A Two-Step Approach for 3D-Guided Patient-Specific Corrective Limb Osteotomies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
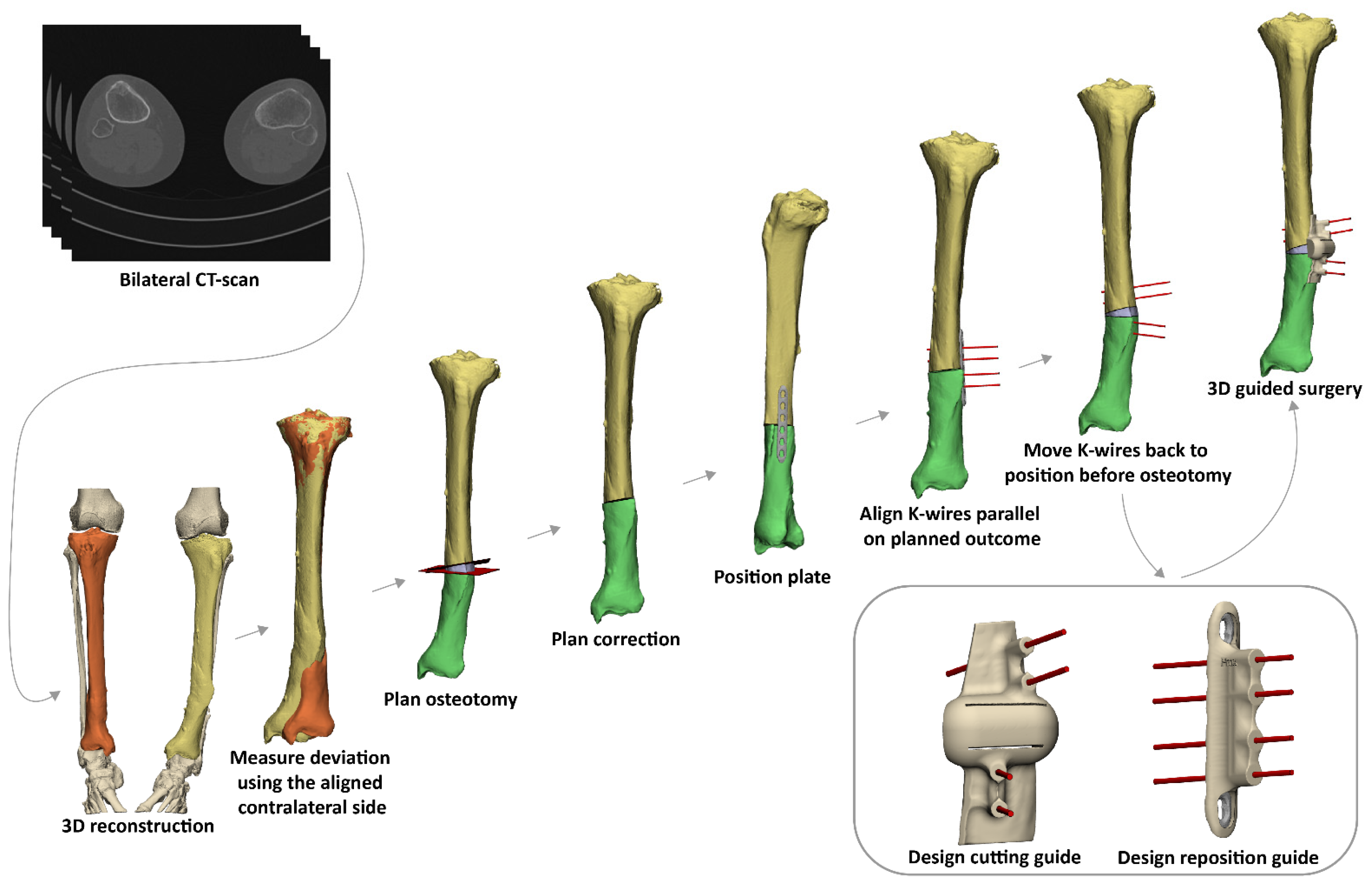
2.2. 3D Virtual Surgical Planning of the Corrective Osteotomy
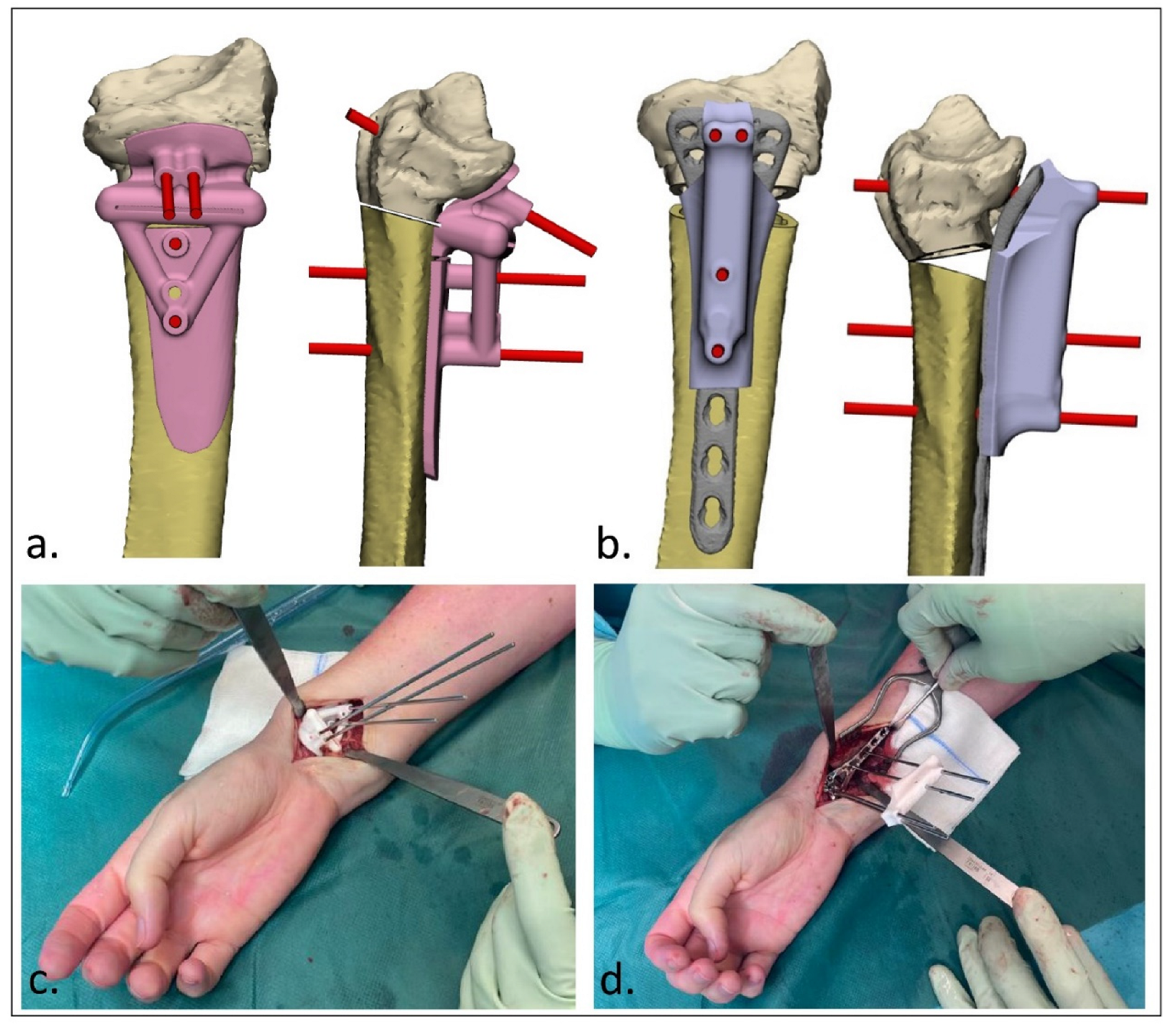
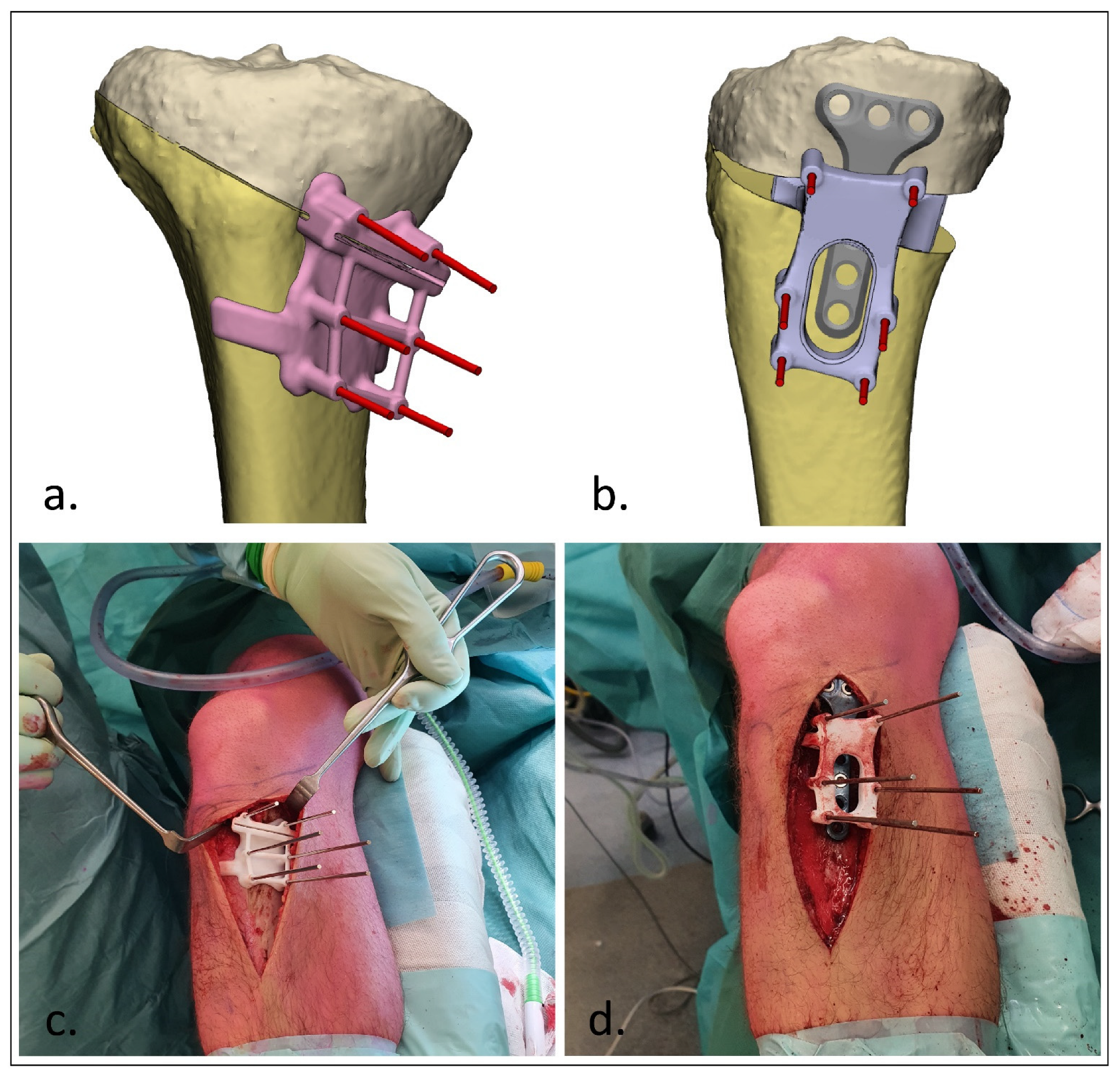
2.3. Surgical Procedure
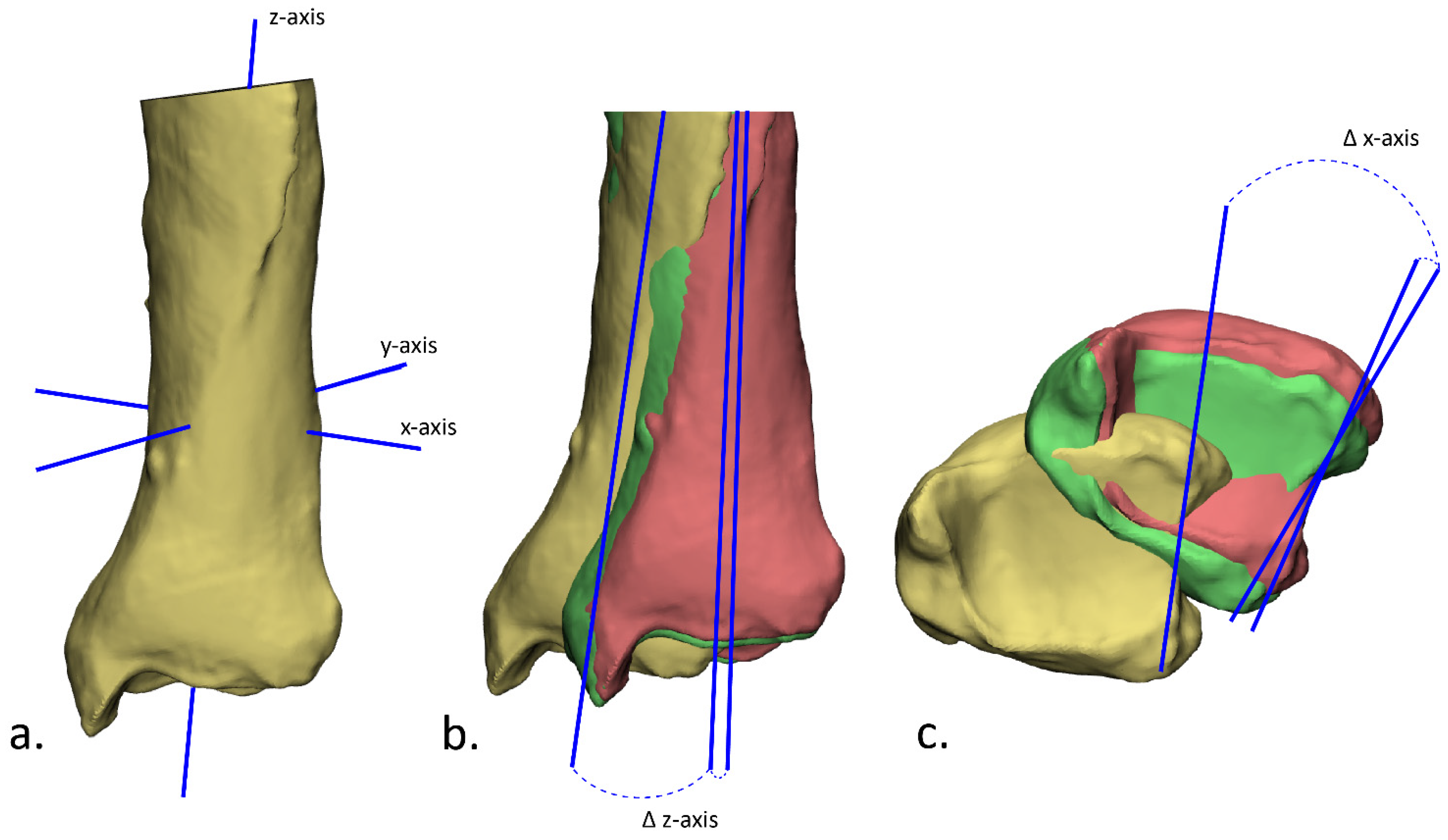
2.4. Postoperative Evaluation
3. Results
3.1. Patients
3.2. Accuracy
3.3. Clinical Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lal, H.; Patralekh, M.K. 3D printing and its applications in orthopaedic trauma: A technological marvel. J. Clin. Orthop. Trauma 2018, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Baraza, N.; Chapman, C.; Zakani, S.; Mulpuri, K. 3D-printed patient specific instrumentation in corrective osteotomy of the femur and pelvis: A review of the literature. 3D Print Med. 2020, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.; Murphy, D.; Gelfer, Y. The effect of three-dimensional (3D) printing on quantitative and qualitative outcomes in paediatric orthopaedic osteotomies: A systematic review. EFORT Open Rev. 2021, 6, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, H.; Rosseels, W.; Sermon, A.; Nijs, S. Corrective limb osteotomy using patient specific 3D-printed guides: A technical note. Injury 2016, 47, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Buijze, G.A.; Leong, N.L.; Stockmans, F.; Axelsson, P.; Moreno, R.; Sörensen, A.I.; Jupiter, J.B. Three-dimensional compared with two-dimensional preoperative planning of corrective osteotomy for extra-articular distal radial malunion: A multicenter randomized controlled trial. J. Bone Jt. Surg. Am. 2018, 100, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, M.; van Haver, A.; Bertrand, V.; Vanhees, M.; Verstreken, F. Corrective osteotomy of distal radius malunions using three-dimensional computer simulation and patient-specific guides to achieve anatomic reduction. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Athlani, L.; Chenel, A.; Detammaecker, R.; de Almeida, Y.-K.; Dautel, G. Computer-assisted 3D preoperative planning of corrective osteotomy for extra-articular distal radius malunion: A 16-patient case series. Hand Surg. Rehabil. 2020, 39, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Oka, K.; Tanaka, H.; Okada, K.; Sahara, W.; Myoui, A.; Yamada, T.; Yamamoto, M.; Kurimoto, S.; Hirata, H.; Murase, T. Three-dimensional corrective osteotomy for malunited fractures of the upper extremity using patient-matched instruments: A prospective, multicenter, open-label, single-arm trial. J. Bone Jt. Surg. Am. 2019, 101, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Rosseels, W.; Herteleer, M.; Sermon, A.; Nijs, S.; Hoekstra, H. Corrective osteotomies using patient-specific 3D-printed guides: A critical appraisal. Eur. J. Trauma Emerg. Surg. 2019, 45, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.C.; van Es, E.M.; Kraan, G.A.; Verhaar, J.A.N.; Stockmans, F.; Colaris, J.W. Outcomes of 3-D corrective osteotomies for paediatric malunited both-bone forearm fractures. J. Hand Surg. 2022, 47, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Dobbe, J.G.G.; Peymani, A.; Roos, H.A.L.; Beerens, M.; Streekstra, G.J.; Strackee, S.D. Patient-specific plate for navigation and fixation of the distal radius: A case series. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Omori, S.; Murase, T.; Oka, K.; Kawanishi, Y.; Oura, K.; Tanaka, H.; Yoshikawa, H. Postoperative accuracy analysis of three-dimensional corrective osteotomy for cubitus varus deformity with a custom-made surgical guide based on computer simulation. J. Shoulder Elbow Surg. 2015, 24, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.E.; Zimmermann, S.; Aichmair, A.; Hingsammer, A.; Schweizer, A.; Nagy, L.; Fürnstahl, P. Conventional versus computer-assisted corrective osteotomy of the forearm: A retrospective analysis of 56 consecutive cases. J. Hand Surg. Am. 2017, 42, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Gerbers, J.G.; Pijpker, P.A.J.; Brouwer, R.W.; van der Veen, H.C. Anterolateral proximal tibial opening wedge osteotomy for biplanar correction in genu valgum recurvatum using patient specific instrumentation (PSI). A technical note. Knee 2021, 33, 58–64. [Google Scholar] [CrossRef] [PubMed]
- van Raaij, T.; van der Wel, H.; Beldman, M.; de Vries, A.; Kraeima, J. Two-Step 3D-Guided Supramalleolar Osteotomy to Treat Varus Ankle osteoarthritis. Foot Ankle Int. 2022, 43, 937–941. [Google Scholar] [CrossRef] [PubMed]
| Case | Sex | Age at Time of Surgery (Years) | Area of Deformity | Deformity | Planned Correction | |
|---|---|---|---|---|---|---|
| Upper limb | 1 | F | 16 | Clavicula | Angulation; Shortening | Closed wedge |
| 2 | F | 23 | Proximal humerus | Varus; Rotation | Closed wedge | |
| 3 | M | 12 | Midshaft radius | Angulation | Closed wedge | |
| 4 | M | 16 | Distal radius | Rotation | Rotation | |
| 5 | M | 17 | Distal radius | Volar angulation | Open wedge | |
| 6 | F | 59 | Distal radius | Volar angulation | Open wedge | |
| Lower limb | 7 | M | 28 | Femur | Rotation | Rotation |
| 8 | M | 17 | Proximal tibia | Varus; Increased tibial slope | Open wedge | |
| 9 | M | 24 | Proximal tibia | Increased tibial slope | Closed wedge | |
| 10 | M | 64 | Distal tibia | Varus; Rotation | Closed wedge |
| Case | Angulation (°) | Rotation (°) | Translation (mm) | |||||
|---|---|---|---|---|---|---|---|---|
| Preoperative vs. Plan | Postoperative vs. Plan | Over/under Correction | Preoperative vs. Plan | Postoperative vs. Plan | Over/under Correction | Preoperative vs. Plan | Postoperative vs. Plan | |
| 1 | 20.5 | 3.7 | Under | - | - | - | 8.1 | 0.3 |
| 2 | 14.7 | 2.7 | Under | 22.8 | 1.3 | Over | 6.2 | 3.2 |
| 3 | 14.7 | 1.3 | Over | - | - | - | 7.2 | 0.8 |
| 4 | - | - | - | 26.1 | 4.3 | Over | 4.4 | 0.5 |
| 5 | 12.5 | 2.7 | Over | - | - | - | 7.5 | 1.6 |
| 6 | 27.5 | 1.8 | Over | 5.3 | 1.6 | Over | 7.1 | 1.0 |
| 7 | - | - | - | 26.0 | 5.1 | Under | 1.1 | 0.8 |
| 8 | 13.5 | 2.6 | Under | - | - | Over | 37.8 | 6.2 |
| 9 | 7.1 | 0.2 | Under | - | - | - | 3.5 | 0.4 |
| 10 | 13.6 | 1.6 | Over | 24.7 | 4.8 | Under | 17.2 | 2.9 |
| Average | 15.5 ± 5.7 | 2.1 ± 1.0 | 21.0 ± 7.9 | 3.4 ± 1.6 | 10.0 ± 10.1 | 1.8 ± 1.8 | ||
| Case | Restricted Joint | Preoperative Range of Restricted Motion | Postoperative Range of Motion (6 Months) | |
|---|---|---|---|---|
| Upper limb | 1 | Clavicula | F/E 110-0-40; Ab/Ad 140-0-30 | F/E 160-0-40; Ab/Ad 160-0-30 |
| 2 | Proximal humerus | F/E 150-0-40; Ab/Ad: 140-0-30; ER/IR: 90-0-L5 | F/E 170-0-40; Ab/Ad: 160-0-30; ER/IR: 80-0-T12 | |
| 3 | Midshaft radius | P/S: 10-0-60 | P/S: 45/0/70 | |
| 4 | Distal radius | P/S: 70-0-55 | P/S: 70-0-80 | |
| 5 | Distal radius | P/S: 70-0-30 | P/S: 70-0-80 | |
| 6 | Distal radius | F/E: 50/0/20 P/S: 20/0/80 | F/E: 40/0/50 P/S: 60/0/80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assink, N.; Meesters, A.M.L.; ten Duis, K.; Harbers, J.S.; IJpma, F.F.A.; van der Veen, H.C.; Doornberg, J.N.; Pijpker, P.A.J.; Kraeima, J. A Two-Step Approach for 3D-Guided Patient-Specific Corrective Limb Osteotomies. J. Pers. Med. 2022, 12, 1458. https://doi.org/10.3390/jpm12091458
Assink N, Meesters AML, ten Duis K, Harbers JS, IJpma FFA, van der Veen HC, Doornberg JN, Pijpker PAJ, Kraeima J. A Two-Step Approach for 3D-Guided Patient-Specific Corrective Limb Osteotomies. Journal of Personalized Medicine. 2022; 12(9):1458. https://doi.org/10.3390/jpm12091458
Chicago/Turabian StyleAssink, Nick, Anne M. L. Meesters, Kaj ten Duis, Jorrit S. Harbers, Frank F. A. IJpma, Hugo C. van der Veen, Job N. Doornberg, Peter A. J. Pijpker, and Joep Kraeima. 2022. "A Two-Step Approach for 3D-Guided Patient-Specific Corrective Limb Osteotomies" Journal of Personalized Medicine 12, no. 9: 1458. https://doi.org/10.3390/jpm12091458






