Thermal Boost Combined with Interstitial Brachytherapy in Early Breast Cancer Conserving Therapy—Initial Group Long-Term Clinical Results and Late Toxicity
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Results
3.2. Cosmetic Outcome
3.3. Late Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.-H.; Wolmark, N. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-Year Follow-up of a Randomized Study Comparing Breast-Conserving Surgery with Radical Mastectomy for Early Breast Cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, H.; Horiot, J.-C.; Poortmans, P.; Struikmans, H.; Bogaert, W.V.D.; Barillot, I.; Fourquet, A.; Borger, J.; Jager, J.; Hoogenraad, W.; et al. Recurrence Rates after Treatment of Breast Cancer with Standard Radiotherapy with or without Additional Radiation. N. Engl. J. Med. 2001, 345, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- eaton, L.; Chan, E.; Tyldesley, S.; Gondara, L.; Speers, C.; Nichol, A. In the Era After the European Organisation for Research and Treatment of Cancer ‘Boost’ Study, is the Additional Radiotherapy to the Breast Tumour Bed Still Beneficial for Young Women? Clin. Oncol. 2020, 32, 373–381. [Google Scholar] [CrossRef]
- Polgár, C.; Fodor, J.; Orosz, Z.; Major, T.; Takácsi-Nagy, Z.; Mangel, L.C.; Sulyok, Z.; Somogyi, A.; Kásler, M.; Németh, G. Electron and high-dose-rate brachytherapy boost in the conservative treatment of stage I-II breast cancer first results of the randomized Budapest boost trial. Strahlenther. Onkol. 2002, 178, 615–623. [Google Scholar] [CrossRef]
- Harms, W.; Krempien, R.; Hensley, F.W.; Berns, C.; Fritz, P.; Wannenmacher, M. 5-year results of pulsed dose rate brachytherapy applied as a boost after breast-conserving therapy in patients at high risk for local recurrence from breast cancer. Strahlenther. Onkol. 2002, 178, 607–614. [Google Scholar] [CrossRef]
- Bartelink, H.; Horiot, J.C.; Poortmans, P.M.; Struikmans, H.; Van den Bogaert, W.; Fourquet, A.; Jager, J.J.; Hoogenraad, W.J.; Oei, S.B.; Wárlám-Rodenhuis, C.C.; et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J. Clin. Oncol. 2007, 25, 3259–3265. [Google Scholar] [CrossRef]
- Vrieling, C.; van Werkhoven, E.; Maingon, P.; Poortmans, P.; Weltens, C.; Fourquet, A.; Schinagl, D.; Oei, B.; Rodenhuis, C.C.; Horiot, J.C.; et al. European Organisation for Research and Treatment of Cancer, Radiation Oncology and Breast Cancer Groups. Prognostic Factors for Local Control in Breast Cancer After Long-term Follow-up in the EORTC Boost vs. No Boost Trial: A Randomized Clinical Trial. JAMA Oncol. 2017, 3, 42–48. [Google Scholar] [CrossRef]
- Polgár, C.; Jánváry, L.; Major, T.; Somogyi, A.; Takácsi-Nagy, Z.; Fröhlich, G.; Fodor, J. The role of high-dose-rate brachytherapy boost in breast-conserving therapy: Long-term results of the Hungarian National Institute of Oncology. Rep. Pract. Oncol. Radiother. 2010, 15, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Beddok, A.; Kirova, Y.; Laki, F.; Reyal, F.; Vincent Salomon, A.; Servois, V.; Fourquet, A. The place of the boost in the breast cancer treatment: State of art. Radiother. Oncol. 2022, 170, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R.; Overgaard, J. Hyperthermia: A potent enhancer of radiotherapy. Clin. Oncol. 2007, 19, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Roti Roti, J.L. Introduction: Radiosensitization by hyperthermia. Int. J. Hyperth. 2004, 20, 109–114. [Google Scholar] [CrossRef]
- Chicheł, A.; Skowronek, J.; Kubaszewska, M.; Kanikowski, M. Hyperthermia–description of a method and a review of clinical applications. Rep. Pract. Oncol. Radiother. 2007, 12, 267–275. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Sneed, P.K.; Stauffer, P.R.; Li, G.C.; Stege, G.J.J. Hyperthermia. In Textbook of Radiation Oncology, 2nd ed.; Leibel, S.A., Phillips, T.L., Eds.; Saunders: Philadelphia, PA, USA, 2004; pp. 1569–1596. [Google Scholar]
- Datta, N.R.; Bodis, S. Hyperthermia with radiotherapy reduces tumour alpha/beta: Insights from trials of thermoradiotherapy vs radiotherapy alone. Radiother. Oncol. 2019, 138, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kubaszewska, M.; Dymnicka, M.; Skowronek, J.; Chicheł, A.; Kanikowski, M. CT-image based conformal high-dose-rate brachytherapy boost in the conservative treatment of stage I–II breast cancer – introducing the procedure. Rep. Pract. Oncol. Radiother. 2008, 13, 227–239. [Google Scholar] [CrossRef]
- Chicheł, A.; Burchardt, W.; Chyrek, A.J.; Bielęda, G.; Zwierzchowski, G.; Stefaniak, P.; Malicki, J. Thermal Boost to Breast Tumor Bed-New Technique Description, Treatment Application and Example Clinical Results. Life 2022, 12, 512. [Google Scholar] [CrossRef]
- Chicheł, A.; Skowronek, J.; Kanikowski, M. Thermal boost combined with interstitial brachytherapy in breast-conserving therapy—Assessment of early toxicity. Rep. Pract. Oncol. Radiother. 2011, 16, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Chichel, A.; Skowronek, J. Thermal Boost Combined with HDR Brachytherapy in Breast-Conserving Therapy—A Study Update After 7-Year Follow-Up. Brachytherapy 2015, 14, S40. [Google Scholar] [CrossRef]
- Emami, B.; Stauffer, P.; Dewhirst, M.W.; Prionas, S.; Ryan, T.; Corry, P.; Herman, T.; Kapp, D.S.; Myerson, R.J.; Samulski, T.; et al. RTOG quality assurance guidelines for interstitial hyperthermia. Int. J. Radiat. Oncol. 1991, 20, 1117–1124. [Google Scholar] [CrossRef]
- Dobšíček Trefná, H.; Schmidt, M.; van Rhoon, G.C.; Kok, H.P.; Gordeyev, S.S.; Lamprecht, U.; Marder, D.; Nadobny, J.; Ghadjar, P.; Abdel-Rahman, S.; et al. Quality assurance guidelines for interstitial hyperthermia. Int. J. Hyperth. 2019, 36, 276–293. [Google Scholar] [CrossRef]
- Harris, J.R.; Levene, M.B.; Svensson, G.; Hellman, S. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int. J. Radiat. Oncol. 1979, 5, 257–261. [Google Scholar] [CrossRef]
- Emami, B.; Scott, C.; Perez, C.A.; Asbell, S.; Swift, P.; Grigsby, P.; Montesano, A.; Rubin, P.; Curran, W.; Delrowe, J.; et al. Phase III study of interstitial thermoradiotherapy compared with interstitial radiotherapy alone in the treatment of recurrent or persistent human tumors. A prospectively controlled randomized study by the Radiation Therapy Group. Int. J. Radiat. Oncol. 1996, 34, 1097–1104. [Google Scholar] [CrossRef]
- Vernon, C.C.; Hand, J.W.; Field, S.B.; Machin, D.; Whaley, J.B.; van der Zee, J.; van Putten, W.L.; van Rhoon, G.C.; van Dijk, J.D.; González González, D.; et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 731–744. [Google Scholar] [CrossRef]
- Dooley, W.C.; Vargas, H.I.; Fenn, A.J.; Tomaselli, M.B.; Harness, J.K. Focused Microwave Thermotherapy for preoperative treatment of invasive breast cancer: A review of clinical studies. Ann. Surg. Oncol. 2009, 17, 1076–1093. [Google Scholar] [CrossRef]
- Hartmann, K.A.; Audretsch, W.; Carl, U.M.; Gripp, S.; Kolotas, C.; Muskalla, K.; Rezai, M.; Schnabel, T.; Waap, I.; Zamboglou, N.; et al. Präoperative Bestrahlung mit interstitiellem Radio-hyperthermie-Boost bei Mammatumoren > or = 3 cm. Düsseldorfer Ergebnisse [Preoperative irradiation and interstitial radiotherapy-hyperthermia boost in breast tumors > or = 3 cm. The Düsseldorf experience]. Strahlenther. Onkol. 1997, 173, 519–523. (In German) [Google Scholar] [CrossRef]
- Kok, H.P.; Wust, P.; Stauffer, P.R.; Bardati, F.; van Rhoon, G.C.; Crezee, J. Current state of the art of regional hyperthermia treatment planning: A review. Radiat. Oncol. 2015, 10, 196. [Google Scholar] [CrossRef]
- Gardner, R.A.; Vargas, H.I.; Block, J.B.; Vogel, C.L.; Fenn, A.J.; Kuehl, G.V.; Doval, M. Focused microwave phased array thermotherapy for primary breast cancer. Ann. Surg. Oncol. 2002, 9, 326–332. [Google Scholar] [CrossRef]
- Vargas, H.I.; Dooley, W.C.; Gardner, R.A.; Gonzalez, K.D.; Venegas, R.; Heywang-Kobrunner, S.H.; Fenn, A.J. Focused microwave phased array thermotherapy for ablation of early-stage breast cancer: Results of thermal dose escalation. Ann. Surg. Oncol. 2004, 11, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.; Dooley, W.; Gardner, R.; Gonzalez, K.; Heywang-Köbrunner, S.; Fenn, A. Success of sentinel lymph node mapping after breast cancer ablation with focused microwave phased array thermotherapy. Am. J. Surg. 2003, 186, 330–332. [Google Scholar] [CrossRef]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumors: A review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- Arunachalam, K.; Maccarini, P.F.; Craciunescu, O.I.; Schlorff, J.L.; Stauffer, P.R. Thermal characteristics of ThermoBrachytherapy Surface Applicators (TBSA) for treating chest wall recurrence. Phys. Med. Biol. 2010, 55, 1949–1969. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.O.; Rademaker, A.; Kiel, K.D.; Jones, E.L.; Marks, L.B.; Croog, V.; McCormick, B.M.; Hirsch, A.; Karkar, A.; Motwani, S.B.; et al. Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int. J. Radiat. Oncol. 2008, 70, 477–484. [Google Scholar] [CrossRef]
- Kapp, D.S.; Hahn, G.M.; Carlson, R.W. Principles of hyperthermia. In Cancer Medicine e. 5, 5th ed.; Bast, R.C., Jr., Kufe, D.W., Pollock, R.E., Eds.; B.C. Decker Inc.: Hamilton, ON, Canada, 2000. [Google Scholar]
- Kapp, D.S.; Cox, R.S.; Barnett, T.A.; Ben-Yosef, R. Thermoradiotherapy for residual microscopic cancer: Elective or post-excisional hyperthermia and radiation therapy in the management of local-regional recurrent breast cancer. Int. J. Radiat. Oncol. 1992, 24, 261–277. [Google Scholar] [CrossRef]
- Bakker, A.; Van Der Zee, J.; Van Tienhoven, G.; Kok, H.P.; Rasch, C.R.N.; Crezee, H. Temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: A systematic review. Int. J. Hyperth. 2019, 36, 1024–1039. [Google Scholar] [CrossRef]
- Datta, N.R.; Puric, E.; Klingbiel, D.; Gomez, S.; Bodis, S. Hyperthermia and Radiation Therapy in Locoregional Recurrent Breast Cancers: A Systematic Review and Meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2015, 94, 1073–1087. [Google Scholar] [CrossRef]
- Medina, M.A.; Oza, G.; Sharma, A.; Arriaga, L.; Hernández, J.M.H.; Rotello, V.M.; Ramirez, J.T. Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. Int. J. Environ. Res. Public Healthy 2020, 17, 2078. [Google Scholar] [CrossRef]
- Kindts, I.; Verhoeven, K.; Laenen, A.; Christiaens, M.; Janssen, H.; Van der Vorst, A.; Van Limbergen, E.; Weltens, C. A comparison of a brachytherapy and an external beam radiotherapy boost in breast-conserving therapy for breast cancer: Local and any recurrences. Strahlenther. Onkol. 2019, 195, 310–317. [Google Scholar] [CrossRef]
- Schroeder, T.M.; Liem, B.; Sampath, S.; Thompson, W.R.; Longhurst, J.; Royce, M. Early breast cancer with positive margins: Excellent local control with an upfront brachytherapy boost. Breast Cancer Res. Treat. 2012, 134, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Quéro, L.; Guillerm, S.; Taright, N.; Michaud, S.; Teixeira, L.; Cahen-Doidy, L.; Bourstyn, E.; Espié, M.; Hennequin, C. 10-Year follow-up of 621 patients treated using high-dose rate brachytherapy as ambulatory boost technique in conservative breast cancer treatment. Radiother. Oncol. 2016, 122, 11–16. [Google Scholar] [CrossRef]
- Guinot, J.-L.; Baixauli-Perez, C.; Soler, P.; Tortajada, M.I.; Moreno, A.; Santos, M.A.; Mut, A.; Gozalbo, F.; Arribas, L. High-dose-rate brachytherapy boost effect on local tumor control in young women with breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Polo, A.; Polgár, C.; Hannoun-Levi, J.-M.; Guinot, J.-L.; Gutierrez, C.; Galalae, R.; Van Limbergen, E.; Strnad, V. Risk factors and state-of-the-art indications for boost irradiation in invasive breast carcinoma. Brachytherapy 2017, 16, 552–564. [Google Scholar] [CrossRef]
- Serkies, K.; Jaśkiewicz, J.; Dziadziuszko, R. Pulsed-dose-rate peri-operative brachytherapy as an interstitial boost in organ-sparing treatment of breast cancer. J. Contemp. Brachytherapy 2016, 8, 492–496. [Google Scholar] [CrossRef]
- Dolezel, M.; Stastny, K.; Odrážka, K.; Vaňásek, J.; Kohlova, T.; Dvorakova, D.; Kolářová, I.; Kroulik, T.; Jalcova, L.; Štǎstný, K. Perioperative interstitial CT-based brachytherapy boost in breast cancer patients with breast conservation after neoadjuvant chemotherapy. Neoplasma 2012, 59, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.B.; Anandan, S.; Hartman, A.L.; McSweeney, M.; Chun, J.; McKee, A.; Yang, R.; Kim, C. Radiographic findings after treatment with balloon brachytherapy accelerated partial breast irradiation. RadioGraphics 2015, 35, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.S.; Tchatalbachev, V.; Nelson, J.; Wang, Z.; Dale, P.; Biedermann, G.B. Long term radiographic findings of breast brachytherapy: Implications of surgical volume. J. Surg. Oncol. 2017, 116, 203–207. [Google Scholar] [CrossRef]
- Esserman, L.E.; Da Costa, D.; d’Almeida, M.; Gombos, E.C.; Keisch, M.E. Imaging findings after breast brachytherapy. Am. J. Roentgenol. 2006, 187, 57–64. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Dipiro, P.J.; Devlin, P.M.; Nguyen, M.L.; Bellon, J.R. Mammographic appearance following accelerated partial breast irradiation by using MammoSite brachytherapy. Radiology 2010, 255, 362–368. [Google Scholar] [CrossRef]



| Feature | HDR-BT Alone Group A | HDR-BT + Thermal Boost Group B |
|---|---|---|
| Age, median (range) years | 53 (32–71) | p = 0.47 * |
| ≤40 | 4 (16.0%) | 1 (3.1%) |
| 41–50 | 5 (20.0%) | 8 (25.0%) |
| 51–60 | 9 (36.0%) | 13 (40.6%) |
| ≥61 | 7 (28.0%) | 10 (31.3%) |
| T stage | p = 0.53 * | |
| T1a | 0 (0.0%) | 2 (6.3%) |
| T1b | 6 (24.0%) | 5 (15.6%) |
| T1c | 16 (64.0%) | 17 (53.1%) |
| T2 | 2 (8.0%) | 6 (18.8%) |
| Tx | 1 (4.0%) | 2 (6.3%) |
| N stage | p = 0.95 * | |
| N0 | 17 (68.0%) | 20 (62.5%) |
| N1 | 5 (20.0%) | 12 (37.5%) |
| N2a | 3 (12.0%) | 0 (0.0%) |
| Clinical stage | p = 0.52 * | |
| I | 14 (56.0%) | 14 (43.8%) |
| IIA | 7 (28.0%) | 14 (43.8%) |
| IIB | 0 (0.0%) | 2 (6.3%) |
| IIIA | 3 (12.0%) | 0 (0.0%) |
| n.d. | 1 (4.0%) | 2 (6.3%) |
| Histology | ^ p = 0.15 | |
| Ductal invasive carcinoma | 21 (84.0%) | 26 (81.3%) |
| Lobular invasive carcinoma | 2 (8.0%) | 0 (0.0%) |
| Tubular carcinoma | 1 (4.0%) | 3 (9.4%) |
| Not specified (post-chemo) | 1 (4.0%) | 3 (9.4%) |
| Grade | p = 0.34 * | |
| G1 | 9 (36.0%) | 11 (34.4%) |
| G2 | 13 (52%) | 11 (34.4%) |
| G3 | 3 (12.0%) | 7 (21.9%) |
| Not specified (post-chemo) | 0 (0.0%) | 3 (9.4%) |
| Estrogen receptor status | ^ p = 0.29 | |
| ER (+) | 18 (72.0%) | 21 (65.6%) |
| ER (−) | 7 (28.0%) | 8 (25.0%) |
| n.d. | 0 (0.0%) | 3 (9.4%) |
| Progesteron receptor status | ^ p = 0.27 | |
| PgR (+) | 16 (64.0%) | 20 (62.5%) |
| PgR (−) | 9 (36.0%) | 9 (28.1%) |
| n.d. | 0 (0.0%) | 3 (9.4%) |
| HER2 status † | ^ p = 0.13 | |
| Positive (+) | 0 (0.0%) | 1 (3.1%) |
| Negative (−) | 22 (88.0%) | 21 (65.6%) |
| n.d. | 3 (12.0%) † | 10 (31.3%) † |
| Lymph node treatment | ^ p = 0.85 | |
| ALND | 23 (92.0%) | 29 (90.6%) |
| SNB ‡ | 2 (8.0%) | 3 (9.4%) |
| Chemotherapy treatment | ^ p = 0.30 | |
| Yes | 13 (52.0%) | 21 (65.6%) |
| No | 12 (48.0%) | 11 (34.4%) |
| External Beam RT regimen | p = 0.66 * | |
| 42.5 Gy/2.5 Gy/17 fx | 15 (60.0%) | 20 (62.5%) |
| 45.0 Gy/2.25 Gy/20 fx | 7 (28.0%) | 12 (37.5%) |
| 50.0 Gy/2.0 Gy/25 fx | 3 (12.0%) | 0 (0.0%) |
| Feature | HDR-BT Alone Group A | HDR-BT + Thermal Boost Group B |
|---|---|---|
| Overall survival | Test log rank | |
| Alive 46 (80.7%) | 19 (76.0%) | 27 (84.4%) |
| Dead 11 (19.3%) | 6 (24.0%) | 5 (15.6%) |
| Local control | Test log rank | |
| Yes 55 (96.5%) | 24 (96.0%) | 31 (96.9%) |
| No 2 (3.5%) | 1 (4.0%) | 1 (3.1%) |
| Distant metastases | Test log rank | |
| No 50 (87.7%) | 22 (88.0%) | 28 (87.5%) |
| Yes 7 (12.3%) | 3 (12.0%) | 4 (12.5%) |
| Feature | HDR-BT Alone Group A | HDR-BT + Thermal Boost Group B |
|---|---|---|
| Cosmetic effect | ^ p = 0.68 | |
| Excellent 32 (56.1%) | 15 (60.0%) | 17 (53.1%) |
| Good 20 (35.1%) | 9 (36.0%) | 11 (34.4%) |
| Satisfactory 4 (7.0%) | 1 (4.0%) | 3 (9.4%) |
| Poor 1 (1.8%) | 0 (0.0%) | 1 (3.1%) |
| Tumor bed hardening | ^ p = 0.70 | |
| No 38 (66.7%) | 16 (64.0%) | 22 (68.7%) |
| Yes 19 (33.3%) | 9 (36.0%) | 10 (31.3%) |
| Teleangiectases | ||
| No 56 (98.2%) | 24 (96.0%) | 23 (100.0%) |
| Yes 1 (1.8%) | 1 (4.0%) | 0 (0.0%) |
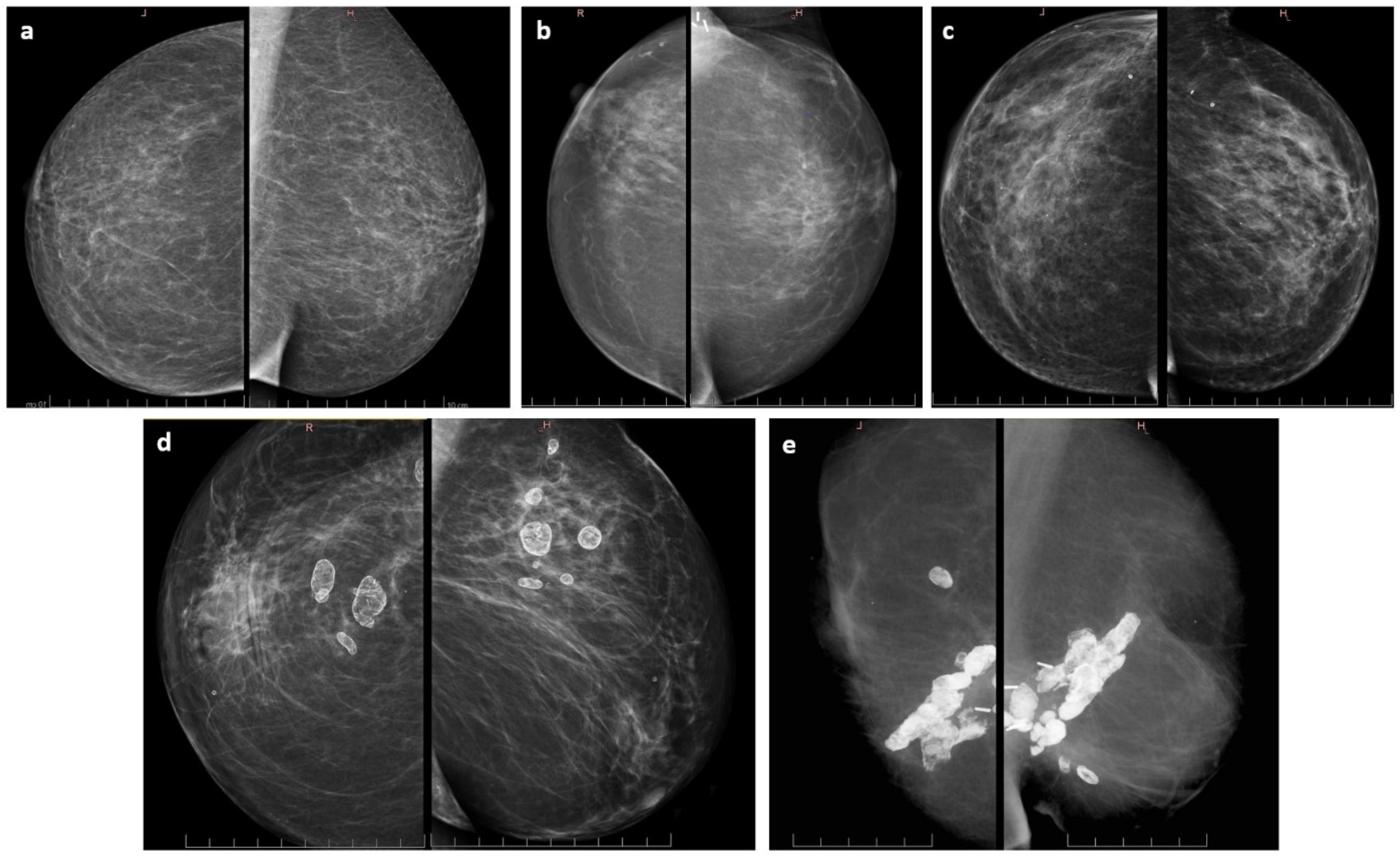
| Mammography findings | ^ p = 0.34 | |
| No changes | 7 (28.0%) | 5 (15.6%) |
| In breast scarring | 10 (40.0%) | 13 (40.7%) |
| Scarring with calcifications | 3 (12.0%) | 9 (28.2%) |
| Asymptomatic fat necrosis | 4 (16.0%) | 2 (6.2%) |
| Symptomatic fat necrosis | 0 (0.0%) | 1 (3.1%) |
| n.d. | 1 (4.0%) | 2 (6.2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chicheł, A.; Burchardt, W.; Chyrek, A.J.; Bielęda, G. Thermal Boost Combined with Interstitial Brachytherapy in Early Breast Cancer Conserving Therapy—Initial Group Long-Term Clinical Results and Late Toxicity. J. Pers. Med. 2022, 12, 1382. https://doi.org/10.3390/jpm12091382
Chicheł A, Burchardt W, Chyrek AJ, Bielęda G. Thermal Boost Combined with Interstitial Brachytherapy in Early Breast Cancer Conserving Therapy—Initial Group Long-Term Clinical Results and Late Toxicity. Journal of Personalized Medicine. 2022; 12(9):1382. https://doi.org/10.3390/jpm12091382
Chicago/Turabian StyleChicheł, Adam, Wojciech Burchardt, Artur J. Chyrek, and Grzegorz Bielęda. 2022. "Thermal Boost Combined with Interstitial Brachytherapy in Early Breast Cancer Conserving Therapy—Initial Group Long-Term Clinical Results and Late Toxicity" Journal of Personalized Medicine 12, no. 9: 1382. https://doi.org/10.3390/jpm12091382






