Deep Learning on Basal Cell Carcinoma In Vivo Reflectance Confocal Microscopy Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
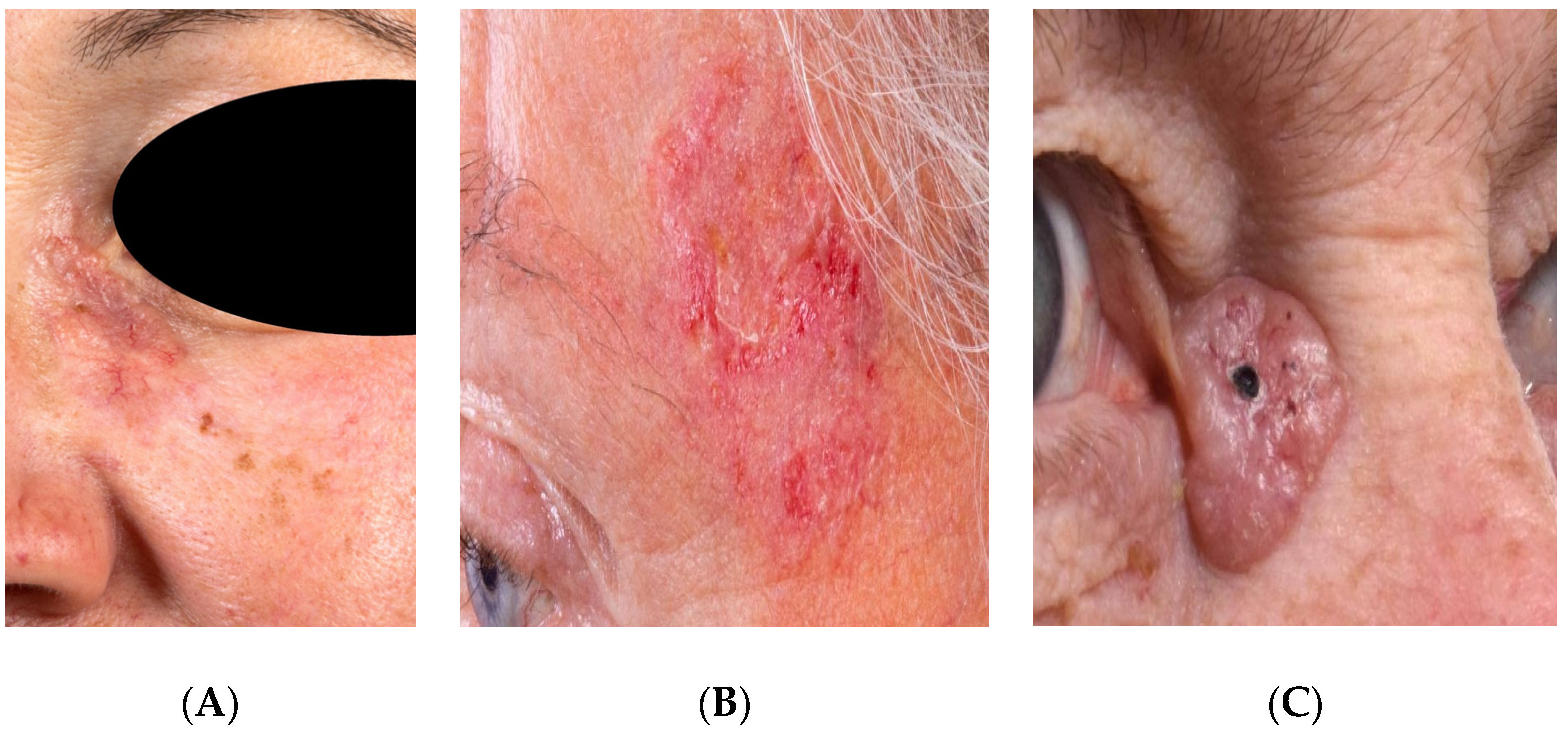
2.2. Imaging
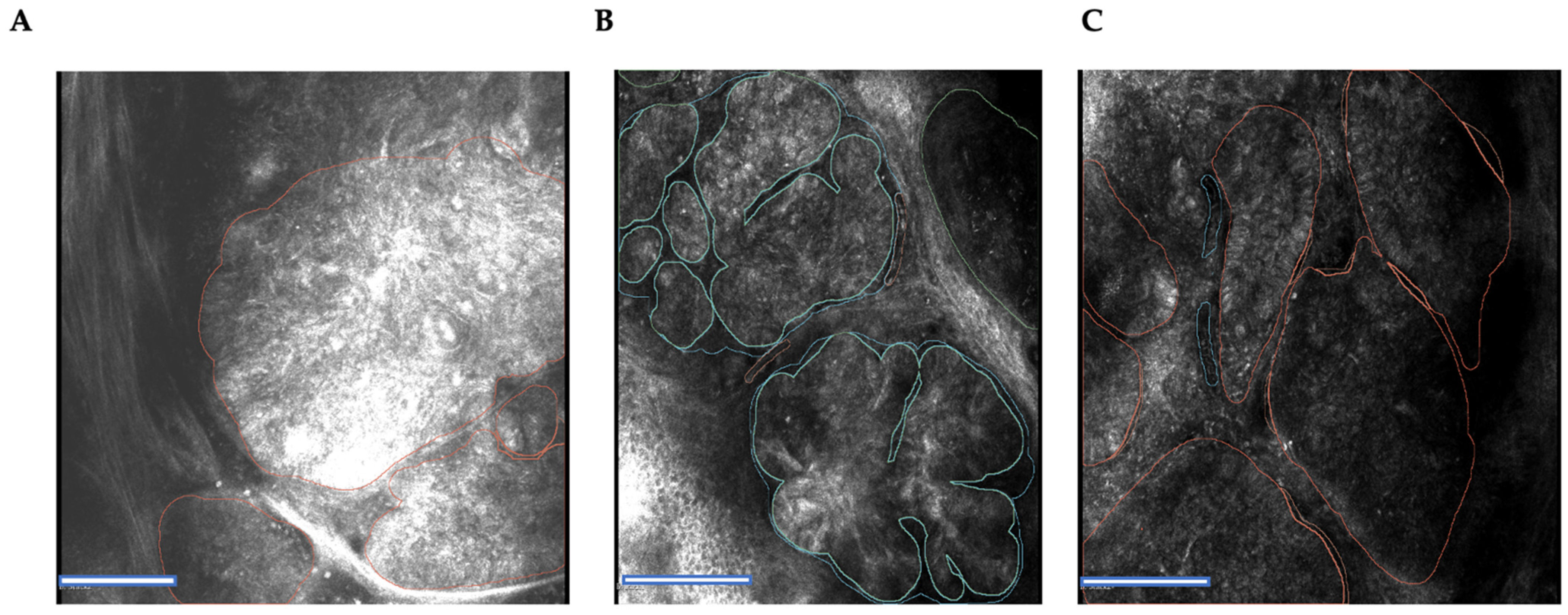
2.3. Tissue Annotation
- (1)
- An epidermal lesion accompanied by cellular pleomorphism and damage to the epidermis above the lesion.
- (2)
- Tumor cells with monomorphic and elongated nuclei.
- (3)
- Nuclei are aligned along a single axis (“nuclear polarization”).
- (4)
- A high ratio of dilated blood vessels and leukocytes.
- (5)
- Inflammation associated with tumor cells.
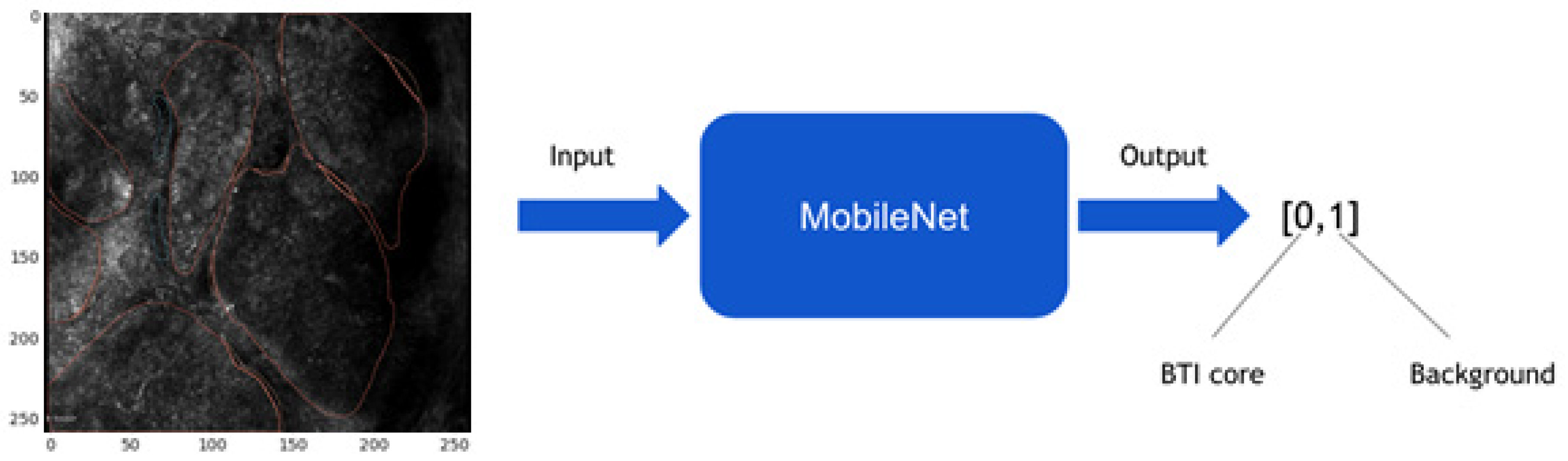
2.4. Image Preprocessing and Convolutional Neural Network Model
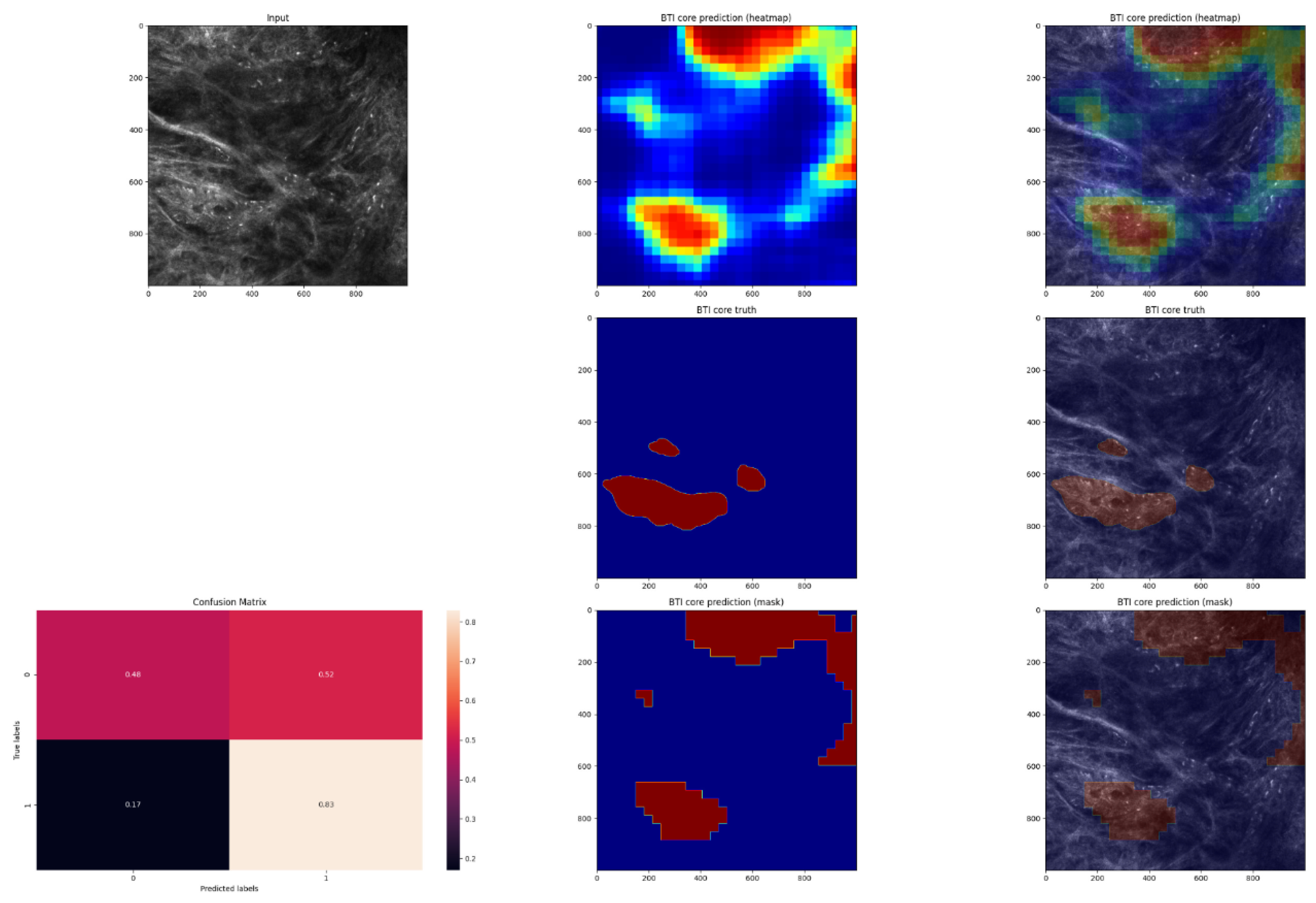
2.5. Expanding MobileNet and Evaluation on the Validation Dataset
- a.
- Improving the visual appearance of the heatmap by using Gaussian filtering.
- b.
- Transforming every pixel with a probability of less than 0.5 to 0 and the rest to 1 using a threshold of 0.5.
- c.
- Erosion (which is intended to remove isolated pixels).
- d.
- The process of dilation (after the erosion operation, the mask is slightly thinner, and the dilation operation restores this property).
2.6. Statistical Analysis
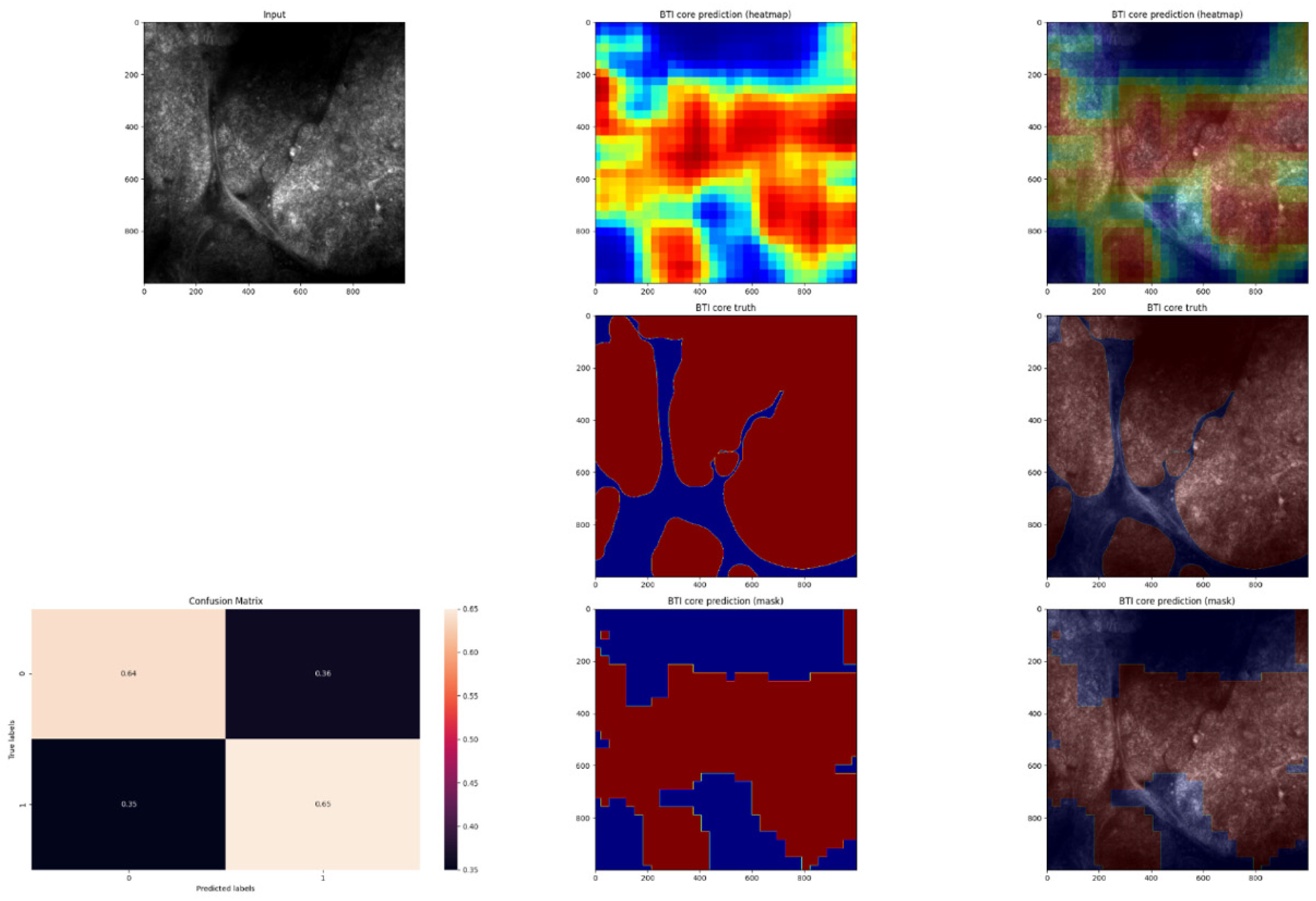
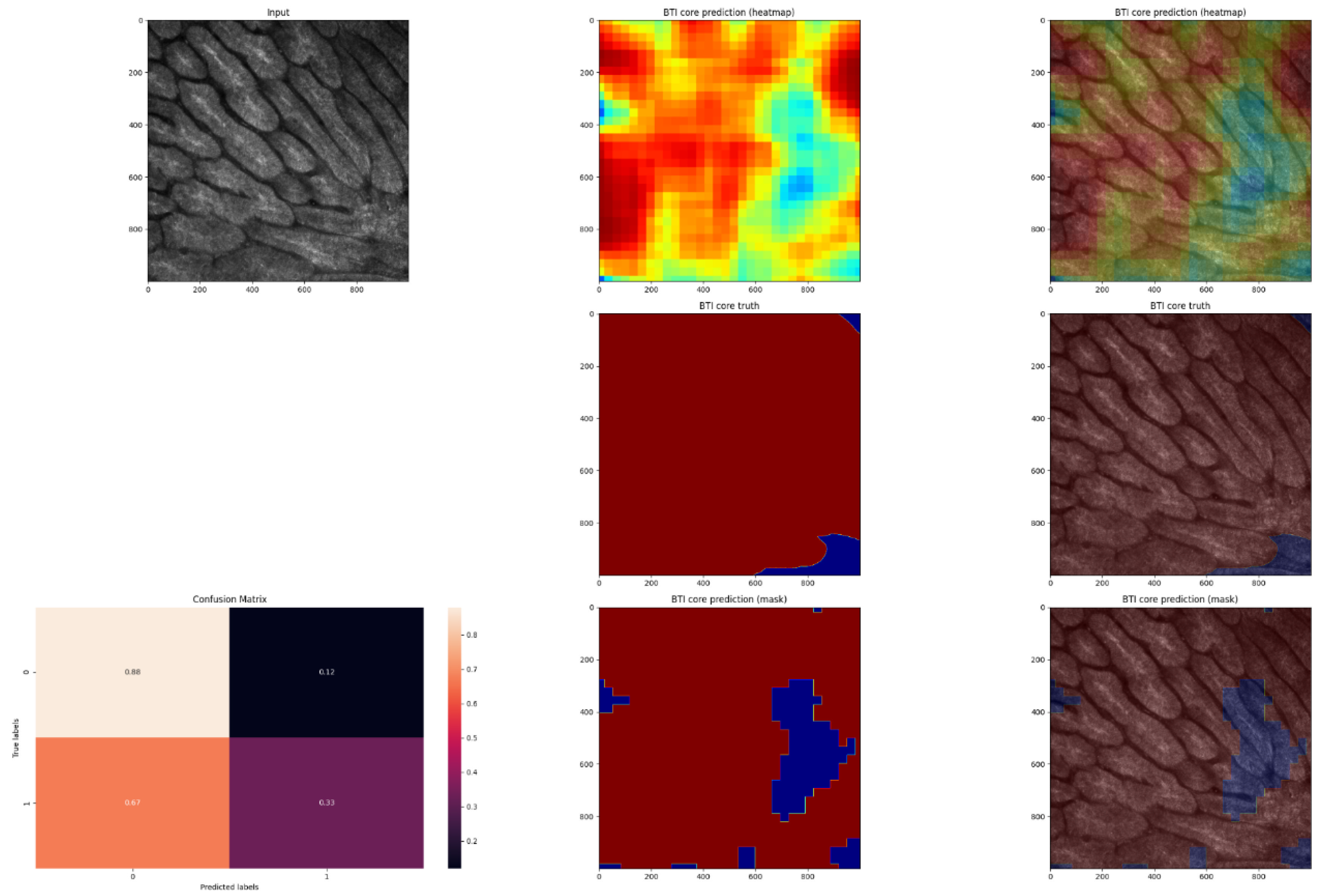
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiu, L.-W.; Hu, S.C.-S. Recent Advances in Noninvasive Imaging of the Skin–Dermoscopy and Optical Coherence Tomography. Dermatol. Sin. 2021, 39, 115. [Google Scholar]
- Nam, S.; Chong, Y.; Jung, C.K.; Kwak, T.-Y.; Lee, J.Y.; Park, J.; Rho, M.J.; Go, H. Introduction to Digital Pathology and Computer-Aided Pathology. J. Pathol. Transl. Med. 2020, 54, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Matheny, M.E.; Whicher, D.; Thadaney Israni, S. Artificial Intelligence in Health Care: A Report From the National Academy of Medicine. JAMA 2020, 323, 509. [Google Scholar] [CrossRef] [PubMed]
- Aubreville, M.; Knipfer, C.; Oetter, N.; Jaremenko, C.; Rodner, E.; Denzler, J. Automatic Classification of Cancerous Tissue in Laserendomicroscopy Images of the Oral Cavity Using Deep Learning. Sci. Rep. 2017, 7, 11979. [Google Scholar] [CrossRef] [PubMed]
- Campanella, G.; Navarrete-Dechent, C.; Liopyris, K.; Monnier, J.; Aleissa, S.; Minhas, B.; Scope, A.; Longo, C.; Guitera, P.; Pellacani, G.; et al. Deep Learning for Basal Cell Carcinoma Detection for Reflectance Confocal Microscopy. J. Investig. Dermatol. 2022, 142, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M. Dermatologist-Level Classification of Skin Cancer with Deep Neural Networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Shavlokhova, V.; Sandhu, S.; Flechtenmacher, C.; Koveshazi, I.; Neumeier, F.; Padrón-Laso, V.; Jonke, Ž.; Saravi, B.; Vollmer, M.; Vollmer, A.; et al. Deep Learning on Oral Squamous Cell Carcinoma Ex Vivo Fluorescent Confocal Microscopy Data: A Feasibility Study. J. Clin. Med. 2021, 10, 5326. [Google Scholar] [CrossRef]
- Benvenuto-Andrade, C.; Dusza, S.W.; Agero, A.L.C.; Scope, A.; Rajadhyaksha, M.; Halpern, A.C. Differences between Polarized Light Dermoscopy and Immersion Contact Dermoscopy for the Evaluation of Skin Lesions. Arch. Dermatol. 2007, 143, 329–338. [Google Scholar] [CrossRef]
- Elliott, A.D. Confocal Microscopy: Principles and Modern Practices. Curr. Protoc. Cytom. 2020, 92, e68. [Google Scholar] [CrossRef]
- Zysk, A.M.; Nguyen, F.T.; Oldenburg, A.L.; Marks, D.L.; Boppart, S.A. Optical Coherence Tomography: A Review of Clinical Development from Bench to Bedside. J. Biomed. Opt. 2007, 12, 051403. [Google Scholar] [CrossRef]
- Manfredini, M.; Arginelli, F.; Dunsby, C.; French, P.; Talbot, C.; König, K.; Pellacani, G.; Ponti, G.; Seidenari, S. High-Resolution Imaging of Basal Cell Carcinoma: A Comparison between Multiphoton Microscopy with Fluorescence Lifetime Imaging and Reflectance Confocal Microscopy. Skin Res. Technol. 2013, 19, e433. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, F.; Migliorati, S.; Farnetani, F.; De Pace, B.; Ciardo, S.; Manfredini, M.; Reggiani Bonetti, L.; Kaleci, S.; Chester, J.; Pellacani, G. Nodular Skin Lesions: Correlation of Reflectance Confocal Microscopy and Optical Coherence Tomography Features. J. Eur. Acad. Derm. Venereol. 2020, 34, 101–111. [Google Scholar] [CrossRef]
- Guitera, P.; Menzies, S.W.; Longo, C.; Cesinaro, A.M.; Scolyer, R.A.; Pellacani, G. In Vivo Confocal Microscopy for Diagnosis of Melanoma and Basal Cell Carcinoma Using a Two-Step Method: Analysis of 710 Consecutive Clinically Equivocal Cases. J. Investig. Dermatol. 2012, 132, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.A.; Caruntu, C.; Lupu, M.; Lixandru, D.; Tampa, M.; Georgescu, S.-R. Current and Future Applications of Confocal Laser Scanning Microscopy Imaging in Skin Oncology. Oncol. Lett. 2019, 17, 4102–4111. [Google Scholar] [CrossRef]
- Kadouch, D.J.; Elshot, Y.S.; Zupan-Kajcovski, B.; van Haersma de With, A.S.E.; van der Wal, A.C.; Leeflang, M. One-Stop-Shop with Confocal Microscopy Imaging vs. Standard Care for Surgical Treatment of Basal Cell Carcinoma: An Open-Label, Noninferiority, Randomized Controlled Multicentre Trial. Br. J. Derm. 2017, 177, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Kadouch, D.J.; Leeflang, M.M.; Elshot, Y.S.; Longo, C.; Ulrich, M.; van der Wal, A.C. Diagnostic Accuracy of Confocal Microscopy Imaging vs. Punch Biopsy for Diagnosing and Subtyping Basal Cell Carcinoma. J. Eur. Acad. Derm. Venereol. 2017, 31, 1641–1648. [Google Scholar] [CrossRef]
- Flores, E.S.; Cordova, M.; Kose, K.; Phillips, W.; Rossi, A.; Nehal, K. Intraoperative Imaging during Mohs Surgery with Reflectance Confocal Microscopy: Initial Clinical Experience. J. Biomed. Opt. 2015, 20, 61103. [Google Scholar] [CrossRef]
- Mogensen, M.; Jemec, G.B.E. Diagnosis of Nonmelanoma Skin Cancer/Keratinocyte Carcinoma: A Review of Diagnostic Accuracy of Nonmelanoma Skin Cancer Diagnostic Tests and Technologies. Derm. Surg. 2007, 33, 1158–1174. [Google Scholar] [CrossRef]
- Lorber, A.; Wiltgen, M.; Hofmann-Wellenhof, R.; Koller, S.; Weger, W.; Ahlgrimm-Siess, V. Correlation of Image Analysis Features and Visual Morphology in Melanocytic Skin Tumours Using in Vivo Confocal Laser Scanning Microscopy. Skin Res. Technol. 2009, 15, 237–241. [Google Scholar] [CrossRef]
- Pan, Z.-Y.; Lin, J.-R.; Cheng, T.-T.; Wu, J.-Q.; Wu, W.-Y. In Vivo Reflectance Confocal Microscopy of Basal Cell Carcinoma: Feasibility of Preoperative Mapping of Cancer Margins. Derm. Surg. 2012, 38, 1945–1950. [Google Scholar] [CrossRef]
- Sahu, A.; Yélamos, O.; Iftimia, N.; Cordova, M.; Alessi-Fox, C.; Gill, M.; Maguluri, G.; Dusza, S.W.; Navarrete-Dechent, C.; González, S. Evaluation of a Combined Reflectance Confocal Microscopy–Optical Coherence Tomography Device for Detection and Depth Assessment of Basal Cell Carcinoma. JAMA Dermatol. 2018, 154, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Peppelman, M.; Wolberink, E.A.W.; Blokx, W.A.M.; van de Kerkhof, P.C.M.; van Erp, P.E.J.; Gerritsen, M.-J.P. In Vivo Diagnosis of Basal Cell Carcinoma Subtype by Reflectance Confocal Microscopy. Dermatology 2013, 227, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.; Popa, I.M.; Voiculescu, V.M.; Boda, D.; Caruntu, C.; Zurac, S. A Retrospective Study of the Diagnostic Accuracy of In Vivo Reflectance Confocal Microscopy for Basal Cell Carcinoma Diagnosis and Subtyping. J. Clin. Med. 2019, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- González, S. Confocal Reflectance Microscopy in Dermatology: Promise and Reality of Non-Invasive Diagnosis and Monitoring. Actas Dermosifiliogr. 2009, 100, 59–69. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Longo, C.; Venturini, M.; Sala, R.; Pellacani, G. Reflectance Confocal Microscopy for in Vivo Skin Imaging. Photochem. Photobiol. 2008, 84, 1421–1430. [Google Scholar] [CrossRef]
- González, S.; Tannous, Z. Real-Time, in Vivo Confocal Reflectance Microscopy of Basal Cell Carcinoma. J. Am. Acad. Dermatol. 2002, 47, 869–874. [Google Scholar] [CrossRef]
- Ulrich, M.; Roewert-Huber, J.; González, S.; Rius-Diaz, F.; Stockfleth, E.; Kanitakis, J. Peritumoral Clefting in Basal Cell Carcinoma: Correlation of in Vivo Reflectance Confocal Microscopy and Routine Histology. J. Cutan. Pathol. 2011, 38, 190–195. [Google Scholar] [CrossRef]
- Nori, S.; Rius-Díaz, F.; Cuevas, J.; Goldgeier, M.; Jaen, P.; Torres, A. Sensitivity and Specificity of Reflectance-Mode Confocal Microscopy for in Vivo Diagnosis of Basal Cell Carcinoma: A Multicenter Study. J. Am. Acad. Dermatol. 2004, 51, 923–930. [Google Scholar] [CrossRef]
- Hofmann-Wellenhof, R. Reflectance Confocal Microscopy for Skin Diseases; Springer: Heidelberg, Germany, 2012; ISBN 978-3-642-21997-9. [Google Scholar]
- Hoffman, A.; Goetz, M.; Vieth, M.; Galle, P.R.; Neurath, M.F.; Kiesslich, R. Confocal Laser Endomicroscopy: Technical Status and Current Indications. Endoscopy 2006, 38, 1275–1283. [Google Scholar] [CrossRef]
- Lang, B.M.; Balermpas, P.; Bauer, A.; Blum, A.; Brölsch, G.F.; Dirschka, T. S2k Guidelines for Cutaneous Basal Cell Carcinoma—Part 2: Treatment, Prevention and Follow-Up. J. Dtsch. Derm. Ges. 2019, 17, 214–230. [Google Scholar] [CrossRef]
- Saravi, B.; Hassel, F.; Ülkümen, S.; Zink, A.; Shavlokhova, V.; Couillard-Despres, S.; Boeker, M.; Obid, P.; Lang, G.M. Artificial Intelligence-Driven Prediction Modeling and Decision Making in Spine Surgery Using Hybrid Machine Learning Models. J. Pers. Med. 2022, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M. A Survey on Deep Learning in Medical Image Analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Samaras, D.; Kurc, T.M.; Gao, Y.; Davis, J.E.; Saltz, J.H. Patch-Based Convolutional Neural Network for Whole Slide. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 2424–2433. [Google Scholar]
- Kraus, O.Z.; Ba, J.L.; Frey, B.J. Classifying and Segmenting Microscopy Images with Deep Multiple Instance Learning. Bioinformatics 2016, 32, i52–i59. [Google Scholar] [CrossRef] [PubMed]
- Langlotz, C.P.; Allen, B.; Erickson, B.J.; Kalpathy-Cramer, J.; Bigelow, K.; Cook, T.S. A Roadmap for Foundational Research on Artificial Intelligence in Medical Imaging: From the 2018 NIH/RSNA/ACR/The Academy Workshop. Radiology. 2019, 291, 781–791. [Google Scholar] [CrossRef]
- Willemink, M.J.; Koszek, W.A.; Hardell, C.; Wu, J.; Fleischmann, D.; Harvey, H. Preparing Medical Imaging Data for Machine Learning. Radiology 2020, 295, 4–15. [Google Scholar] [CrossRef]


| Sex | Age (Mean) | Subtype | Percentage |
|---|---|---|---|
| male (56%) | 71.87 | nodular BCC | 69% |
| female (44%) | superficial/plaque/trunk skin BCC | 22% | |
| sclerosing/morpheaform/infiltrative BCC | 4% | ||
| infundibulocystic BCC | 3% | ||
| pigmented BCC | 1% |
| Truth/Prediction | P = Noncancerous | P = Cancerous |
|---|---|---|
| T = noncancerous | 233,794,335 (TN) 0.85 | 40,618,083 (FP) 0.15 |
| T = cancerous | 58,199,803 (FN) 0.54 | 49,387,779 (TP) 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shavlokhova, V.; Vollmer, M.; Gholam, P.; Saravi, B.; Vollmer, A.; Hoffmann, J.; Engel, M.; Freudlsperger, C. Deep Learning on Basal Cell Carcinoma In Vivo Reflectance Confocal Microscopy Data. J. Pers. Med. 2022, 12, 1471. https://doi.org/10.3390/jpm12091471
Shavlokhova V, Vollmer M, Gholam P, Saravi B, Vollmer A, Hoffmann J, Engel M, Freudlsperger C. Deep Learning on Basal Cell Carcinoma In Vivo Reflectance Confocal Microscopy Data. Journal of Personalized Medicine. 2022; 12(9):1471. https://doi.org/10.3390/jpm12091471
Chicago/Turabian StyleShavlokhova, Veronika, Michael Vollmer, Patrick Gholam, Babak Saravi, Andreas Vollmer, Jürgen Hoffmann, Michael Engel, and Christian Freudlsperger. 2022. "Deep Learning on Basal Cell Carcinoma In Vivo Reflectance Confocal Microscopy Data" Journal of Personalized Medicine 12, no. 9: 1471. https://doi.org/10.3390/jpm12091471
APA StyleShavlokhova, V., Vollmer, M., Gholam, P., Saravi, B., Vollmer, A., Hoffmann, J., Engel, M., & Freudlsperger, C. (2022). Deep Learning on Basal Cell Carcinoma In Vivo Reflectance Confocal Microscopy Data. Journal of Personalized Medicine, 12(9), 1471. https://doi.org/10.3390/jpm12091471







