Immunoglobulins G of Patients with Schizophrenia Protects from Superoxide: Pilot Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Blood Sampling
2.3. Laboratory Methods
2.4. Statistics
3. Results
3.1. General and Clinical Characteristics of Study Groups
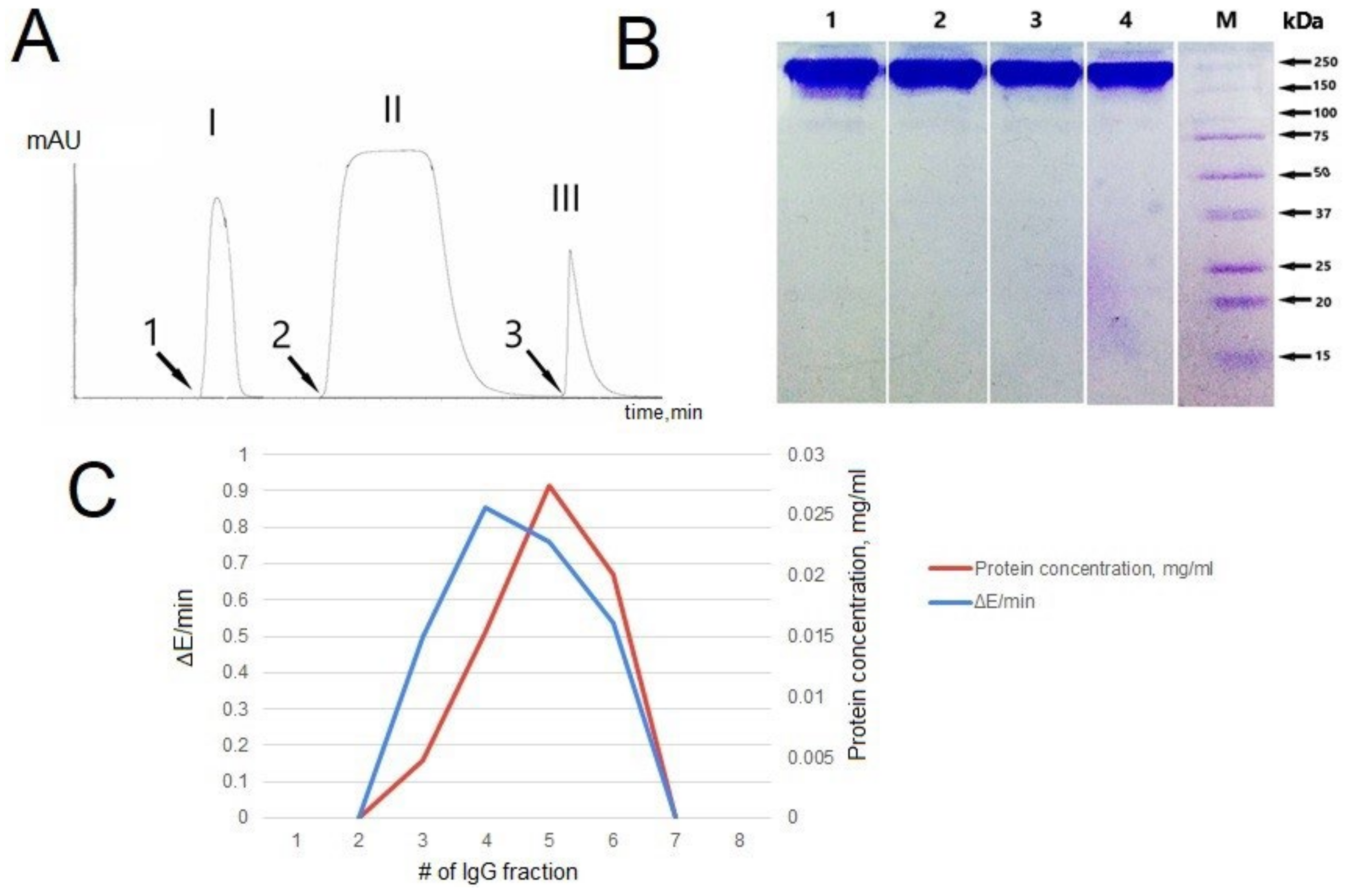
3.2. IgG Purification and Analysis
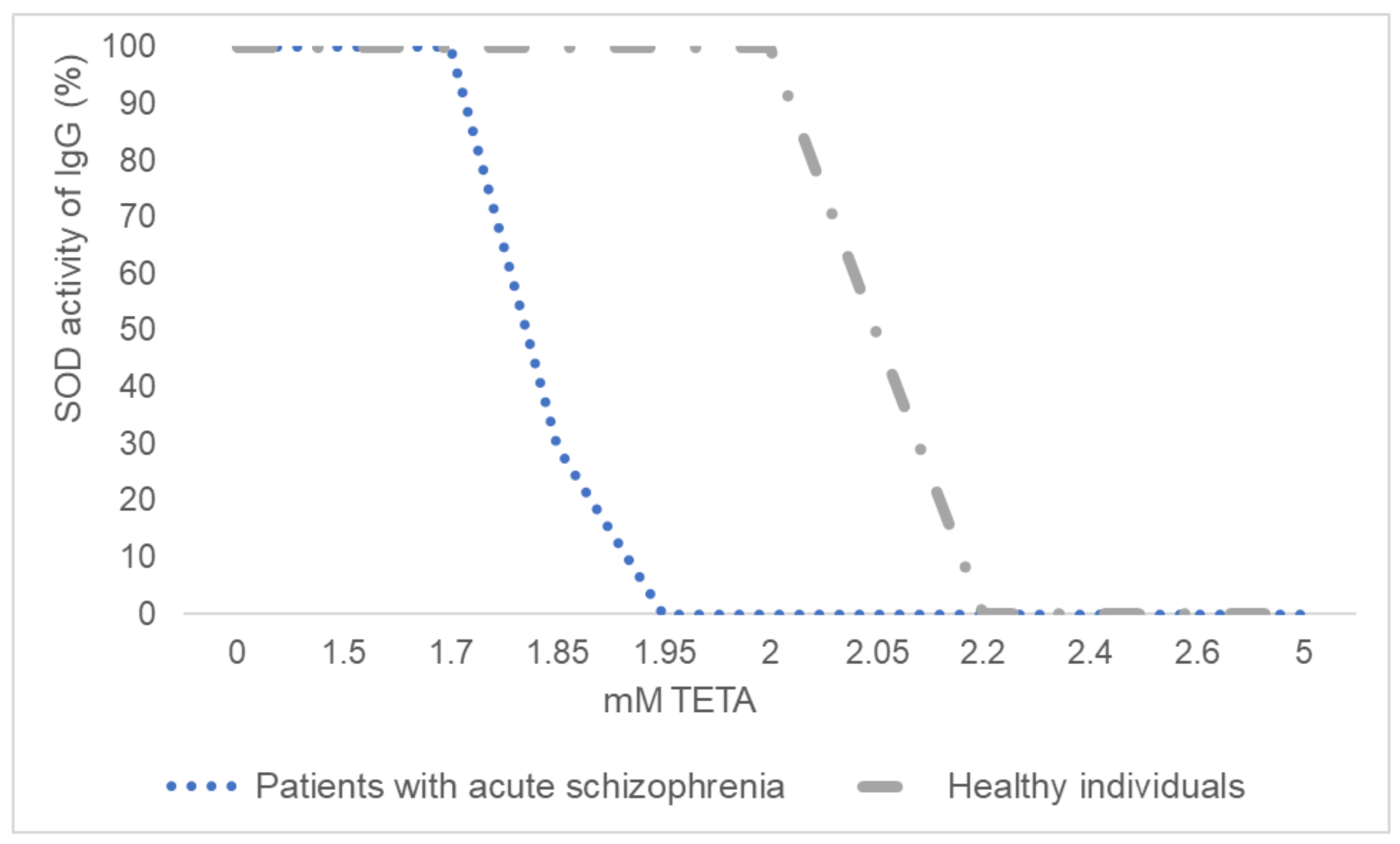
3.3. Inhibitory Analysis of IgGs’ SOD Activity
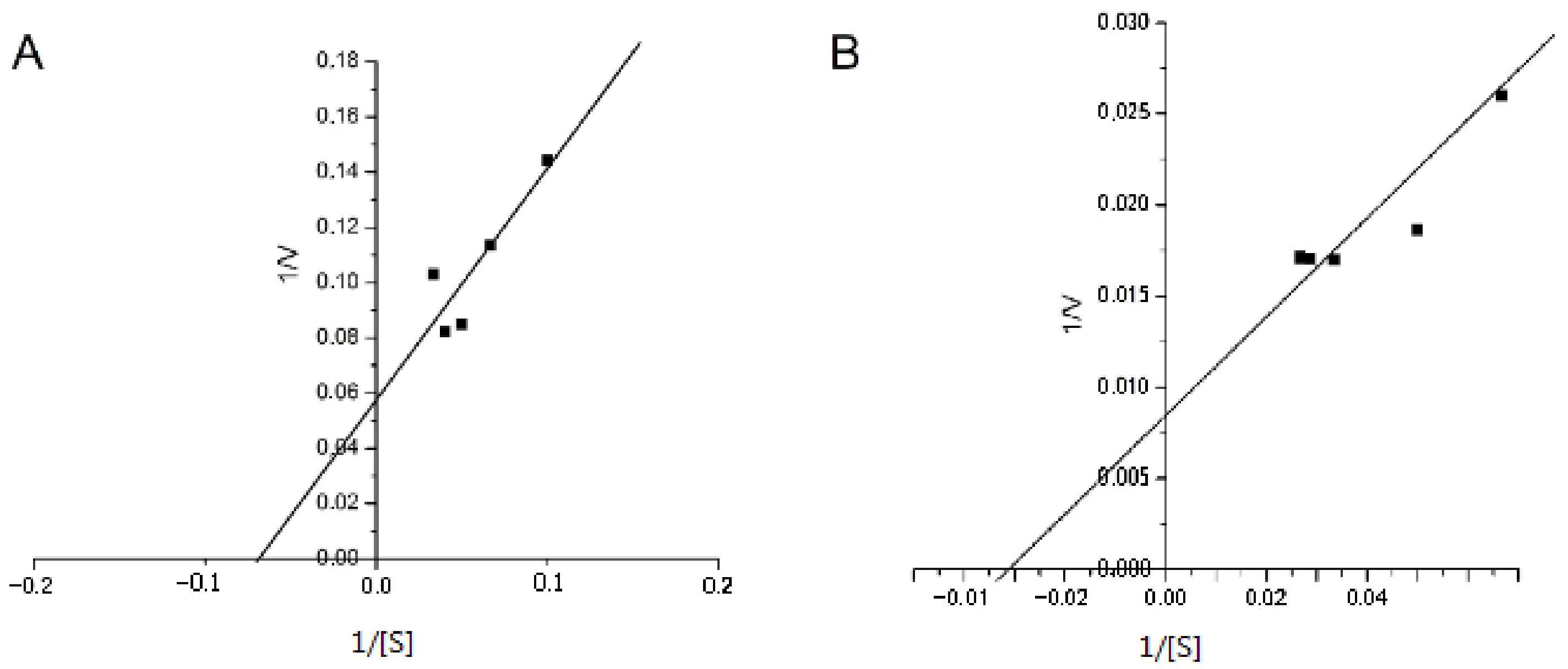
3.4. Determination of the Kinetic Parameters of IgGs’ SOD Activity
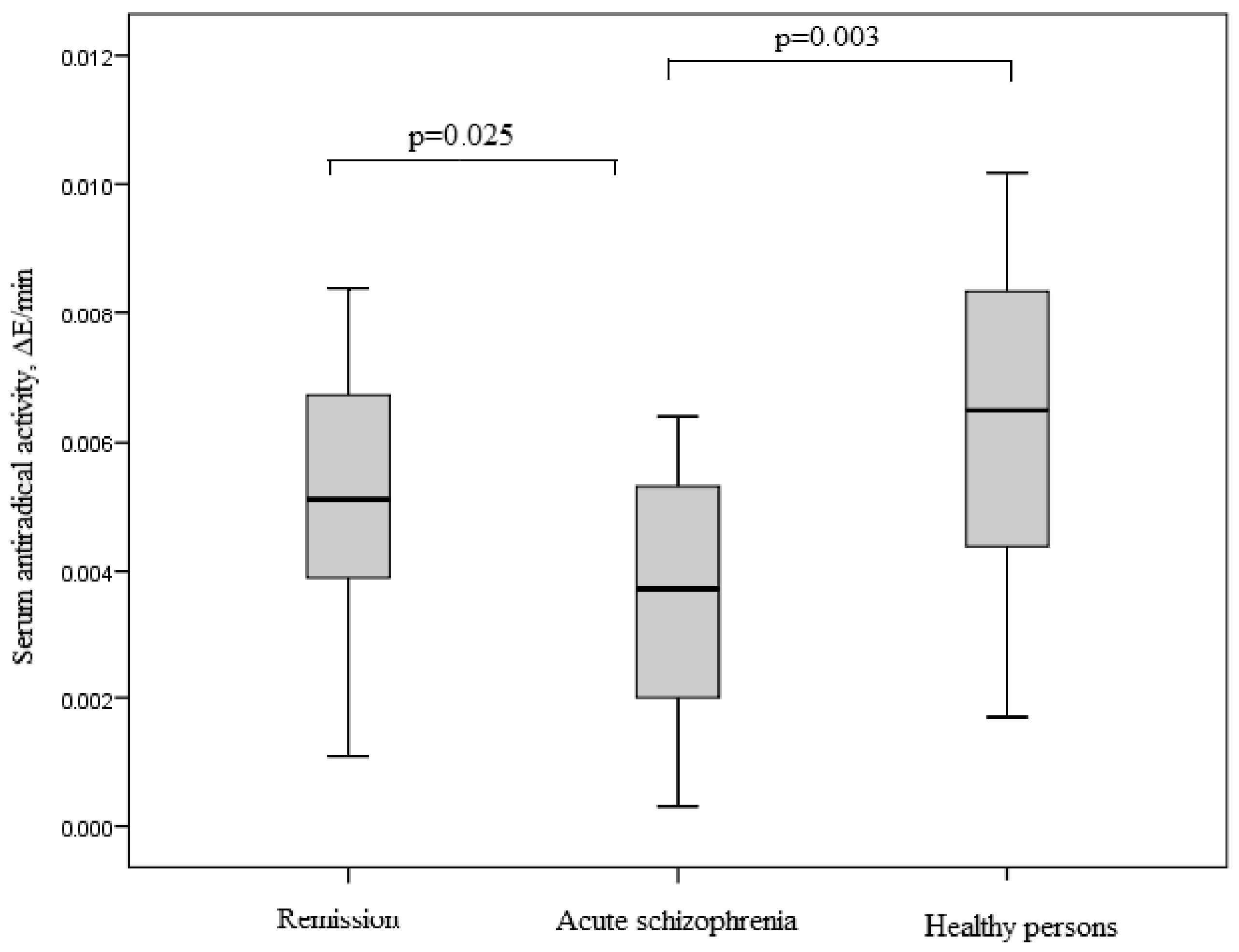
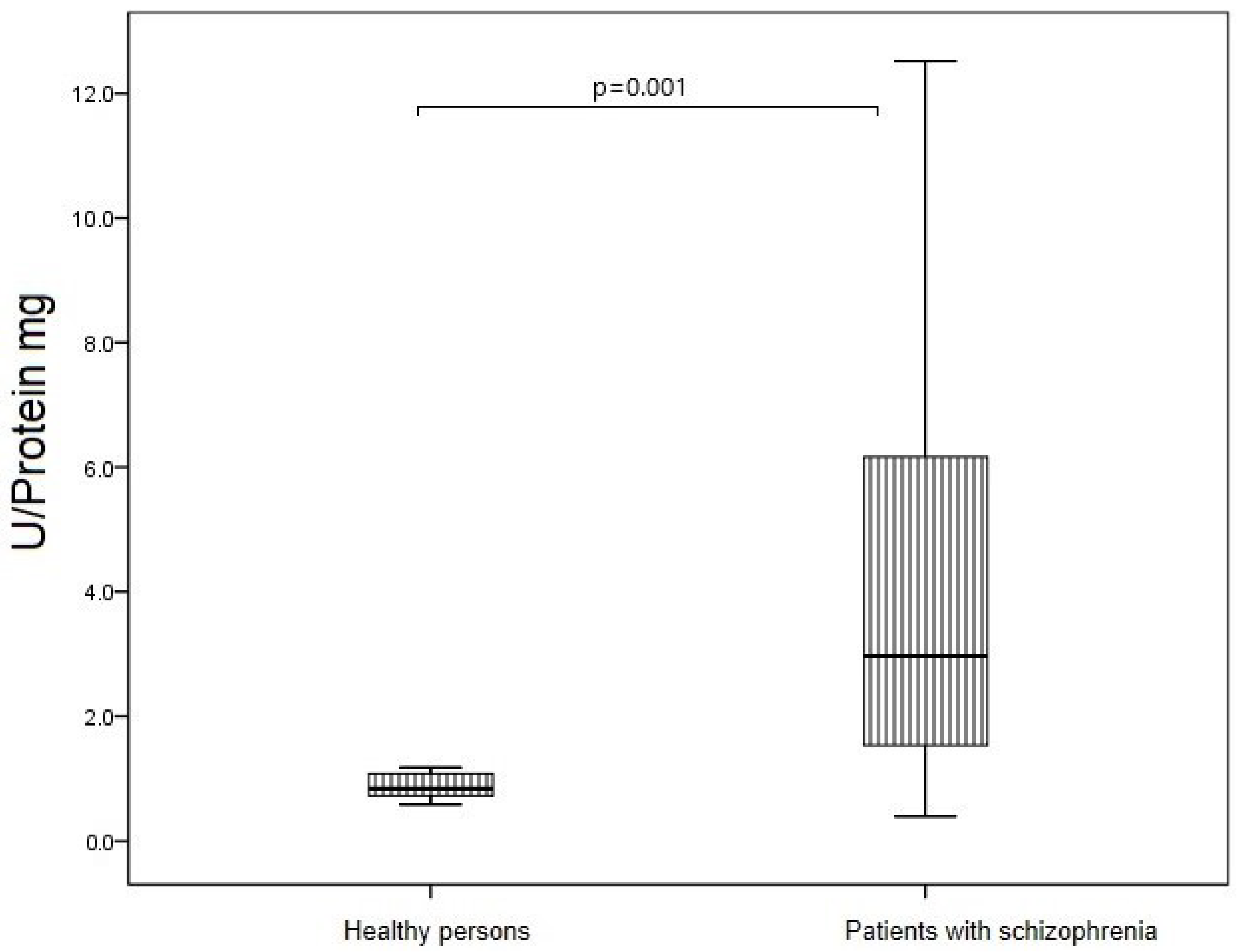
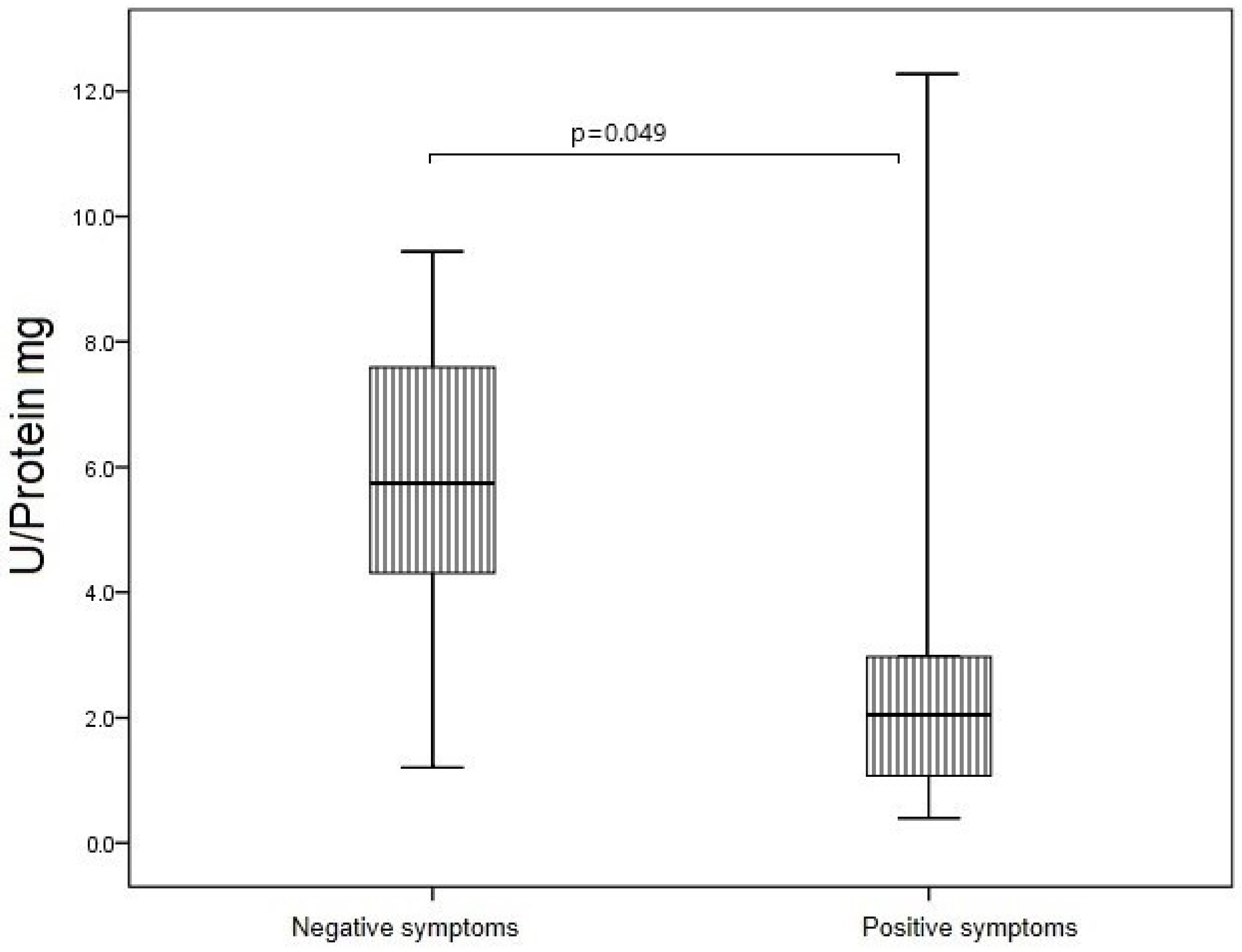
3.5. SOD Activity of IgG in Study Groups
3.6. Antioxidant System in Study Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shukla, V.; Mishra, S.K.; Pant, H.C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 2011, 572634. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Dmitrieva, E.M.; Parshukova, D.A.; Kazantseva, D.V.; Vasilieva, A.R.; Smirnova, L.P. Oxidative Stress-Related Mechanisms in Schizophrenia Pathogenesis and New Treatment Perspectives. Oxidative Med. Cell. Longev. 2021, 2021, 8881770. [Google Scholar] [CrossRef]
- Huang, D.; Liu, S. Oxidative stress and schizophrenia. J. Psychiatry Brain Sci. 2017, 2, 4. [Google Scholar] [CrossRef]
- Ivanova, S.A.; Smirnova, L.P.; Shchigoreva, Y.G.; Boiko, A.S.; Semke, A.V.; Uzbekov, M.G.; Bokhan, N.A. Glucose-6-phosphate dehydrogenase and catalase activities in erythrocytes of schizophrenic patients under pharmacotherapy with traditional antipsychotics. Neurochem. J. 2014, 8, 66–70. [Google Scholar] [CrossRef]
- Ivanova, S.A.; Smirnova, L.P.; Shchigoreva, Y.G.; Semke, A.V.; Bokhan, N.A. Serum glutathione in patients with schizophrenia in dynamics of antipsychotic therapy. Bull. Exp. Biol. Med. 2015, 160, 283–285. [Google Scholar] [CrossRef]
- Kropp, S.; Kern, V.; Lange, K.; Degner, D.; Hajak, G.; Kornhuber, J.; Rüther, E.; Emrich, H.M.; Schneider, U.; Bleich, S. Oxidative stress during treatment with first- and second-generation antipsychotics. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.-L.; Li, X.-S.; Chen, G.-Y.; Du, Y.; Wei, Z.-X.; Chen, X.; Zheng, G.-E.; Deng, W.; Cheng, Y. Serum oxidative stress marker levels in unmedicated and medicated patients with schizophrenia. J. Mol. Neurosci. 2018, 66, 428–436. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tan, Y.L.; Cao, L.Y.; Wu, G.Y.; Xu, Q.; Shen, Y.; Zhou, D.F. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr. Res. 2006, 81, 291–300. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Z.; He, L.; Wan, C. A meta–analysis of oxidative stress markers in schizophrenia. Sci. China Life Sci. 2010, 53, 112–124. [Google Scholar] [CrossRef]
- Flatow, J.; Buckley, P.; Miller, B.J. Meta-analysis of oxidative stress in schizophrenia. Biol. Psychiatry 2013, 74, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Raffa, M.; Barhoumi, S.; Atig, F.; Fendri, C.; Kerkeni, A.; Mechri, A. Reduced antioxidant defense systems in schizophrenia and bipolar I disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 39, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Reyazuddin, M.; Azmi, S.A.; Islam, N.; Rizvi, A. Oxidative stress and level of antioxidant enzymes in drug-naive schizophrenics. Indian J. Psychiatry 2014, 56, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Kuloglu, M.; Ustundag, B.; Atmaca, M.; Canatan, H.; Ertan Tezcan, A.; Cinkilinc, N. Lipid Peroxidation and Antioxidant Enzyme Levels in Patients with Schizophrenia and Bipolar Disorder. Cell Biochem. Funct. 2002, 20, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Gama, C.S.; Andreazza, A.C.; Salvador, M.; Ceresér, K.M.; Gomes, F.A.; Belmonte-de-Abreu, P.S.; Berk, M.; Kapczinski, F. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhou, D.F.; Cao, L.Y.; Zhang, P.Y.; Wu, G.Y. Elevated blood superoxide dismutase in neuroleptic-free schizophrenia: Association with positive symptoms. Psychiatry Res. 2003, 117, 85–88. [Google Scholar] [CrossRef]
- Wang, D.M.; Chen, D.C.; Wang, L.; Zhang, X.Y. Sex differences in the association between symptoms and superoxide dismutasein patients with never-treated first-episode schizophrenia. World J. Biol. Psychiatry 2021, 22, 325–334. [Google Scholar] [CrossRef]
- Michel, T.M.; Thome, J.; Martin, D.; Nara, K.; Zwerina, S.; Tatschner, T.; Weijers, H.G.; Koutsilieri, E. Cu, Zn- and Mn-superoxide dismutase levels in brains of patients with schizophrenic psychosis. J. Neural Transm. 2004, 111, 1191–1201. [Google Scholar] [CrossRef]
- Pauling, L. Molecular architecture and biological reactions. Chem. Eng. News 1946, 24, 1375–1377. [Google Scholar] [CrossRef]
- Jencks, W.P. Catalysis in Chemistry and Enzymology; Courier Corporation: Chelmsford, MA, USA; McGraw-Hill: New York, NY, USA, 1987; pp. 1–806. [Google Scholar]
- Nevinsky, G.A.; Buneva, V.N. Natural Catalytic Antibodies in Norm, Autoimmune, Viral, and bacterial Diseases. Sci. World J. 2010, 10, 1203–1233. [Google Scholar] [CrossRef]
- Taguchi, H.; Planque, S.; Nishiyama, Y.; Symersky, J.; Boivin, S.; Szabo, P.; Friedland, R.P.; Ramsland, P.A.; Edmundson, A.B.; Weksler, M.E.; et al. Autoantibody-catalyzed hydrolysis of amyloid β peptide. J. Biol. Chem. 2008, 283, 4714–4722. [Google Scholar] [CrossRef] [Green Version]
- Ermakov, E.A.; Smirnova, L.P.; Parkhomenko, T.A.; Dmitrenok, P.S.; Krotenko, N.M.; Fattakhov, N.S.; Bokhan, N.A.; Semke, A.V.; Ivanova, S.A.; Buneva, V.N.; et al. DNA-hydrolysing activity of IgG antibodies from the sera of patients with schizophrenia. Open Biol. 2015, 5, 150064. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, E.A.; Kabirova, E.M.; Buneva, V.N.; Nevinsky, G.A. IgGs-Abzymes from the sera of patients with multiple sclerosis recognize and hydrolyze miRNAs. Int. J. Mol. Sci. 2021, 22, 2812. [Google Scholar] [CrossRef] [PubMed]
- Parshukova, D.A.; Smirnova, L.P.; Kornetova, E.G.; Semke, A.V.; Buneva, V.N.; Ivanova, S.A. Igg-Dependent Hydrolysis of Myelin Basic Protein of Patients with Different Courses of Schizophrenia. J. Immunol. Res. 2020, 2020, 8986521. [Google Scholar] [CrossRef] [PubMed]
- Kulberg, A.; Petyaev, I.M.; Zamotaeva, N.G. Catalytic properties and catalytic destruction of cellular receptors (R-proteins). Immunology 1988, 3, 37–40. [Google Scholar]
- Lerner, R.A.; Tramontano, A. Antibodies as enzymes. Trends Biochem. Sci. 1987, 12, 427–438. [Google Scholar] [CrossRef]
- Tolmacheva, A.S.; Blinova, E.A.; Ermakov, E.A.; Buneva, V.N.; Vasilenko, N.L.; Nevinsky, G.A. IgG abzymes with peroxidase and oxidoreductase activities from the sera of healthy humans. J. Mol. Recognit. 2015, 28, 565–580. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Smirnova, L.P.; Bokhan, N.A.; Semke, A.V.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Catalase activity of IgG antibodies from the sera of healthy donors and patients with schizophrenia. PLoS ONE 2017, 12, e0183867. [Google Scholar] [CrossRef]
- Smirnova, L.P.; Mednova, I.A.; Krotenko, N.M.; Alifirova, V.M.; Ivanova, S.A. IgG-Dependent Dismutation of Superoxide in Patients with Different Types of Multiple Sclerosis and Healthy Subjects. Oxidative Med. Cell. Longev. 2020, 2020, 8171020. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Glavind, J. Antioxidants in animal tissue. Acta Chem. Scand. 1963, 17, 1635–1640. [Google Scholar] [CrossRef]
- Gülçin, İ.; Elias, R.; Gepdiremen, A.; Boyer, L. Antioxidant Activity of Lignans from Fringe Tree (Chionanthus virginicus L.). Eur. Food Res. Technol. 2006, 223, 759–767. [Google Scholar] [CrossRef]
- Smirnova, L.P.; Kondakova, I.V.; Borunov, E.V. Method for Determining Superoxide Dismutase Activity. Patent RF No. 2272074, 20 March 2006. (In Russian). [Google Scholar]
- Nevinsky, G.A.; Kanyshkova, T.G.; Buneva, V.N. Natural catalytic antibodies (abzymes) in normalcy and pathology. Biochemistry 2000, 65, 1245–1255. [Google Scholar]
- Voet, D. Biochemistry, 4th ed.; John Wiley& Sons Inc.: New York, NY, USA, 2011; ISBN 0470917458. [Google Scholar]
- Wentworth, A.D.; Jones, L.H.; Wentworth, P.; Janda, K.D.; Lerner, R.A. Antibodies have the intrinsic capacity to destroy antigens. Proc. Natl. Acad. Sci. USA 2000, 97, 10930–10935. [Google Scholar] [CrossRef] [PubMed]
- Ikhmyangan, E.N.; Vasilenko, N.L.; Buneva, V.N.; Nevinsky, G.A. Metal ions-dependent peroxidase and oxidoreductase activities of polyclonal IgGs from the sera of Wistar rats. J. Mol. Recognit. 2006, 19, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Keinan, E.E. (Ed.) Catalytic Antibodies; Wiley: Weinheim, Germany, 2005; 586p. [Google Scholar] [CrossRef]
- Case, A.J. On the Origin of Superoxide Dismutase: An Evolutionary Perspective of Superoxide-Mediated Redox Signaling. Antioxidants 2017, 6, 82. [Google Scholar] [CrossRef]
- Moskowitz, S.I.; Basu, S.B.; Bergold, P.J. Chronic and cyclical neuronal loss in hippocampal slice cultures following transient inhibition of the type 1 isoform of superoxide dismutase. Brain Res. 2001, 913, 207–219. [Google Scholar] [CrossRef]
- Brennan, A.M.; Suh, S.W.; Won, S.J.; Narasimhan, P.; Kauppinen, T.M.; Lee, H.; Edling, Y.; Chan, P.H.; Swanson, R.A. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat. Neurosci. 2009, 12, 857–863. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhou, D.F.; Su, J.M.; Zhang, P.Y. The effect of extract of ginkgo biloba added to haloperidol on superoxide dismutase in inpatients with chronic schizophrenia. J. Clin. Psychopharmacol. 2001, 21, 85–88. [Google Scholar] [CrossRef]
- Parikh, V.; Khan, M.M.; Mahadik, S.P. Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J. Psychiatr. Res. 2003, 37, 43–51. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhou, D.F.; Shen, Y.C.; Zhang, P.Y.; Zhang, W.F.; Liang, J.; Chen, D.C.; Xiu, M.H.; Kosten, T.A.; Kosten, T.R. Effects of risperidone and haloperidol on superoxide dismutase and nitric oxide in schizophrenia. Neuropharmacology 2012, 62, 1928–1934. [Google Scholar] [CrossRef]
- Arnaiz, S.L.; Coronel, M.F.; Boveris, A. Nitric oxide, superoxide, and hydrogen peroxide production in brain mitochondria after haloperidol treatment. Nitric Oxide 1999, 3, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhou, D.F.; Qi, L.Y.; Chen, S.; Cao, L.Y.; Chen, D.C.; Xiu, M.H.; Wang, F.; Wu, G.Y.; Lu, L.; et al. Superoxide dismutase and cytokines in chronic patients with schizophrenia: Association with psychopathology and response to antipsychotics. Psychopharmacology 2009, 204, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Virit, O.; Altindag, A.; Yumru, M.; Dalkilic, A.; Savas, H.A.; Selek, S.; Erel, O.; Herken, H. A defect in the antioxidant defense system in schizophrenia. Neuropsychobiology 2009, 60, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, Y.; Ünsal, C.; Beyazyüz, M.; Ünal, A.; Kuloğlu, M. Reduced total antioxidant level and increased oxidative stress in patients with deficit schizophrenia: A preliminary study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 144–149. [Google Scholar] [CrossRef]
- Morera-Fumero, A.L.; Diaz-Mesa, E.; Abreu-Gonzalez, P.; Fernandez-Lopez, L.; del Rosario Cejas-Mendez, M. Low levels of serum total antioxidant capacity and presence at admission and absence at discharge of a day/night change as a marker of acute paranoid schizophrenia relapse. Psychiatry Res. 2017, 249, 200–205. [Google Scholar] [CrossRef]
- Al-Chalabi, B.M.; Thanoon, I.A.; Ahmed, F.A. Potential effect of olanzapine on total antioxidant status and lipid peroxidation in schizophrenic patients. Neuropsychobiology 2009, 59, 8–11. [Google Scholar] [CrossRef]



| Parameter | Patients with Schizophrenia | Healthy Persons | p-Value | |
|---|---|---|---|---|
| Acute Schizophrenia | Remission | |||
| Sex (men/women) | 18 (52.9%)/16 (47.1%) | 17 (42.9%)/16 (48.5%) | 6 (42.9%)/8 (57.1%) | 0.913 |
| Age, years | 40.00 ± 10.97 | 38.9 ± 8.93 | 33.7 ± 8.81 | 0.132 |
| Age of manifestation, years | 22 (19; 30) | 22 (20; 27) | - | 0.802 |
| Duration of schizophrenia, years | 15 (12; 18) | 15 (11.5; 20) | - | 0.545 |
| PANSS total score | 97.5 (89; 105.5) | 62,1 (51.3; 71) | - | 0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mednova, I.A.; Smirnova, L.P.; Vasilieva, A.R.; Kazantseva, D.V.; Epimakhova, E.V.; Krotenko, N.M.; Semke, A.V.; Ivanova, S.A. Immunoglobulins G of Patients with Schizophrenia Protects from Superoxide: Pilot Results. J. Pers. Med. 2022, 12, 1449. https://doi.org/10.3390/jpm12091449
Mednova IA, Smirnova LP, Vasilieva AR, Kazantseva DV, Epimakhova EV, Krotenko NM, Semke AV, Ivanova SA. Immunoglobulins G of Patients with Schizophrenia Protects from Superoxide: Pilot Results. Journal of Personalized Medicine. 2022; 12(9):1449. https://doi.org/10.3390/jpm12091449
Chicago/Turabian StyleMednova, Irina A., Liudmila P. Smirnova, Alisa R. Vasilieva, Daria V. Kazantseva, Elena V. Epimakhova, Nina M. Krotenko, Arkadiy V. Semke, and Svetlana A. Ivanova. 2022. "Immunoglobulins G of Patients with Schizophrenia Protects from Superoxide: Pilot Results" Journal of Personalized Medicine 12, no. 9: 1449. https://doi.org/10.3390/jpm12091449






