000 and m/z 200, and isolation width was 2 m/z. Normalized collision energy was 30 eV, and underfill ratio, which determines the minimum percentage of the target value possible to be gained at the maximum fill time, was determined as 0.1%. The instrument was performed with peptide identification mode permitted [33].
2.8. Proteomic and Bioinformatic Analysis
Protein characterization was conducted using Mascot® (version 2.2; Matrix Science, MA, USA) and the Proteome Discoverer™ software (version 1.4; Thermo Scientific, Waltham, MA, USA) using the sequences from the UniProt Human Database (133,549 sequences, downloaded on 3 March 2013). In this method, the parameters used were mass tolerance = 20 ppm, MS/MS tolerance = 0.1 Da, enzyme = trypsin, missed cleavage—2, oxidation (M), iTRAQ 8plex (Y) as the possible variable modifications, and carbomidomethyl (C), iTRAQ 8plex (N-term), iTRAQ 8plex (K) as the permanent modifications. The calculation for false discovery rate (FDR) of peptide characterization used a bait database search at a filtering basis of FDR ≤0.01. The iTRAQ ratio between the two groups of >1.2 or <0.83 defined the differential protein expression, and all of the diversely expressed RfaC, RfaG, and RfaL proteins were examined using UniProt (http://www.uniprot.org/; accessed on 28 December 2021) [34].
A Venn diagram was generated to elaborate the general diversely expressed proteins between the OMVs released by E. coli wild-type (BW25113) and its mutant strains. The cross-comparison of the gene names generated Venn diagrams and sets of gene lists are shown in Table 2. GO analysis (version go_201608.obo; www.geneontology.org; accessed on 28 December 2021) was used to examine the biological importance of the distinct expressed proteins. Furthermore, the distinct expressed proteins were entangled in the identical process; function and components were distributed into corresponding clusters. KEGG pathway analysis was carried out to explore the potential of the biological pathways using the online software (KEGG Automatic Annotation Server (KAAAS)) [33].

Table 2.
Unique and shared genes between ΔrfaC/BW25113: ΔrfaG/BW25113: ΔrfaL/BW25113.
2.9. Statistical Analysis
All data were statistically analyzed using IBM® SPSS® Software Version 18.0 (IBM Corp., Armonk, NY, USA) and expressed as mean ± standard error. Data were analyzed using one-way analysis of variance or a two-tailed paired t-test. Significant differences between groups were detected using * p < 0.05 and ** p < 0.01 to indicate statistical significance.
3. Results
3.1. TEM Analysis of OMVs Released from E. Coli BW25113 and Mutants
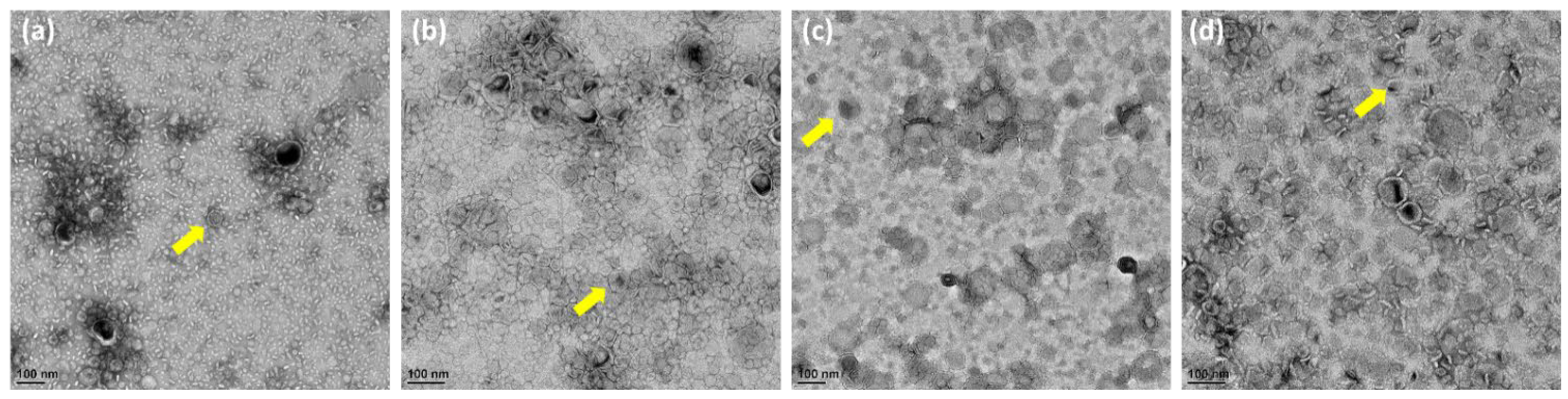
Generally, in Figure 2a–d, OMVs were not uniform in size. High magnification TEM images of OMVs showed spherical particles. However, the overall sample quantification data showed that the size of the OMVs released by the ΔrfaG strain was significantly larger than that of E. coli BW25113, whereas OMVs released by the ΔrfaC and ΔrfaL strains were significantly smaller than that of E. coli BW25113.
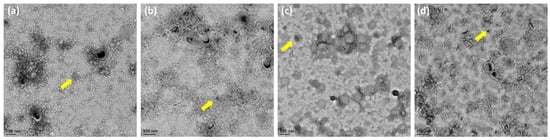
Figure 2.
Morphology and size of OMVs from WT and mutant E. coli. (a–d) Transmission electron microscopy observation of E. coli BW25113, rfaC knockout (ΔrfaC), rfaG knockout (ΔrfaG), and rfaL knockout (ΔrfaL) strains, respectively. The yellow arrows show the example of observed OMV.
3.2. NTA Analysis of OMVs from WT and Mutant E. Coli
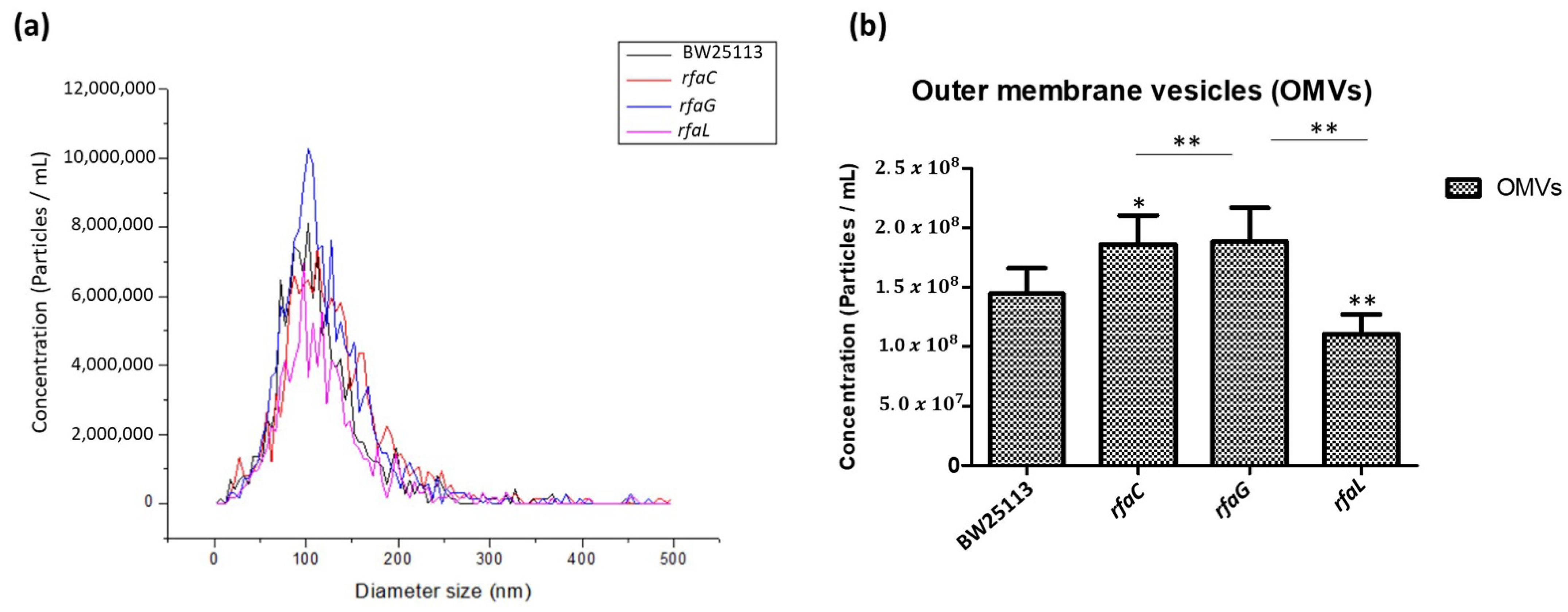
To further determine the distribution of OMV size released by the E. coli BW25113 and its mutant strains, we analyzed the heterogenous population of OMVs using NTA. The results revealed that the size distribution of OMVs in each group ranged from 50 to 200 nm in diameter, but the majority were 100 nm (Figure 3a). Furthermore, the histogram data showed that OMVs released by the ΔrfaC and ΔrfaG strains were significantly larger in diameter compared with those of the ΔrfaL strain (Figure 3b).
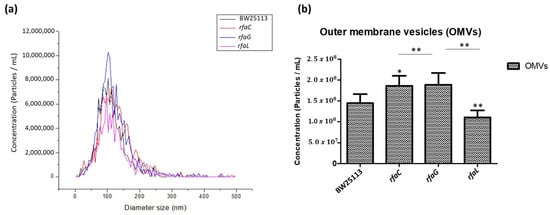
Figure 3.
Small and large outer membrane vesicle (OMV) populations purified from a heterogeneous population of OMVs. (a) OMV population diameter sizes (nm) of E. coli BW25113, rfaC knockout (ΔrfaC), rfaG knockout (ΔrfaG), and rfaL knockout (ΔrfaL) strains, respectively, were evaluated using Nanoparticle Tracking Analysis (NTA); (b) average diameter size of E. coli BW25113, rfaC knockout (ΔrfaC), rfaG knockout (ΔrfaG), and rfaL knockout (ΔrfaL) OMVs presented in nm in each concentration (particle/mL). * p < 0.05 and ** p < 0.01 indicate statistical significance.
3.3. Proteomic Analysis of the OMV Fraction from E. Coli
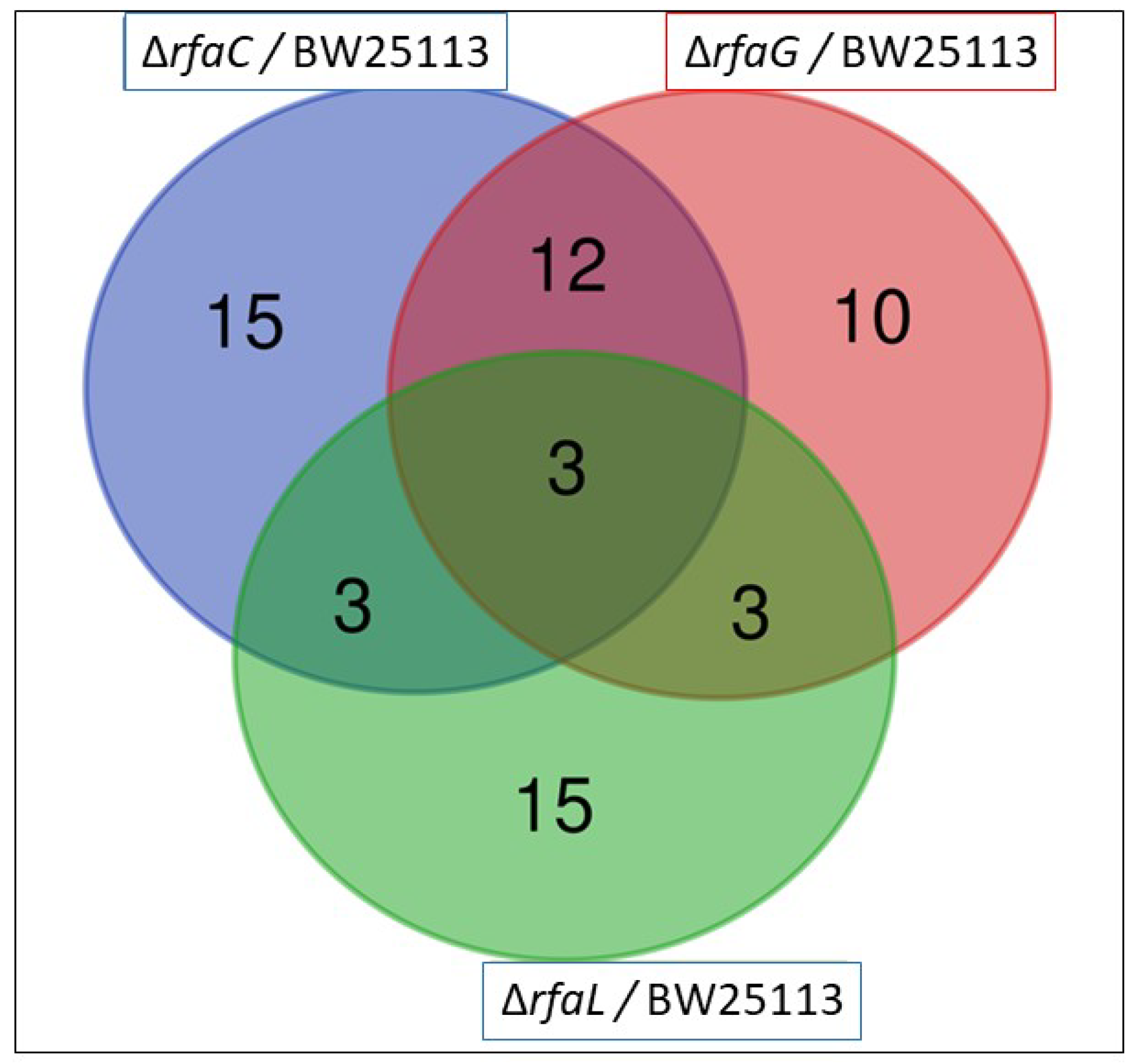
The OMV pan-proteome was determined after the identification of clusters of orthologous groups (COG) (Figure 4 and Table 2). Individual cross-comparison of shared genes from Venn diagrams are summarized in Table 2. The identified proteins were grouped into 33, 28, and 24 clusters of orthologous genes for OMVs purified from E. coli BW25113 and mutant strains (ΔrfaC, ΔrfaG, and ΔrfaL genes, respectively) (Figure 4). Only 3 clusters containing RpsT, GalF, and RpmB proteins were shared among all the strains (Table 2). The RpsT protein, which defines the 30S ribosomal protein S20 OS, was downregulated in E. coli mutants (ΔrfaC log2 ratio = −2.124; ΔrfaC normalized ratio = 0.229; ΔrfaG log2 ratio = −1.986; ΔrfaG normalized ratio = 0.253; ΔrfaL log2 ratio = −1.968; and ΔrfaC normalized ratio = 0.256). The 50S ribosomal protein L28 OS, defined by the RpmB protein, was also downregulated in all mutants (ΔrfaC log2 ratio = −2.181; ΔrfaC normalized ratio = 0.220; ΔrfaG log2 ratio = −3.315; ΔrfaG normalized ratio = 0.100; ΔrfaL log2 ratio = −2.844; and ΔrfaL normalized ratio = 0.139). Further, the GalF protein upregulated UTP-glucose−1-phosphate uridylyl transferase OS in E. coli BW25113 mutants (ΔrfaC log2 ratio = 1.792; ΔrfaC normalized ratio = 3.463; ΔrfaG log2 ratio = 1.588; ΔrfaC normalized ratio = 3.006; ΔrfaL log2 ratio = 1.317; and ΔrfaL normalized ratio = 2.492). Venn diagrams showing shared and unique genes in E. coli BW25113, rfaC knockout (ΔrfaC), rfaG knockout (ΔrfaG), and rfaL knockout (ΔrfaC) strains were rpsT, galF, and rpmB, respectively. The log2 fold values were shown in Table 3.
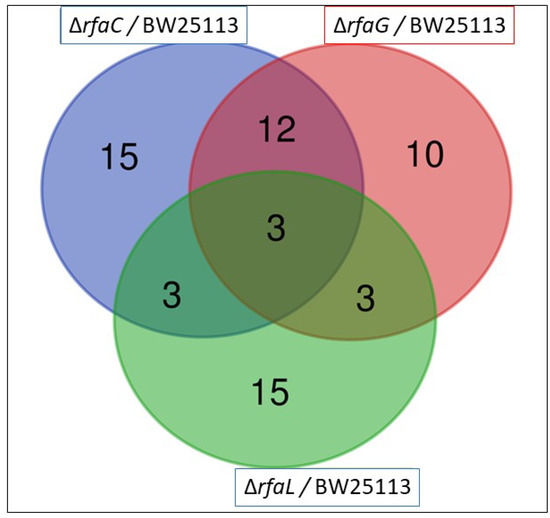
Figure 4.
Venn diagram of the existence of the OMV genes in E. coli BW25113, rfaC knockout (ΔrfaC), rfaG knockout (ΔrfaG), and rfaL knockout (ΔrfaC) strains. Three strains encompassing rpsT, galF, and rpmB existed in the three E. coli mutants.

Table 3.
Differentially expressed genes related with OMVs of genes in E. coli BW25113, rfaC knockout (ΔrfaC), rfaG knockout (ΔrfaG), and rfaL knockout (ΔrfaL) strains.
3.4. Heat Map Analysis of Mutant Strains Compared to E. Coli BW25113 Strain
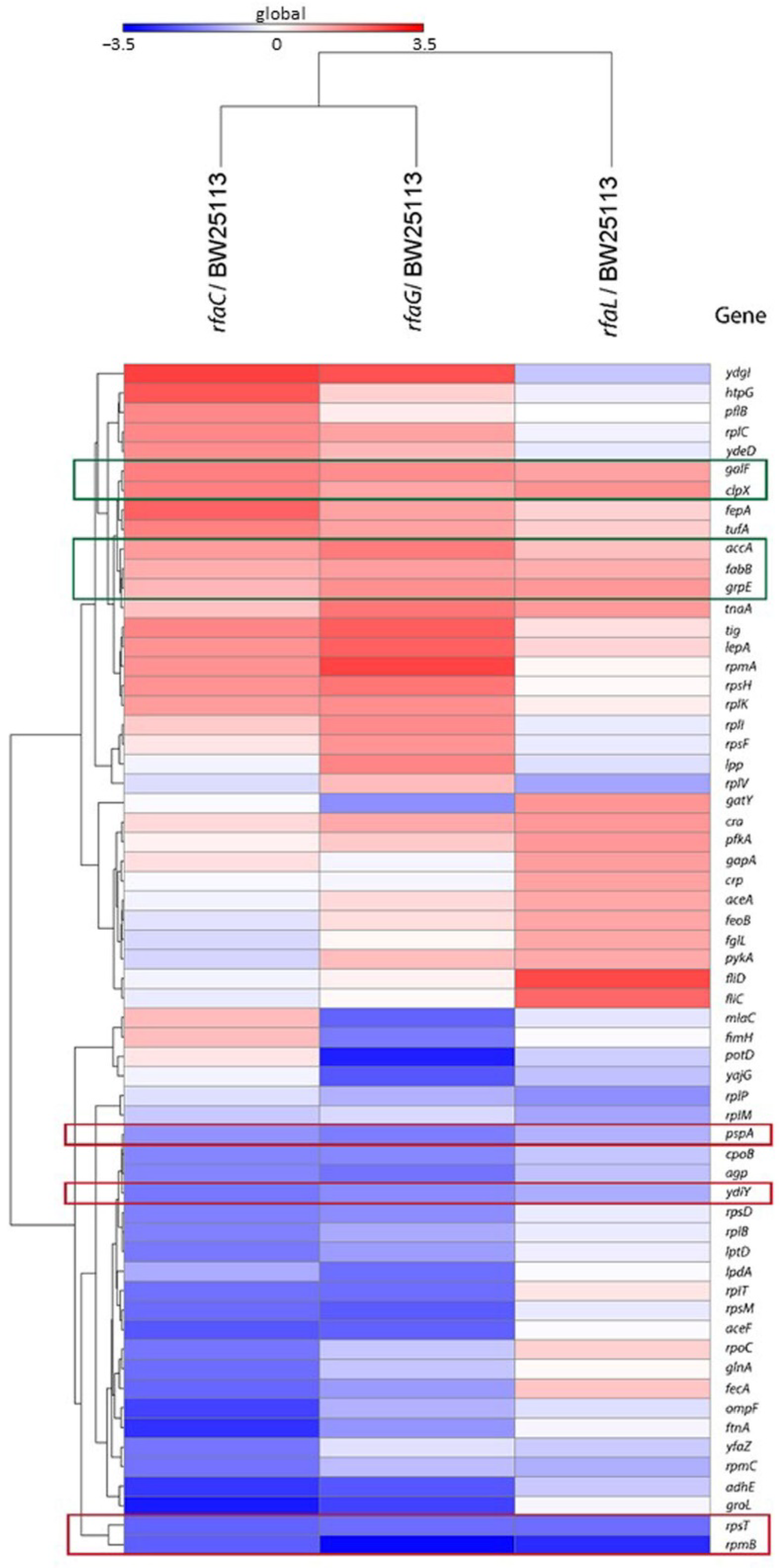
Heat map analysis showed that GalF, ClpX, AccA, FabB, and GrpE proteins were upregulated among the OMVs released by the mutant strains compared with the E. coli BW25113 (Figure 5). In contrast, PspA, YdiY, RpsT, and RpmB proteins were found to be downregulated in mutant strains compared with E. coli BW25113.
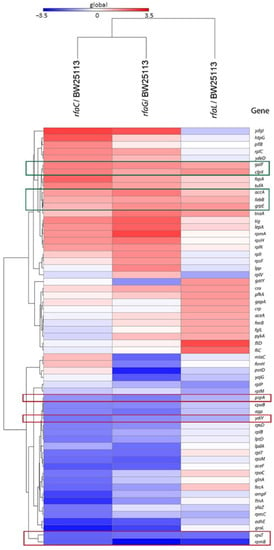
Figure 5.
Heat map analysis of E. coli BW25113 mutant strains (rfaC knockout (ΔrfaC), rfaG knockout (ΔrfaG), and rfaL knockout (ΔrfaL) strains) genes shared with WT E. coli BW25113 strain. Red frames are for downregulated genes, whereas the green frames are the upregulated genes.
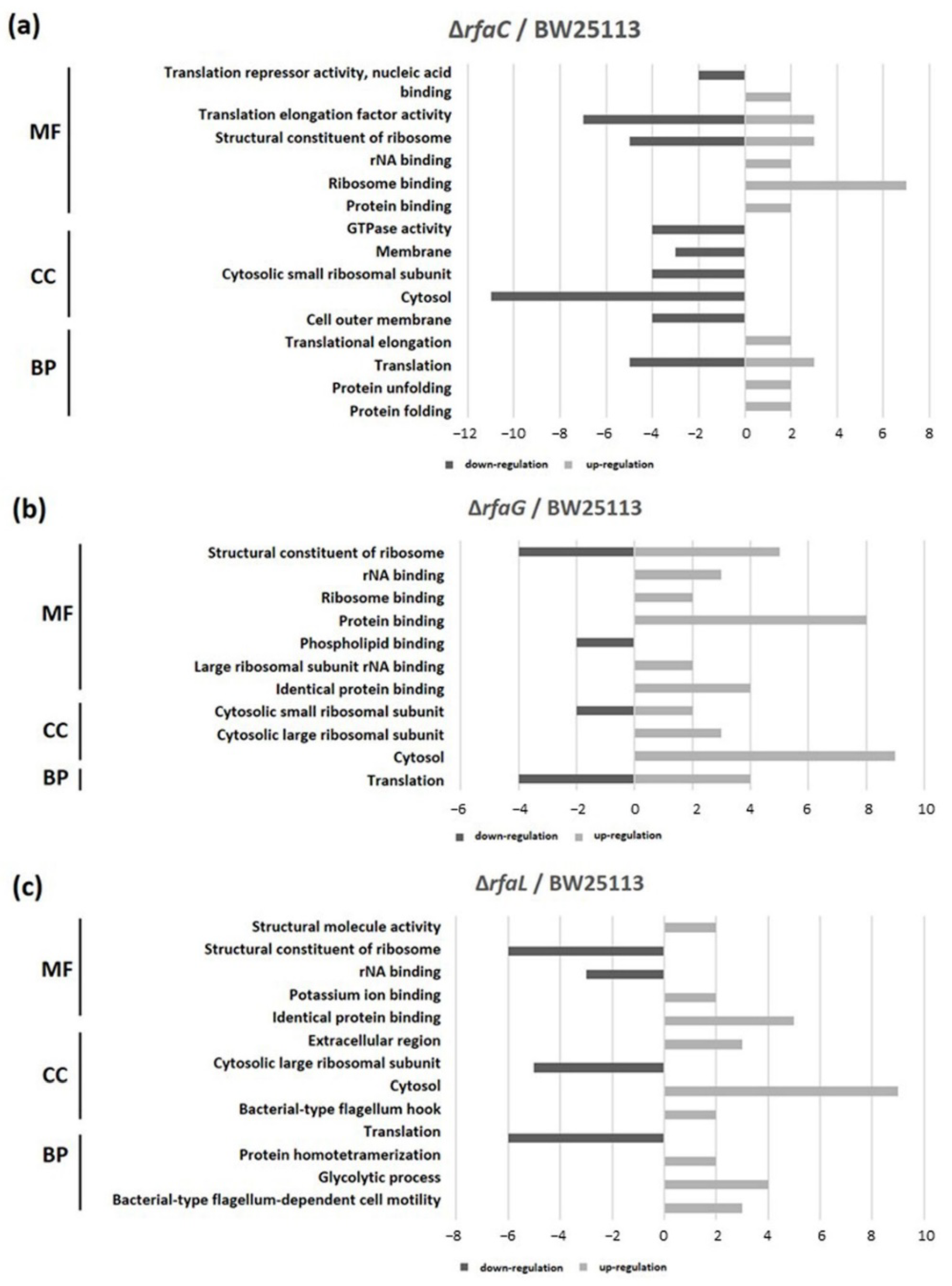
3.5. GO Bar Chart of Mutants Versus E. Coli BW25113 strains
GO classification revealed that protein binding was the most shared in the OMVs of ΔrfaC and that of BW25113 strain, whereas structural constituent of ribosome and rRNA binding were the least shared in molecular function (MF). Cytosol and translation clusters of the ΔrfaC strain were downregulated in the cellular component (CC) and biological process (BP), respectively (Figure 6a). Between ΔrfaG and BW25113 strains, protein binding and structural constituent of ribosome were upregulated in MF, and cytosol was abundant in CC (Figure 6b). Additionally, for the ΔrfaL strain versus wild-type BW25113, cytosol and identical protein binding were the most prevalent in CC and MF, respectively. In addition, the structural constituent of ribosome, translation, and cytosolic large ribosomal subunit were strongly downregulated between ΔrfaL and BW25113 strains (Figure 6c).
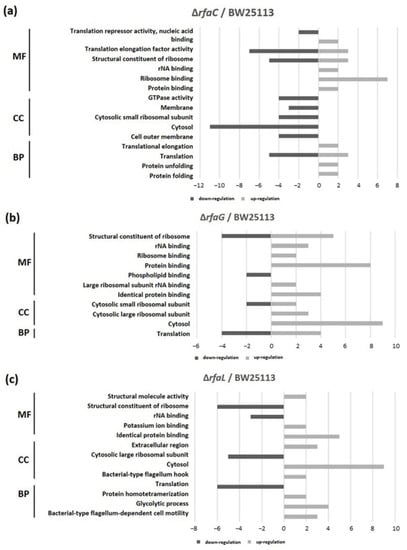
Figure 6.
Functional classification of OMV proteins. Gene ontology (GO) classification was conducted to determine protein content. (a) E. coli BW25113 ΔrfaC strain and E. coli BW25113 shared clusters. (b) E. coli BW25113 ΔrfaG strain and E. coli BW25113 shared clusters. (c) E. coli BW25113 ΔrfaL strain and E. coli BW25113 shared clusters. MF: Molecular Function; CC: Cellular Component; BP: Biological Process.
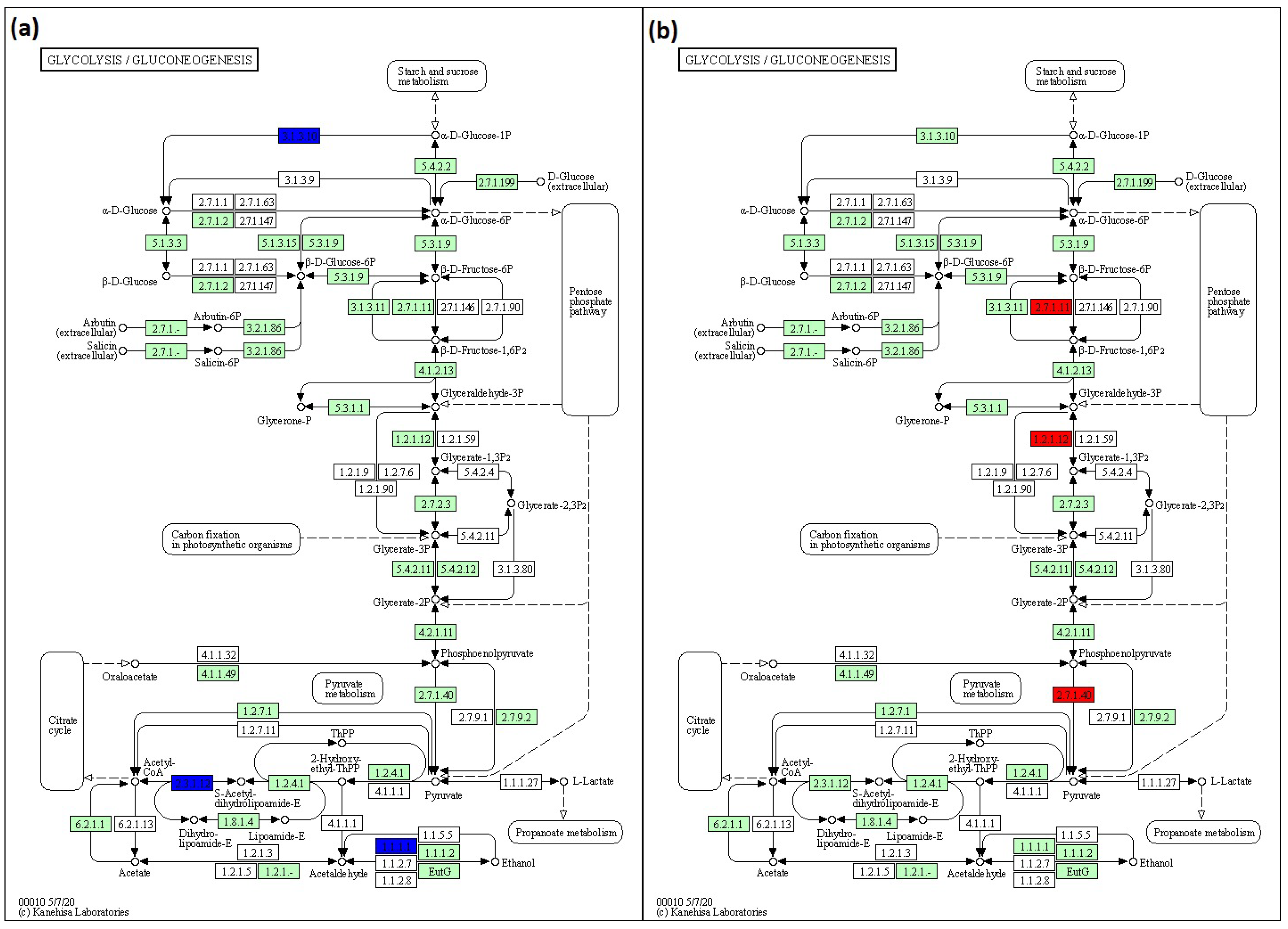
3.6. Pathways Predicted by KEGG
To predict pathways, KEGG analysis was conducted. In glycolysis/glucogenesis, lack of rfaC and rfaG genes in E. coli BW25113 was found to downregulate acyl carrier protein (ACP), acetyltransferase component of pyruvate dehydrogenase (ACEF), and aldehyde-alcohol dehydrogenase (ADHE) genes. The downregulation pathways in BW25113 mutants influenced by the lack of rfaC and rfaG genes were depicted in KEGG pathway maps as 3.1.3.10, 2.3.1.12, and 1.1.1.1 for ACP, ACEF, and ADHE, respectively (Figure 7a). The normalized ratios of the rfaC gene versus BW25113 were 0.319, 0.205, and 0.152 for ACP, ACEF, and ADHE, respectively. The normalized ratios of the rfaG gene lacking from BW25113 were 0.270, 0.227, and 0.207 for ACP, ACEF, and ADHE, respectively. In addition, the lack of the rfaL gene upregulated phosphofructokinase isozyme (PFKA), glyceraldehyde-3-phosphate dehydrogenase-A (GAPA), and pyruvate kinase isozyme (PYKA) in the BW25113 mutant in glycolysis/glucogenesis. The items in the KEGG pathway map displayed the abundance of those pathways using 2.7.1.11, 1.2.1.12, and 2.7.1.40 map items (Figure 7b). A deleted rfaL gene resulted in 2.721, 2.589, and 2.313 normalized ratios for PFKA, GAPA, and PYKA, respectively.
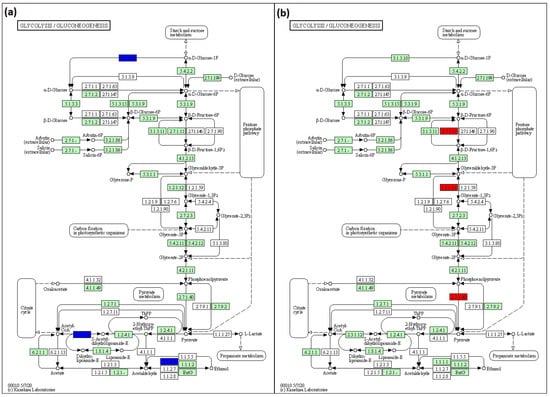
Figure 7.
KEGG pathway maps for predicting pathways influenced by the lack of (a) rfaC and rfaG, and (b) rfaL OMV genes in E. coli BW25113. This pathway is produced using KEGG Mapper online software (https://www.genome.jp/kegg/mapper/color.html; accessed on 28 December 2021).
4. Discussion
Vesicles obtained from the outer membrane of Gram-negative bacteria are known as OMVs and are highly diverse in size, composition, and function [35]. In this study, TEM was used to determine the appearance and size of OMVs released by E. coli BW25113 and rfaC, rfaG, and rfaL knockout strains (Figure 2a–d). Moreover, using NTA, the diameter of OMVs in all strains was shown to range from 50–200 nm (Figure 2a), with the highest mostly found in E. coli lacking rfaC, and rfaG, significantly different from OMVs released by E. coli BW25113 and E. coli lacking rfaL (Figure 2b). In addition, our NTA results showed that OMVs released by E. coli BW25113 and its mutant strains ranged between 50–200 nm in diameter, with the majority being 100 nm (Figure 3a). Additionally, the OMVs of ΔrfaC and ΔrfaG were found to be larger than those of BW25113 and ΔrfaL (Figure 3b). These results indicate that secreted OMVs have diverse functions. Turner et al. mentioned that OMV size is dependent on protein content and composition [30]. The smaller OMV fraction contained less protein than the larger OMV fraction and the heterogenous population of OMVs. Generally, the larger OMVs contained more adhesion protein for virulence, whereas small OMVs contained protein predominantly for metabolism. The size of OMVs also determined the mechanism and efficiency of cellular entry of the OMV into the host cell. Smaller OMVs are known to enter the cell through the micropinocytosis pathway, whereas larger OMVs enter the cell preferentially via the clathrin and dynamin-mediated pathway. Inhibition of clathrin-mediated endocytosis has no effect on enterotoxigenicity of E. coli OMVs [30,36].
Furthermore, in the proteomics study, the genes rpsT, galF, and rpmB were identified as shared among cluster mutants and E. coli BW25113 (Figure 4). Our results revealed that rpsT, which defines the 30S ribosomal protein S20 OS, and 50S ribosomal protein L28 OS that is encoded by the rpmB gene, were downregulated in both mutant and E. coli BW25113 strains. On the other hand, galF upregulated UTP-glucose-1-phosphate uridylyl transferase OS in E. coli BW25113 mutants. These proteomic data were consistent with the heat map analysis results. GalF, ClpX, AccA, FabB, and GrpE appeared to be upregulated among all OMV proteins in the E. coli BW25113 and mutant strains (Figure 5). This result likely suggests that rfaC, rfaG, and rfaL are not directly responsible for fatty acid biosynthesis and elongation together with AccA and FabB, respectively [37,38]. Furthermore, GalF proteins for cellular UDP-glucose formation and GrpE for protein folding and thermos-sensing demonstrate a high protein expression following OMV protein knockout (ΔrfaC, ΔrfaG, ΔrfaL) in mutant and E. coli BW25113 strains, indicating that UDP-glucose formation and heat shock response genes continue to function well without the presence of OMV proteins [39,40]. However, the downregulation of stress-related proteins such as PspA and YdiY [41,42] is likely attributable to their relationship with OMV protein construction, and the suppression of the aforementioned gene transcripts or protein expression by OMV protein knockout. Moreover, upregulation of the ClpX protein and downregulation of ribosomal proteins such as RpsT and RpmB are suggested to occur because of the involvement of these OMV genes in ribosomal 30S and 50S synthesis, respectively [43,44].
The results of GO exhibited that the binding protein is the most widely shared protein in ΔrfaC and E. coli BW25113 strains, whereas the structural constituent of the ribosome and rRNA binding are the least conserved. Moreover, cytosol and translation clusters of ΔrfaC in CC and BP, respectively are downregulated (Figure 6a). These data are reasonable considering the importance of OMVs in supporting the unique architectures corresponding to protein transport, genetic material transfer, interkingdom communication, antibacterial activity, neutralizing phage decoy activity, virulence factor delivery, and immune response modulation that involve binding activity [8,9,10,11,12,13,14]. Furthermore, the expression of protein binding and structural constituent of ribosomes is upregulated in MF and cytosol was abundant in CC between ΔrfaG and E. coli BW25113 strains (Figure 6b). These results indicate that rfaG is not directly involved in outer membrane and OMV synthesis, and possibly acts in other pathways owing to its glycosyltransferase activity [45]. However, the ΔrfaL strain versus E. coli BW25113 strain data showed substantial downregulation of the structural constituent of the ribosome, translation, and cytosolic large ribosomal subunit (Figure 6c). RfaL is known to encode a component of the O ligase, which transfers the completed O-antigen from the ACL to the core of a suitable LPS acceptor. Furthermore, it is involved in core modification, and plays a significant role in producing core heterogeneity, as well as O-antigen attachment [46]. The O-antigen is important for Gram-negative bacteria as it functions in targeting both the innate and adaptive immune systems in pathogenicity [47].
KEGG was also used to predict the pathway of glycolysis/glucogenesis since rfaC, rfaG, and rfaL were involved in LPS core glycosylation. The data revealed that ΔrfaC and ΔrfaG downregulate ACP, ACEF, and ADHE genes (Figure 7a). These results were likely attributable to the roles of ACP, ACEF, and ADHE in the transfer of acyl fatty acid during phospholipid synthesis, pyruvate dehydrogenase, and aldehyde dehydrogenase, respectively [48,49,50]. These proteins are possibly involved in LPS construction, which is implicated in the regulation of rfaC, rfaG, and rfaL genes. While the misfolded protein produced by a truncated set of genes will be retained in the ER and processed for ER-related degradation [51], the deletion of rfa genes may lead to the further interference of the membrane’s subsequent function [50]. Moreover, glycosylation deficiencies in the LPS protein production also disrupt protein localization in ER, which affects the functional membrane of protein [52]. In addition, the KEGG prediction showed high expression of PFKA, GAPA, and PYKA in E. coli by rfaL gene knockout (Figure 7b). PFKA, GAPA, and PYKA are involved in fructose phosphorylation, pyruvate formation, and GAPDH production (glycolysis pathway), respectively [53,54,55]. From these results, it is suggested that rfaL gene is not involved in energy production because of its role in E. coli LPS synthesis.
Regarding the involvement of rfaC, rfaG, and rfaL genes in E. coli LPS synthesis, a recent study mentioned that the small amount of clinically relevant Gram-negative human pathogen bacterial inoculum may cause bacteremia and eventually lead to death. E. coli infection in burn wound has been shown to lead to bacteremia at 24 to 48 h and death after 3 to 4 days [56]. This finding was also supported by Crompton et al., who found that in the early phase of healing, wounds treated by LPS exhibited apoptosis and reduction in local proliferation, thereby showing that contact between LPS and the wound delayed the healing process [57]. In addition, some previous studies mentioned links between glycolysis and immune system response. OMV LPS was capable of inducing macrophage metabolism shift from oxidative phosphorylation (OXPHOS) to glycolysis, which was supported by decreased mitochondrial oxygen consumption and reduced respiration activity, as well as increased mitochondrial reactive oxygen species production [58]. Thus, LPS-induced glycolysis at the wound site heightened infection by inhibiting dendritic cell maturation and inducing inflammasome activation [59,60].
Considering bacterial involvement in infection, biofilm formation could lead to further wound chronicity and delayed healing [61,62]. A previous study reported that absence of the genes responsible for generating OMV LPS, such as rfaC, leads to an increase in biofilm production and bacterial pathogenicity [21]. Biofilm formation may inhibit wound healing owing to a 10-fold increase in interleukin-1β (IL-1β), interleukin-6 (IL-6), and matrix metalloprotease-10 (MMP-10) expression, indicative of persistent inflammatory response and delayed healing process [63,64]. Furthermore, chronic wound biofilms can be highly tolerant and resistant to antibiotics owing to the formation of a shield to protect bacteria from the phagocytic activity of invading polymorphonuclear neutrophils (PMNs). rfaG gene mutation also resulted in OMV destabilization due to reduced core phosphorylation and LPS length [65,66]. This mutation also leads to a L-glycero-α-D-manno heptose (heptose) deficit that impairs the growth of E. coli [67]. Furthermore, due to the function of the rfaL gene in O-antigen biosynthesis, mutation of rfaL gene interferes with the transfer process of polymerized O-antigen to the lipid A core to form LPS [68]. Moreover, O-glycosylation-defective Gram-negative bacteria did not show any growth deficiency, but a tremendously diminished capacity to generate biofilm, thereby reducing its resistance against antibiotics, and affecting its survival ability in the host [69]. Hence, by deleting rfa genes in E. coli, we identified their association with OMV LPS biosynthesis, O-antigen glycosylation and biofilm formation ability. These findings can provide excellent targets for the identification of rfa gene inhibitors for the development of new antibiotics to enhance the wound healing process.
5. Conclusions
This study concludes that the OMV defects of constituent proteins RfaC, RfaG, and RfaL were upregulated, and downregulated important cellular proteins, and that they may play important roles in the glycolysis/glucogenesis pathway in E. coli. Hence, all findings in this study highlight that rfaG, rfaC, and rfaL genes are responsible for LPS synthesis as the component of OMV. Our findings also suggest that OMV contact with the injury area may inhibit the wound healing process by hindering the inflammatory response at the wound site.
Author Contributions
Conceptualization, Y.-C.W. and Y.-K.C.; methodology, Y.-C.W.; software, Y.-K.C. and T.Y.; validation, S.-J.C. and Y.-C.W.; formal analysis, Y.-K.C., T.Y. and Y.-T.L.; investigation, Y.-K.C. and Y.-C.W.; resources, Y.-C.W. and K.-H.M.; data curation, T.Y., Y.-T.L. and S.-J.C.; writing—original draft preparation, Y.-K.C.; writing—review and editing, K.-H.M.; visualization, Y.-K.C.; supervision, K.-H.M.; project administration, Y.-C.W.; funding acquisition, Y.-K.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministry of National Defense—Medical Affairs Bureau (MND-MAB-110-153) and Tri-Service General Hospital (TSGH-SS-D-109015; TSGH-SS-D-109001; TSGH-SS-D-110015; TSGH-E-110205; TSGH-E-111245).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Acknowledgments
We thank the Academia Sinica Biological Electron Microscopy Core Facility for EM technical support. The core facility is funded by the Academia Sinica Core Facility and Innovative Instrument Project (AS-CFII-111-203).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Socorro, R.M.D.; Avila-Calderón, E.D.; Aguilera-Arreola, M.G.; López-Merino, A.; Ruiz, E.A.; Morales-García, M.D.R.; López-Villegas, E.O.; Gomez-Lunar, Z.; Arellano-Reynoso, B.; Contreras-Rodríguez, A. Comparative proteomic analysis of outer membrane vesicles from Brucella suis, Brucella ovis, Brucella canis and Brucella neotomae. Arch. Microbiol. 2021, 203, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Bang, J.Y.; Park, G.W.; Choi, D.S.; Kang, J.S.; Kim, H.J.; Park, K.S.; Lee, J.O.; Kim, Y.K.; Kwon, K.H.; et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 2007, 7, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, M.J.; Kesty, N.C. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005, 19, 2645–2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horstman, A.L.; Kuehn, M.J. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 2000, 275, 12489–12496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wai, S.N.; Lindmark, B.; Söderblom, T.; Takade, A.; Westermark, M.; Oscarsson, J.; Jass, J.; Richter-Dahlfors, A.; Mizunoe, Y.; Uhlin, B.E. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 2003, 115, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Nevot, M.; Deroncelé, V.; Messner, P.; Guinea, J.; Mercadé, E. Characterization of outer membrane vesicles released by the psychrotolerant bacterium Pseudoalteromonas antarctica NF3. Environ. Microbiol. 2006, 8, 1523–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauman, S.J.; Kuehn, M.J. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 2006, 8, 2400–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Veith, P.D.; Chen, Y.Y.; Gorasia, D.G.; Chen, D.; Glew, M.D.; O’Brien-Simpson, N.M.; Cecil, J.D.; Holden, J.A.; Reynolds, E.C. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J. Proteome Res. 2014, 13, 2420–2432. [Google Scholar] [CrossRef]
- Bitto, N.J.; Chapman, R.; Pidot, S.; Costin, A.; Lo, C.; Choi, J.; D’Cruze, T.; Reynolds, E.C.; Dashper, S.G.; Turnbull, L.; et al. Bacterial membrane vesicles transport their DNA cargo into host cells. Sci. Rep. 2017, 7, 7072. [Google Scholar] [CrossRef]
- Augustyniak, D.; Seredyński, R.; McClean, S.; Roszkowiak, J.; Roszniowski, B.; Smith, D.L.; Drulis-Kaw, Z.; Mackiewicz, P. Virulence factors of Moraxella catarrhalis outer membrane vesicles are major targets for cross-reactive antibodies and have adapted during evolution. Sci. Rep. 2018, 8, 4955. [Google Scholar] [CrossRef] [Green Version]
- Backert, S.; Bernegger, S.; Skórko-Glonek, J.; Wessler, S. Extracellular HtrA serine proteases: An emerging new strategy in bacterial pathogenesis. Cell Microbiol. 2018, 20, 12845. [Google Scholar] [CrossRef] [Green Version]
- Maerz, J.K.; Steimle, A.; Lange, A.; Bender, A.; Fehrenbacher, B.; Frick, J.S. Outer membrane vesicles blebbing contributes to B. vulgatus mpk-mediated immune response silencing. Gut Microbes 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Robles, T.; Dillard, R.S.; Cairns, L.S.; Silva-Valenzuela, C.A.; Housman, M.; Ali, A.; Wright, E.R.; Camilli, A. Vibrio cholerae outer membrane vesicles inhibit bacteriophage infection. J. Bacteriol. 2018, 200, e00792-17. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.R.; Stewart, N.A.; Veenstra, T.D. Chapter 8—Proteomics: The Deciphering of the Functional Genome. In Essentials of Genomic and Personalized Medicine; Ginsburg, G.S., Willard, H.F., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 89–96. ISBN 9780123749345. [Google Scholar]
- Karp, P.D.; Riley, M.; Saier, M.; Paulsen, I.T.; Collado-Vides, J.; Paley, S.M.; Pellegrini-Toole, A.; Bonavides, C.; Gama-Castro, S. The EcoCyc database. Nucleic Acids Res. 2002, 30, 56–58. [Google Scholar] [CrossRef] [Green Version]
- Pagnout, C.; Sohm, B.; Razafitianamaharavo, A.; Caillet, C.; Offroy, M.; Leduc, M.; Gendre, H.; Jomini, S.; Beaussart, A.; Bauda, P.; et al. Pleiotropic effects of rfa-gene mutations on Escherichia coli envelope properties. Sci. Rep. 2019, 9, 9696. [Google Scholar] [CrossRef] [Green Version]
- Schnaitman, C.A.; Klena, J.D. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol. Rev. 1993, 57, 655–682. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog. Lipid Res. 2010, 49, 97–107. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Ren, G.; Li, Y.; Wang, X. Influence of core oligosaccharide of lipopolysaccharide to outer membrane behavior of Escherichia coli. Mar. Drugs 2015, 13, 3325–3339. [Google Scholar] [CrossRef] [Green Version]
- Nakao, R.; Ramstedt, M.; Wai, S.N.; Uhli, B.E. Enhanced biofilm formation by Escherichia coli LPS mutants defective in Hep biosynthesis. PLoS ONE 2012, 7, e51241. [Google Scholar] [CrossRef] [Green Version]
- Das, T.; Sharma, P.K.; Busscher, H.J.; van der Mei, H.C.; Krom, B.P. Role of extracellular DNA in initial bacterial adhesion and surface aggregation. Appl. Environ. Microbiol. 2010, 76, 3405–3408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalf, D.G.; Bowler, P.G. Biofilm delays wound healing: A review of the evidence. Burn. Trauma 2013, 1, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Usui, M.L.; Underwood, R.A.; Singh, P.K.; James, G.A.; Stewart, P.S.; Fleck, P.; Olerud, J.E. Time course study of delayed wound healing in a biofilm-challenged diabetic mouse model. Wound Repair Regen. 2012, 20, 342–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, A.M.; Cerqueira, M.A.; Bañobre-Lópes, M.; Pastrana, L.M.; Sillankorva, S. Bacteriophages for chronic wound treatment: From traditional to novel delivery systems. Viruses 2020, 12, 235. [Google Scholar] [CrossRef] [Green Version]
- Bessa, L.J.; Fazii, P.; Di-Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; van Duin, D. Bacterial infections after burn injuries: Impact of multidrug resistance. Clin. Infect. Dis. 2017, 65, 2130–2136. [Google Scholar] [CrossRef]
- Feldman, M.F.; Wacker, M.; Hernandez, M.; Hitchen, P.G.; Marolda, C.L.; Kowarik, M.; Morris, H.R.; Dell, A.; Valvano, M.A.; Aebi, M. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc. Natl. Acad. Sci. USA 2005, 102, 3016–3021. [Google Scholar] [CrossRef] [Green Version]
- Ritcher, R.; Kamal, M.A.M.; Koch, M.; Niebuur, B.J.; Huber, A.L.; Goes, A.; Volz, C.; Vergalli, J.; Kraus, T.; Müller, R.; et al. An outer membrane vesicle-based permeation assay (OMPA) for assessing bacterial bioavaibility. Adv. Health Mat. 2021, 11, 2101180. [Google Scholar]
- Turner, L.; Bitto, N.J.; Steer, D.L.; Lo, C.; D’Costa, K.; Ramm, G.; Shambrook, M.; Hill, A.F.; Ferrero, R.L.; Kaparakis-Liaskos, M. Helicobacter pylori outer membrane vesicle size determines their mechanisms of host cell entry and protein content. Front. Immunol. 2018, 9, 1456. [Google Scholar] [CrossRef] [Green Version]
- Reimer, S.L.; Beniac, D.R.; Hiebert, S.L.; Booth, T.F.; Chong, P.M.; Westmacott, G.R.; Zhanel, G.G.; Bay, D.C. Comparative analysis of outer membrane vesicle isolation methods with an Escherichia coli tolA mutant reveals a hypervesiculating phenotype with outer-inner membrane vesicle content. Front. Microbiol. 2021, 12, 628801. [Google Scholar] [CrossRef]
- Miao, J.; Chen, F.; Duan, S.; Gao, X.; Liu, G.; Chen, Y.; Dixon, W.; Xiao, H.; Cao, Y. iTRAQ-based quantitative proteomic analysis of the antimicrobial mechanism of peptide F1 against Escherichia coli. J. Agric. Food Chem. 2015, 63, 7190–7197. [Google Scholar] [CrossRef]
- Wang, Y.; Arthur, E.W.; Liu, N.; Li, X.; Xiang, W.; Maxwell, A.; Li, Z.; Zhou, Z. iTRAQ-based quantitative proteomics analysis of hela cells infected with Chlamydia muridarum TC0668 mutant and wild-type strains. Front. Microbiol. 2019, 10, 2553. [Google Scholar] [CrossRef] [Green Version]
- UniProt. Available online: http://www.uniprot.org/ (accessed on 8 March 2022).
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [Green Version]
- Bomberger, J.M.; MacEachran, D.P.; Coutermarsh, B.A.; Ye, S.; O’Toole, G.A.; Stanton, B.A. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009, 5, e1000382. [Google Scholar] [CrossRef] [Green Version]
- Hillman, T. Antisense Inhibition of accA in E. coli Suppressed luxS Expression and Increased Antibiotic Susceptibility. Available online: https://www.biorxiv.org/content/10.1101/747980v7.full.pdf+html (accessed on 8 March 2022).
- Kassab, E.; Fuchs, M.; Haack, M.; Mehlmer, N.; Brueck, T.B. Engineering Escherichia coli FAB system using synthetic plant genes for the production of long chain fatty acids. Microb. Cell Fact. 2019, 18, 163. [Google Scholar] [CrossRef] [Green Version]
- Marolda, C.L.; Valvano, M.A. The GalF protein of Escherichia coli is not a UDP-glucose pyrophosphorylase but interacts with the GalU protein possibly to regulate cellular levels of UDP-glucose. Mol. Microbiol. 1996, 22, 827–840. [Google Scholar] [CrossRef]
- Bracher, A.; Verghese, J. The nucleotide exchange factors of Hsp70 molecular chaperones. Front. Mol. Biosci. 2015, 2, 10. [Google Scholar] [CrossRef]
- Jovanovic, G.; Lloyd, L.J.; Stumpf, M.P.H.; Mayhew, A.J.; Buck, M. Induction and function of the phage shock protein extracytoplasmic stress response in Escherichia coli. J. Biol. Chem. 2006, 281, 21147–21161. [Google Scholar] [CrossRef] [Green Version]
- Yung, P.Y.; Grasso, L.L.; Mohidin, A.F.; Acerbi, E.; Hinks, J.; Seviour, T.; Marsili, E.; Lauro, F.M. Global transcriptomic responses of Escherichia coli K-12 to volatile organic compounds. Sci. Rep. 2016, 6, 19899. [Google Scholar] [CrossRef] [Green Version]
- Knöppel, A.; Andersson, D.I.; Näsvall, J. Synonymous mutations in rpsT lead to ribosomal assembly defects that can be compensated by mutations in fis and rpoA. Front. Microbiol. 2020, 11, 340. [Google Scholar] [CrossRef]
- Aseev, L.V.; Koledinskaya, L.S.; Boni, I.V. Regulation of ribosomal protein operons rplM-rpsI, rpmB-rpmG, and rplU-rpmA at the transcriptional and translational levels. J. Bacteriol. 2016, 198, 2494–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, C.T.; Kloser, A.W.; Schnaitman, C.A.; Stein, M.A.; Gottesman, S.; Gibson, B.W. Role of the rfaG and rfaP genes in determining the lipopolysaccharide core structure and cell surface properties of Escherichia coli K-12. J. Bacteriol. 1992, 174, 2525–2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klena, J.D.; Ashford, R.S.; Schnaitman, C.A. Role of Escherichia coli K-12 rfa genes and the rfp gene of Shigella dysenteriae 1 in generation of lipopolysaccharide core heterogeneity and attachment of O antigen. J. Bacteriol. 1992, 174, 7297–7307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DebRoy, C.; Roberts, E.; Fratamico, P.M. Detection of O antigens in Escherichia coli. Anim. Health Res. Rev. 2011, 12, 169–185. [Google Scholar] [CrossRef]
- McAllister, K.A.; Peery, R.B.; Zhao, G. Acyl carrier protein synthases from gram-negative, gram-positive, and atypical bacterial species: Biochemical and structural properties and physiological implications. J. Bacteriol. 2006, 188, 4737–4748. [Google Scholar] [CrossRef] [Green Version]
- Guest, J.R.; Stephens, P.E. Molecular cloning of the pyruvate dehydrogenase complex genes of Escherichia coli. J. Gen. Microbiol. 1980, 121, 277–292. [Google Scholar] [CrossRef] [Green Version]
- Membrillo-Hernandez, J.; Echave, P.; Cabiscol, E.; Tamarit, J.; Ros, J.; Lin, E.C. Evolution of the adhE gene product of Escherichia coli from a functional reductase to a dehydrogenase: Genetic and biochemical studies of the mutant proteins. J. Biol Chem. 2000, 275, 33869–33875. [Google Scholar] [CrossRef] [Green Version]
- Araki, K.; Nagata, K. Protein Folding and Quality Control in theER. Cold Spring Harb. Perspect. Biol. 2012, 4, a015438. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhang, J.; Li, X.; Meng, X.; Fang, X. Identification of the Endoplasmic Reticulum Localization Sequence and N-Glycosylation of Matrix Metalloproteinase 26. RSC Adv. 2019, 9, 23053–23060. [Google Scholar] [CrossRef] [Green Version]
- Sundara, B.S.; Seol, E.; Raj, S.M.; Park, S. Co-production of hydrogen and ethanol by pfkA-deficient Escherichia coli with activated pentose-phosphate pathway: Reduction of pyruvate accumulation. Biotechnol. Biofuels 2016, 9, 95. [Google Scholar] [CrossRef] [Green Version]
- Siddiquee, K.A.; Arauzo-Bravo, M.J.; Shimizu, K. Effect of a pyruvate kinase (pykF-gene) knockout mutation on the control of gene expression and metabolic fluxes in Escherichia coli. FEMS Microbiol. Lett. 2004, 235, 25–33. [Google Scholar] [CrossRef]
- Charpentier, B.; Branlant, C. The Escherichia coli gapA gene is transcribed by the vegetative RNA polymerase holoenzyme E sigma 70 and by the heat shock RNA polymerase E sigma 32. J. Bacteriol. 1994, 176, 830–839. [Google Scholar] [CrossRef] [Green Version]
- Busch, N.A.; Zanzot, E.M.; Loiselle, P.M.; Carter, E.A.; Allaire, J.E.; Yarmush, M.L.; Warren, H.S. A model of infected burn wounds using Escerichia coli 018:K1:H7 for study gram negative bacteremia and sepsis. Infect. Immun. 2000, 68, 3349–3351. [Google Scholar] [CrossRef] [Green Version]
- Crompton, R.; Williams, H.; Campbell, L.; Holden, K.; Crickshank, S.; Haedman, M.J. Oestrogen promotes healing in a bacterial LPS model of delayed cutaneous wound repair. Lab. Investig. 2016, 96, 439–449. [Google Scholar] [CrossRef]
- Fleetwood, A.J.; Lee, M.K.S.; Singleton, W.; Achuthan, A.; Lee, M.C.; O’Brie-Simpson, N.M.; Cook, A.D.; Murphy Aj Dashper, S.G.; Reynolds, E.C.; Hamilton, J.A. Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by porphyromonas gingivalis and its outer membrane vesicles. Front. Cell Infect. Microbiol. 2017, 7, 351. [Google Scholar] [CrossRef]
- Masters, S.L.; Dunne, A.; Subramanian, S.L.; Hull, R.L.; Tannahill, G.M.; Sharp, F.A.; Becker, C.; Franchi, L.; Yoshihara, E.; Chen, Z.; et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat. Immunol. 2010, 11, 897–904. [Google Scholar] [CrossRef]
- Everts, B.; Amiel, E.; Huang, S.C.; Smith, A.M.; Chang, C.H.; Lam, W.Y.; Redmann, V.; Freitas, T.C.; Blagih, J.; van der Windt, G.J.; et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014, 15, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Tomic-Canic, M.; Agren, M.A.; Alvarez OMRovie, D.T.; Maibach, H. Epidermal repair and chronic wounds. In The Epidermis in Wound Healing; Rovee, D.T., Maibach, H.I., Eds.; CRS Press: New York, NY, USA, 2008; pp. 26–57. [Google Scholar]
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressing. J. Am. Acad. Dermatol. 2008, 58, 185–206. [Google Scholar] [CrossRef]
- Schultz, G.; Bjarnsholt, T.; James, G.A.; Leaper, D.J.; McBain, A.J.; Malone, M.; Stoodley, P.; Swanson, T.; Tachi, M.; Wolcott, R.D.; et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017, 25, 744–757. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Kirketerp-Moller, K.; Jensen, P.; Kit, M.; Krogfelt, K.; Phipps, R.; Krogfelt, K.; Høiby, N.; Givskov, M. Why chronic wounds won’t heal: A novel hypothesis. Wound Repair Regen. 2008, 1, 2–10. [Google Scholar] [CrossRef]
- Chang, V.; Chen, L.Y.; Wang, A.; Yuan, X. The effect of lipopolysaccharide core structure defects on transformation efficiency in isogenic Escherichia coli BW25113 rfaG, rfaP, and rfaC Mutants. J. Exp. Microbiol. Immunol. 2010, 14, 101–107. [Google Scholar]
- Yethon, J.A.; Vinogradov, E.; Perry, M.B.; Whitfield, C. Mutation of the lipopolysaccharide core glucosyltransferase encoded by waaG destabilizes the outer membrane of Escherichia coli by interfering with core phosphorylation. J. Bacteriol. 2000, 182, 5620. [Google Scholar] [CrossRef] [Green Version]
- Murata, M.; Fujimoto, H.; Nishimura, K.; Charoensuk, K.; Nagamitsu, H.; Raina, S.; Kosaka, T.; Oshima, T.; Ogasawara, N.; Yamada, M. Molecular strategy for survival at a critical high temperature in Escherichia coli. PLoS ONE 2011, 6, e20063. [Google Scholar] [CrossRef] [Green Version]
- Valvano, M.A. Export of O-specific lipopolysaccharide. Front. Biosci. 2003, 1, s452–s471. [Google Scholar] [CrossRef] [Green Version]
- Iwashkiw, J.A.; Seper, A.; Weber, B.S.; Scott, N.E.; Vinogradov, E.; Stratilo, C.; Reiz, B.; Cordwell, S.J.; Whittal, R.; Schild, S.; et al. Identification of a general O-linked protein glycosylation system in Acinetobacter baumannii and its role in virulence and biofilm formation. PLoS Pathog. 2012, 8, e1002758. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).