Ultrathin Struts Drug-Eluting Stents: A State-of-the-Art Review
Abstract
1. Introduction
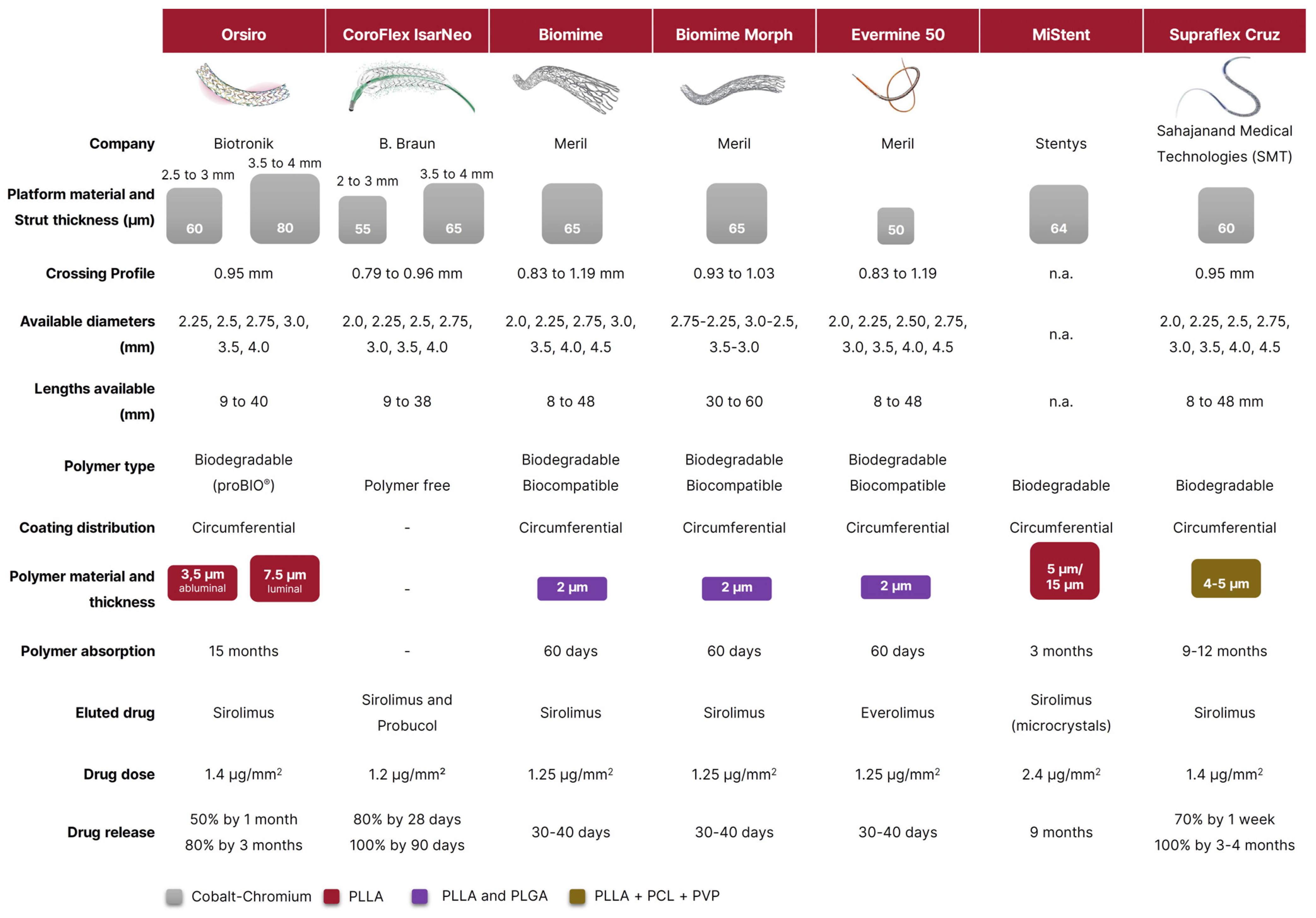
2. Orsiro
3. Coroflex ISAR Neo
4. Biomime, Biomime Morph, and Evermine 50
5. Mi Stent
6. The Supraflex Family
7. Benefit of Strut Thickness Reduction: A Class Effect?
8. Ultrathin Stents in High-Risk Subgroups
8.1. STEMI
8.2. Chronic Total Occlusions
8.3. Diabetes Mellitus
8.4. Small Vessel Disease
8.5. In-Stent Restenosis
8.6. Limitations of Ultrathin DES
9. Future Directions
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMS | bare metal stents |
| BP | biodegradable polymer |
| CI | confidence interval |
| CoCr | cobalt-chromium |
| CTO | chronic total occlusions |
| DES | drug-eluting stents |
| DOCE | device-oriented composite endpoint |
| DP | durable polymer |
| EES | everolimus-eluting stent |
| HBR | high bleeding risk |
| LLL | late lumen loss |
| MACE | major adverse cardiac events |
| MI | myocardial infarction |
| PCI | percutaneous coronary interventions |
| PLGA | poly-d,l-lactide-co-glycolide |
| PLLA | poly-l-lactic acid |
| PtCR | platinum-chromium |
| SES | sirolimus-eluting stent |
| ST | stent thrombosis |
| STEMI | ST-segment elevation myocardial infarction |
| TLF | target-lesion failure |
| TLR | target-lesion revascularization |
| TVF | target-vessel failure |
| TV-MI | target-vessel myocardial infarction |
| ZES | zotarolimus-eluting stent. |
References
- Piccolo, R.; Giustino, G.; Mehran, R.; Windecker, S. Stable Coronary Artery Disease: Revascularisation and Invasive Strategies. Lancet 2015, 386, 702–713. [Google Scholar] [CrossRef]
- Piccolo, R.; Pilgrim, T.; Heg, D.; Franzone, A.; Rat-Wirtzler, J.; Räber, L.; Silber, S.; Serruys, P.W.; Jüni, P.; Windecker, S. Comparative Effectiveness and Safety of New-Generation Versus Early-Generation Drug-Eluting Stents According to Complexity of Coronary Artery Disease. JACC Cardiovasc. Interv. 2015, 8, 1657–1666. [Google Scholar] [CrossRef]
- Piccolo, R.; Franzone, A.; Windecker, S. From Bare Metal to Barely Anything: An Update on Coronary Stenting. Heart 2018, 104, 533–540. [Google Scholar] [CrossRef]
- Tada, T.; Byrne, R.A.; Simunovic, I.; King, L.A.; Cassese, S.; Joner, M.; Fusaro, M.; Schneider, S.; Schulz, S.; Ibrahim, T.; et al. Risk of Stent Thrombosis Among Bare-Metal Stents, First-Generation Drug-Eluting Stents, and Second-Generation Drug-Eluting Stents. JACC Cardiovasc. Interv. 2013, 6, 1267–1274. [Google Scholar] [CrossRef]
- Chisari, A.; Pistritto, A.; Piccolo, R.; la Manna, A.; Danzi, G. The Ultimaster Biodegradable-Polymer Sirolimus-Eluting Stent: An Updated Review of Clinical Evidence. Int. J. Mol. Sci. 2016, 17, 1490. [Google Scholar] [CrossRef]
- Piccolo, R.; Bonaa, K.H.; Efthimiou, O.; Varenne, O.; Baldo, A.; Urban, P.; Kaiser, C.; Remkes, W.; Räber, L.; de Belder, A.; et al. Coronary Stent Trialists’ Collaboration. Drug-Eluting or Bare-Metal Stents for Percutaneous Coronary Intervention: A Systematic Review and Individual Patient Data Meta-Analysis of Randomised Clinical Trials. Lancet 2019, 393, 2503–2510. [Google Scholar] [CrossRef]
- Piscione, F.; Piccolo, R.; Cassese, S.; Galasso, G.; Chiariello, M. Clinical Impact of Sirolimus-Eluting Stent in ST-Segment Elevation Myocardial Infarction: A Meta-Analysis of Randomized Clinical Trials. Catheter. Cardiovasc. Interv. 2009, 74, 323–332. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Iglesias, J.F.; Muller, O.; Zuffi, A.; Eeckhout, E. Performance of the Orsiro Hybrid Drug-Eluting Stent in High-Risk Subgroups. Minerva. Cardioangiol. 2016, 64, 55–73. [Google Scholar]
- Hamon, M.; Niculescu, R.; Deleanu, D.; Dorobantu, M.; Weissman, N.J.; Waksman, R. Clinical and Angiographic Experience with a Third-Generation Drug-Eluting Orsiro Stent in the Treatment of Single de Novo Coronary Artery Lesions (BIOFLOW-I): A Prospective, First-in-Man Study. EuroIntervention 2013, 8, 1006–1011. [Google Scholar] [CrossRef]
- Windecker, S.; Haude, M.; Neumann, F.-J.; Stangl, K.; Witzenbichler, B.; Slagboom, T.; Sabaté, M.; Goicolea, J.; Barragan, P.; Cook, S.; et al. Comparison of a Novel Biodegradable Polymer Sirolimus-Eluting Stent with a Durable Polymer Everolimus-Eluting Stent: Results of the Randomized BIOFLOW-II Trial. Circ. Cardiovasc. Interv. 2015, 8, e001441. [Google Scholar] [CrossRef]
- Lefèvre, T.; Haude, M.; Neumann, F.-J.; Stangl, K.; Skurk, C.; Slagboom, T.; Sabaté, M.; Goicolea, J.; Barragan, P.; Cook, S.; et al. Comparison of a Novel Biodegradable Polymer Sirolimus-Eluting Stent With a Durable Polymer Everolimus-Eluting Stent: 5-Year Outcomes of the Randomized BIOFLOW-II Trial. JACC Cardiovasc. Interv. 2018, 11, 995–1002. [Google Scholar] [CrossRef]
- Saito, S.; Toelg, R.; Witzenbichler, B.; Haude, M.; Masotti, M.; Salmeron, R.; Witkowski, A.; Uematsu, M.; Takahashi, A.; Waksman, R.; et al. BIOFLOW-IV, a Randomised, Intercontinental, Multicentre Study to Assess the Safety and Effectiveness of the Orsiro Sirolimus-Eluting Stent in the Treatment of Subjects with de Novo Coronary Artery Lesions: Primary Outcome Target Vessel Failure at 12 Month. EuroIntervention 2019, 15, e1006–e1013. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Mauri, L.; Koolen, J.J.; Massaro, J.M.; Doros, G.; Garcia-Garcia, H.M.; Bennett, J.; Roguin, A.; Gharib, E.G.; Cutlip, D.E.; et al. BIOFLOW V Investigators. Ultrathin, Bioresorbable Polymer Sirolimus-Eluting Stents versus Thin, Durable Polymer Everolimus-Eluting Stents in Patients Undergoing Coronary Revascularisation (BIOFLOW V): A Randomised Trial. Lancet 2017, 390, 1843–1852. [Google Scholar] [CrossRef]
- Doros, G.; Massaro, J.M.; Kandzari, D.E.; Waksman, R.; Koolen, J.J.; Cutlip, D.E.; Mauri, L. Rationale of a Novel Study Design for the BIOFLOW V Study, a Prospective, Randomized Multicenter Study to Assess the Safety and Efficacy of the Orsiro Sirolimus-Eluting Coronary Stent System Using a Bayesian Approach. Am. Heart J. 2017, 193, 35–45. [Google Scholar] [CrossRef]
- Pilgrim, T.; Heg, D.; Roffi, M.; Tüller, D.; Muller, O.; Vuilliomenet, A.; Cook, S.; Weilenmann, D.; Kaiser, C.; Jamshidi, P.; et al. Ultrathin Strut Biodegradable Polymer Sirolimus-Eluting Stent versus Durable Polymer Everolimus-Eluting Stent for Percutaneous Coronary Revascularisation (BIOSCIENCE): A Randomised, Single-Blind, Non-Inferiority Trial. Lancet 2014, 384, 2111–2122. [Google Scholar] [CrossRef]
- Iglesias, J.F.; Muller, O.; Heg, D.; Roffi, M.; Kurz, D.J.; Moarof, I.; Weilenmann, D.; Kaiser, C.; Tapponnier, M.; Stortecky, S.; et al. Biodegradable Polymer Sirolimus-Eluting Stents versus Durable Polymer Everolimus-Eluting Stents in Patients with ST-Segment Elevation Myocardial Infarction (BIOSTEMI): A Single-Blind, Prospective, Randomised Superiority Trial. Lancet 2019, 394, 1243–1253. [Google Scholar] [CrossRef]
- Pilgrim, T.; Rothenbühler, M.; Siontis, G.C.; Kandzari, D.E.; Iglesias, J.F.; Asami, M.; Lefèvre, T.; Piccolo, R.; Koolen, J.; Saito, S.; et al. Biodegradable Polymer Sirolimus-Eluting Stents vs Durable Polymer Everolimus-Eluting Stents in Patients Undergoing Percutaneous Coronary Intervention: A Meta-Analysis of Individual Patient Data from 5 Randomized Trials. Am. Heart J. 2021, 235, 140–148. [Google Scholar] [CrossRef]
- Von Birgelen, C.; Kok, M.M.; van der Heijden, L.C.; Danse, P.W.; Schotborgh, C.E.; Scholte, M.; Gin, R.M.T.J.; Somi, S.; van Houwelingen, K.G.; Stoel, M.G.; et al. Very Thin Strut Biodegradable Polymer Everolimus-Eluting and Sirolimus-Eluting Stents versus Durable Polymer Zotarolimus-Eluting Stents in Allcomers with Coronary Artery Disease (BIO-RESORT): A Three-Arm, Randomised, Non-Inferiority Trial. Lancet 2016, 388, 2607–2617. [Google Scholar] [CrossRef]
- Kok, M.M.; Zocca, P.; Buiten, R.A.; Danse, P.W.; Schotborgh, C.E.; Scholte, M.; Hartmann, M.; Stoel, M.G.; van Houwelingen, G.; Linssen, G.C.M.; et al. Two-Year Clinical Outcome of All-Comers Treated with Three Highly Dissimilar Contemporary Coronary Drug-Eluting Stents in the Randomised BIO-RESORT Trial. EuroIntervention 2018, 14, 915–923. [Google Scholar] [CrossRef]
- Buiten, R.A.; Ploumen, E.H.; Zocca, P.; Doggen, C.J.M.; Danse, P.W.; Schotborgh, C.E.; Scholte, M.; van Houwelingen, K.G.; Stoel, M.G.; Hartmann, M.; et al. Thin, Very Thin, or Ultrathin Strut Biodegradable or Durable Polymer-Coated Drug-Eluting Stents: 3-Year Outcomes of BIO-RESORT. J. Am. Coll Cardiol. Intv. 2019, 12, 1650–1660. [Google Scholar] [CrossRef]
- Von Birgelen, C.; Zocca, P.; Buiten, R.A.; Jessurun, G.A.J.; Schotborgh, C.E.; Roguin, A.; Danse, P.W.; Benit, E.; Aminian, A.; van Houwelingen, K.G.; et al. Thin Composite Wire Strut, Durable Polymer-Coated (Resolute Onyx) versus Ultrathin Cobalt-Chromium Strut, Bioresorbable Polymer-Coated (Orsiro) Drug-Eluting Stents in Allcomers with Coronary Artery Disease (BIONYX): An International, Single-Blind, Randomi. Lancet 2018, 392, 1235–1245. [Google Scholar] [CrossRef]
- Jensen, L.O.; Thayssen, P.; Maeng, M.; Ravkilde, J.; Krusell, L.R.; Raungaard, B.; Junker, A.; Terkelsen, C.J.; Veien, K.T.; Villadsen, A.B.; et al. Randomized Comparison of a Biodegradable Polymer Ultrathin Strut Sirolimus-Eluting Stent With a Biodegradable Polymer Biolimus-Eluting Stent in Patients Treated With Percutaneous Coronary Intervention: The SORT OUT VII Trial. Circ. Cardiovasc. Interv. 2016, 9, e003610. [Google Scholar] [CrossRef]
- Jensen, L.O.; Maeng, M.; Raungaard, B.; Engstrøm, T.; Hansen, H.S.; Jensen, S.E.; Bøtker, H.E.; Kahlert, J.; Lassen, J.F.; Christiansen, E.H. Comparison of the Polymer-Free Biolimus-Coated BioFreedom Stent with the Thin-Strut Biodegradable Polymer Sirolimus-Eluting Orsiro Stent in an All-Comers Population Treated with Percutaneous Coronary Intervention: Rationale and Design of the Randomized SO. Am. Heart J. 2019, 213, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Jeong, M.H.; Cha, K.S.; Hyun, D.W.; Hur, S.H.; Kim, K.B.; Hong, Y.J.; Park, H.W.; Kim, J.H.; Ahn, Y.K.; et al. Effect of Anti-Oxidant (Carvedilol and Probucol) Loaded Stents in a Porcine Coronary Restenosis Model. Circ. J. 2005, 69, 101–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Massberg, S.; Byrne, R.A.; Kastrati, A.; Schulz, S.; Pache, J.; Hausleiter, J.; Ibrahim, T.; Fusaro, M.; Ott, I.; Schömig, A.; et al. Polymer-free sirolimus- and probucol-eluting versus new generation zotarolimus-eluting stents in coronary artery disease: The Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus-and Probucol-Eluting versus Zotarolimus-eluting Stents (ISAR-TEST 5) trial. Circulation 2011, 124, 624–632. [Google Scholar] [CrossRef]
- Kufner, S.; Sorges, J.; Mehilli, J.; Cassese, S.; Repp, J.; Wiebe, J.; Lohaus, R.; Lahmann, A.; Rheude, T.; Ibrahim, T.; et al. ISAR-TEST-5 Investigators. Randomized Trial of Polymer-Free Sirolimus- and Probucol-Eluting Stents Versus Durable Polymer Zotarolimus-Eluting Stents: 5-Year Results of the ISAR-TEST-5 Trial. JACC Cardiovasc. Interv. 2016, 9, 784–792. [Google Scholar] [CrossRef]
- Kufner, S.; Ernst, M.; Cassese, S.; Joner, M.; Mayer, K.; Colleran, R.; Koppara, T.; Xhepa, E.; Koch, T.; Wiebe, J.; et al. ISAR-TEST-5 Investigators. 10-Year Outcomes From a Randomized Trial of Polymer-Free Versus Durable Polymer Drug-Eluting Coronary Stents. J. Am. Coll Cardiol. 2020, 76, 146–158. [Google Scholar] [CrossRef]
- Dani, S.; Costa, R.A.; Joshi, H.; Shah, J.; Pandya, R.; Virmani, R.; Sheiban, I.; Bhatt, S.; Abizaid, A. First-in-Human Evaluation of the Novel BioMime Sirolimus-Eluting Coronary Stent with Bioabsorbable Polymer for the Treatment of Single de Novo Lesions Located in Native Coronary Vessels-Results from the MeriT-1 Trial. EuroIntervention 2013, 9, 493–500. [Google Scholar] [CrossRef]
- Jain, R.K.; Chakravarthi, P.; Shetty, R.; Ramchandra, P.; Polavarapu, R.S.; Wander, G.S.; Mohan, B.; Banker, D.N.; Dharmadhikari, A.; Bansal, S.S.; et al. One-Year Outcomes of a BioMimeTM Sirolimus-Eluting Coronary Stent System with a Biodegradable Polymer in All-Comers Coronary Artery Disease Patients: The MeriT-3 Study. Indian Heart J. 2016, 68, 599–603. [Google Scholar] [CrossRef][Green Version]
- TCT-650. Impact of the New BioMimeTM Sirolimus-Eluting Stent in Comlex Patients of Daily Practice—Preliminary Results of the MeriT-2 Study. J. Am. Coll Cardiol. 2012, 17, B189. [Google Scholar]
- Abizaid, A.; Kedev, S.; Kedhi, E.; Talwar, S.; Erglis, A.; Hlinomaz, O.; Masotti, M.; Fath-Ordoubadi, F.; Lemos, P.A.; Milewski, K.; et al. Randomised Comparison of a Biodegradable Polymer Ultra-Thin Sirolimus-Eluting Stent versus a Durable Polymer Everolimus-Eluting Stent in Patients with de Novo Native Coronary Artery Lesions: The MeriT-V Trial. EuroIntervention 2018, 14, e1207–e1214. [Google Scholar] [CrossRef] [PubMed]
- Ormiston, J.; Webster, M.; Stewart, J.; Vrolix, M.; Whitbourn, R.; Donohoe, D.; Knape, C.; Lansky, A.; Attizzani, G.F.; Fitzgerald, P.; et al. First-in-Human Evaluation of a Bioabsorbable Polymer-Coated Sirolimus-Eluting Stent: Imaging and Clinical Results of the DESSOLVE I Trial (DES with Sirolimus and a Bioabsorbable Polymer for the Treatment of Patients with de Novo Lesion in the Native Coron. JACC Cardiovasc. Interv. 2013, 6, 1026–1034. [Google Scholar] [CrossRef]
- Wijns, W.; Vrolix, M.; Verheye, S.; Schoors, D.; Slagboom, T.; Gosselink, M.; Benit, E.; Kandzari, D.; Donohoe, D.; Ormiston, J.A. Long-Term Clinical Outcomes of a Crystalline Sirolimus-Eluting Coronary Stent with a Fully Bioabsorbable Polymer Coating: Five-Year Outcomes from the DESSOLVE I and II Trials. EuroIntervention 2018, 13, 2147–2151. [Google Scholar] [CrossRef] [PubMed]
- De Winter, R.J.; Katagiri, Y.; Asano, T.; Milewski, K.P.; Lurz, P.; Buszman, P.; Jessurun, G.A.J.; Koch, K.T.; Troquay, R.P.T.; Hamer, B.J.B.; et al. A Sirolimus-Eluting Bioabsorbable Polymer-Coated Stent (MiStent) versus an Everolimus-Eluting Durable Polymer Stent (Xience) after Percutaneous Coronary Intervention (DESSOLVE III): A Randomised, Single-Blind, Multicentre, Non-Inferiority, Phase 3 Trial. Lancet 2018, 391, 431–440. [Google Scholar] [CrossRef]
- Lemos, P.A.; Chandwani, P.; Saxena, S.; Ramachandran, P.K.; Abhyankar, A.; Campos, C.M.; Marchini, J.F.; Galon, M.Z.; Verma, P.; Sandhu, M.S.; et al. Clinical Outcomes in 995 Unselected Real-World Patients Treated with an Ultrathin Biodegradable Polymer-Coated Sirolimus-Eluting Stent: 12-Month Results from the FLEX Registry. BMJ Open 2016, 6, e010028. [Google Scholar] [CrossRef]
- Choudhury, A.; Garg, S.; Smith, J.; Sharp, A.; Nabais de Araujo, S.; Chauhan, A.; Patel, N.; Wrigley, B.; Chattopadhyay, S.; Zaman, A.G. Prospective Evaluation of an Ultrathin Strut Biodegradable Polymer-Coated Sirolimus-Eluting Stent: 12 Months’ Results from the S-FLEX UK Registry. BMJ Open 2019, 9, e026578. [Google Scholar] [CrossRef]
- Zaman, A.; de Winter, R.J.; Kogame, N.; Chang, C.C.; Modolo, R.; Spitzer, E.; Tonino, P.; Hofma, S.; Zurakowski, A.; Smits, P.C.; et al. TALENT trial investigators. Safety and Efficacy of a Sirolimus-Eluting Coronary Stent with Ultra-Thin Strut for Treatment of Atherosclerotic Lesions (TALENT): A Prospective Multicentre Randomised Controlled Trial. Lancet 2019, 393, 987–997. [Google Scholar] [CrossRef]
- Gao, C.; Kogame, N.; Sharif, F.; Smits, P.C.; Tonino, P.; Hofma, S.; Moreno, R.; Choudhury, A.; Petrov, I.; Cequier, A.; et al. Prospective Multicenter Randomized All-Comers Trial to Assess the Safety and Effectiveness of the Ultra-Thin Strut Sirolimus-Eluting Coronary Stent Supraflex: Two-Year Outcomes of the TALENT Trial. Circ. Cardiovasc. Interv. 2021, 14, e010312. [Google Scholar] [CrossRef] [PubMed]
- De Winter, R.J.; Zaman, A.; Hara, H.; Gao, C.; Ono, M.; Garg, S.; Smits, P.C.; Tonino, P.A.L.; Hofma, S.H.; Moreno, R.; et al. Sirolimus-Eluting Stents with Ultrathin Struts versus Everolimus-Eluting Stents for Patients Undergoing Percutaneous Coronary Intervention: Final Three-Year Results of the TALENT Trial. EuroIntervention 2022, 18, 492–502. [Google Scholar] [CrossRef]
- Biscaglia, S.; Guiducci, V.; Santarelli, A.; Amat Santos, I.; Fernandez-Aviles, F.; Lanzilotti, V.; Varbella, F.; Fileti, L.; Moreno, R.; Giannini, F.; et al. Physiology-Guided Revascularization versus Optimal Medical Therapy of Nonculprit Lesions in Elderly Patients with Myocardial Infarction: Rationale and Design of the FIRE Trial. Am. Heart J. 2020, 229, 100–109. [Google Scholar] [CrossRef]
- Hara, H.; Gao, C.; Kogame, N.; Ono, M.; Kawashima, H.; Wang, R.; Morel, M.-A.; O’Leary, N.; Sharif, F.; Möllmann, H.; et al. A Randomised Controlled Trial of the Sirolimus-Eluting Biodegradable Polymer Ultra-Thin Supraflex Stent versus the Everolimus-Eluting Biodegradable Polymer SYNERGY Stent for Three-Vessel Coronary Artery Disease: Rationale and Design of the Multivessel TAL. EuroIntervention 2020, 16, e997–e1004. [Google Scholar] [CrossRef]
- Bangalore, S.; Toklu, B.; Patel, N.; Feit, F.; Stone, G.W. Newer-Generation Ultrathin Strut Drug-Eluting Stents Versus Older Second-Generation Thicker Strut Drug-Eluting Stents for Coronary Artery Disease. Circulation 2018, 138, 2216–2226. [Google Scholar] [CrossRef]
- Madhavan, M.V.; Howard, J.P.; Naqvi, A.; Ben-Yehuda, O.; Redfors, B.; Prasad, M.; Shahim, B.; Leon, M.B.; Bangalore, S.; Stone, G.W.; et al. Long-Term Follow-up after Ultrathin vs. Conventional 2nd-Generation Drug-Eluting Stents: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. Heart J. 2021, 42, 2643–2654. [Google Scholar] [CrossRef]
- Palmerini, T.; Biondi-Zoccai, G.; Della Riva, D.; Mariani, A.; Sabaté, M.; Smits, P.C.; Kaiser, C.; D’Ascenzo, F.; Frati, G.; Mancone, M.; et al. Clinical Outcomes with Bioabsorbable Polymer- versus Durable Polymer-Based Drug-Eluting and Bare-Metal Stents: Evidence from a Comprehensive Network Meta-Analysis. J. Am. Coll Cardiol. 2014, 63, 299–307. [Google Scholar] [CrossRef]
- Bangalore, S.; Toklu, B.; Amoroso, N.; Fusaro, M.; Kumar, S.; Hannan, E.L.; Faxon, D.P.; Feit, F. Bare Metal Stents, Durable Polymer Drug Eluting Stents, and Biodegradable Polymer Drug Eluting Stents for Coronary Artery Disease: Mixed Treatment Comparison Meta-Analysis. BMJ 2013, 347, f6625. [Google Scholar] [CrossRef]
- Kolandaivelu, K.; Swaminathan, R.; Gibson, W.J.; Kolachalama, V.B.; Nguyen-Ehrenreich, K.-L.; Giddings, V.L.; Coleman, L.; Wong, G.K.; Edelman, E.R. Stent Thrombogenicity Early in High-Risk Interventional Settings Is Driven by Stent Design and Deployment and Protected by Polymer-Drug Coatings. Circulation 2011, 123, 1400–1409. [Google Scholar] [CrossRef]
- Pilgrim, T.; Piccolo, R.; Heg, D.; Roffi, M.; Tüller, D.; Muller, O.; Moarof, I.; Siontis, G.C.M.; Cook, S.; Weilenmann, D.; et al. Ultrathin-Strut, Biodegradable-Polymer, Sirolimus-Eluting Stents versus Thin-Strut, Durable-Polymer, Everolimus-Eluting Stents for Percutaneous Coronary Revascularisation: 5-Year Outcomes of the BIOSCIENCE Randomised Trial. Lancet 2018, 392, 737–746. [Google Scholar] [CrossRef]
- Piccolo, R.; Heg, D.; Franzone, A.; Roffi, M.; Tüller, D.; Vuilliomenet, A.; Muller, O.; Cook, S.; Weilenmann, D.; Kaiser, C.; et al. Biodegradable-Polymer Sirolimus-Eluting Stents Versus Durable-Polymer Everolimus-Eluting Stents in Patients With Acute ST-Segment Elevation Myocardial Infarction: Insights From the 2-Year Follow-Up of the BIOSCIENCE Trial. JACC Cardiovasc. Interv. 2016, 9, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Pilgrim, T.; Piccolo, R.; Heg, D.; Roffi, M.; Tüller, D.; Vuilliomenet, A.; Muller, O.; Cook, S.; Weilenmann, D.; Kaiser, C.; et al. Biodegradable Polymer Sirolimus-Eluting Stents versus Durable Polymer Everolimus-Eluting Stents for Primary Percutaneous Coronary Revascularisation of Acute Myocardial Infarction. EuroIntervention 2016, 12, e1343–e1354. [Google Scholar] [CrossRef] [PubMed]
- Teeuwen, K.; van der Schaaf, R.J.; Adriaenssens, T.; Koolen, J.J.; Smits, P.C.; Henriques, J.P.S.; Vermeersch, P.H.M.J.; Tjon Joe Gin, R.M.; Schölzel, B.E.; Kelder, J.C.; et al. Randomized Multicenter Trial Investigating Angiographic Outcomes of Hybrid Sirolimus-Eluting Stents With Biodegradable Polymer Compared With Everolimus-Eluting Stents With Durable Polymer in Chronic Total Occlusions: The PRISON IV Trial. JACC Cardiovasc. Interv. 2017, 10, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.O.; Maeng, M.; Raungaard, B.; Hansen, K.N.; Kahlert, J.; Jensen, S.E.; Hansen, H.S.; Lassen, J.F.; Bøtker, H.E.; Christiansen, E.H. Two-Year Outcome after Biodegradable Polymer Sirolimus- and Biolimus-Eluting Coronary Stents (from the Randomised SORT OUT VII Trial). EuroIntervention 2018, 13, 1587–1590. [Google Scholar] [CrossRef]
- Waksman, R.; Shlofmitz, E.; Windecker, S.; Koolen, J.J.; Saito, S.; Kandzari, D.; Kolm, P.; Lipinski, M.J.; Torguson, R. Efficacy and Safety of Ultrathin, Bioresorbable-Polymer Sirolimus-Eluting Stents Versus Thin, Durable-Polymer Everolimus-Eluting Stents for Coronary Revascularization of Patients With Diabetes Mellitus. Am. J. Cardiol. 2019, 124, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Ellert, J.; Christiansen, E.H.; Maeng, M.; Raungaard, B.; Jensen, S.E.; Kristensen, S.D.; Veien, K.T.; Junker, A.B.; Jakobsen, L.; Aarøe, J.; et al. Impact of Diabetes on Clinical Outcomes after Revascularization with Sirolimus-Eluting and Biolimus-Eluting Stents with Biodegradable Polymer from the SORT OUT VII Trial. Catheter. Cardiovasc. Interv. 2019, 93, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Siontis, G.C.M.; Piccolo, R.; Praz, F.; Valgimigli, M.; Räber, L.; Mavridis, D.; Jüni, P.; Windecker, S. Percutaneous Coronary Interventions for the Treatment of Stenoses in Small Coronary Arteries. JACC Cardiovasc. Interv. 2016, 9, 1324–1334. [Google Scholar] [CrossRef]
- Buiten, R.A.; Ploumen, E.H.; Zocca, P.; Doggen, C.J.M.; van der Heijden, L.C.; Kok, M.M.; Danse, P.W.; Schotborgh, C.E.; Scholte, M.; de Man, F.H.A.F.; et al. Outcomes in Patients Treated With Thin-Strut, Very Thin-Strut, or Ultrathin-Strut Drug-Eluting Stents in Small Coronary Vessels: A Prespecified Analysis of the Randomized BIO-RESORT Trial. JAMA Cardiol. 2019, 4, 659–669. [Google Scholar] [CrossRef]
- Iglesias, J.F.; Heg, D.; Roffi, M.; Tüller, D.; Noble, S.; Muller, O.; Moarof, I.; Cook, S.; Weilenmann, D.; Kaiser, C.; et al. Long-Term Effect of Ultrathin-Strut Versus Thin-Strut Drug-Eluting Stents in Patients With Small Vessel Coronary Artery Disease Undergoing Percutaneous Coronary Intervention: A Subgroup Analysis of the BIOSCIENCE Randomized Trial. Circ. Cardiovasc. Interv. 2019, 12, e008024. [Google Scholar] [CrossRef]
- Piccolo, R.; Galasso, G.; Piscione, F.; Esposito, G.; Trimarco, B.; Dangas, G.D.; Mehran, R. Meta-Analysis of Randomized Trials Comparing the Effectiveness of Different Strategies for the Treatment of Drug-Eluting Stent Restenosis. Am. J. Cardiol. 2014, 114, 1339–1346. [Google Scholar] [CrossRef]
- Jensen, C.J.; Richardt, G.; Tölg, R.; Erglis, A.; Skurk, C.; Jung, W.; Neumann, F.J.; Stangl, K.; Brachmann, J.; Fischer, D.; et al. Angiographic and Clinical Performance of a Paclitaxel-Coated Balloon Compared to a Second-Generation Sirolimus-Eluting Stent in Patients with in-Stent Restenosis: The BIOLUX Randomised Controlled Trial. EuroIntervention 2018, 14, 1096–1103. [Google Scholar] [CrossRef]
- Öner, A.; Rosam, P.; Borowski, F.; Grabow, N.; Siewert, S.; Schmidt, W.; Schmitz, K.-P.; Stiehm, M. Side-Branch Expansion Capacity of Contemporary DES Platforms. Eur. J. Med. Res. 2021, 26, 121. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Omedè, P.; de Filippo, O.; Cerrato, E.; Autelli, M.; Trabattoni, D.; Ryan, N.; Venuti, G.; Muscoli, S.; Montabone, A.; et al. Impact of Final Kissing Balloon and of Imaging on Patients Treated on Unprotected Left Main Coronary Artery With Thin-Strut Stents (From the RAIN-CARDIOGROUP VII Study). Am. J. Cardiol. 2019, 123, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Gaido, L.; D’Ascenzo, F.; Imori, Y.; Wojakowski, W.; Saglietto, A.; Figini, F.; Mattesini, A.; Trabattoni, D.; Rognoni, A.; Tomassini, F.; et al. Impact of Kissing Balloon in Patients Treated With Ultrathin Stents for Left Main Lesions and Bifurcations. Circ. Cardiovasc. Interv. 2020, 13, e008325. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, M.; D’Ascenzo, F.; Gallone, G.; Mitomo, S.; Parma, R.; Trabattoni, D.; Ryan, N.; Muscoli, S.; Venuti, G.; Montabone, A.; et al. Impact of Structural Features of Very Thin Stents Implanted in Unprotected Left Main or Coronary Bifurcations on Clinical Outcomes. Catheter. Cardiovasc. Interv. 2020, 96, 1–9. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leone, A.; Simonetti, F.; Avvedimento, M.; Angellotti, D.; Immobile Molaro, M.; Franzone, A.; Esposito, G.; Piccolo, R. Ultrathin Struts Drug-Eluting Stents: A State-of-the-Art Review. J. Pers. Med. 2022, 12, 1378. https://doi.org/10.3390/jpm12091378
Leone A, Simonetti F, Avvedimento M, Angellotti D, Immobile Molaro M, Franzone A, Esposito G, Piccolo R. Ultrathin Struts Drug-Eluting Stents: A State-of-the-Art Review. Journal of Personalized Medicine. 2022; 12(9):1378. https://doi.org/10.3390/jpm12091378
Chicago/Turabian StyleLeone, Attilio, Fiorenzo Simonetti, Marisa Avvedimento, Domenico Angellotti, Maddalena Immobile Molaro, Anna Franzone, Giovanni Esposito, and Raffaele Piccolo. 2022. "Ultrathin Struts Drug-Eluting Stents: A State-of-the-Art Review" Journal of Personalized Medicine 12, no. 9: 1378. https://doi.org/10.3390/jpm12091378
APA StyleLeone, A., Simonetti, F., Avvedimento, M., Angellotti, D., Immobile Molaro, M., Franzone, A., Esposito, G., & Piccolo, R. (2022). Ultrathin Struts Drug-Eluting Stents: A State-of-the-Art Review. Journal of Personalized Medicine, 12(9), 1378. https://doi.org/10.3390/jpm12091378






