Simultaneous Motor and Visual Intraoperative Neuromonitoring in Asleep Parietal Lobe Surgery: Dual Strip Technique
Abstract
:1. Introduction
2. Methods
3. Intraoperative Neuromonitoring
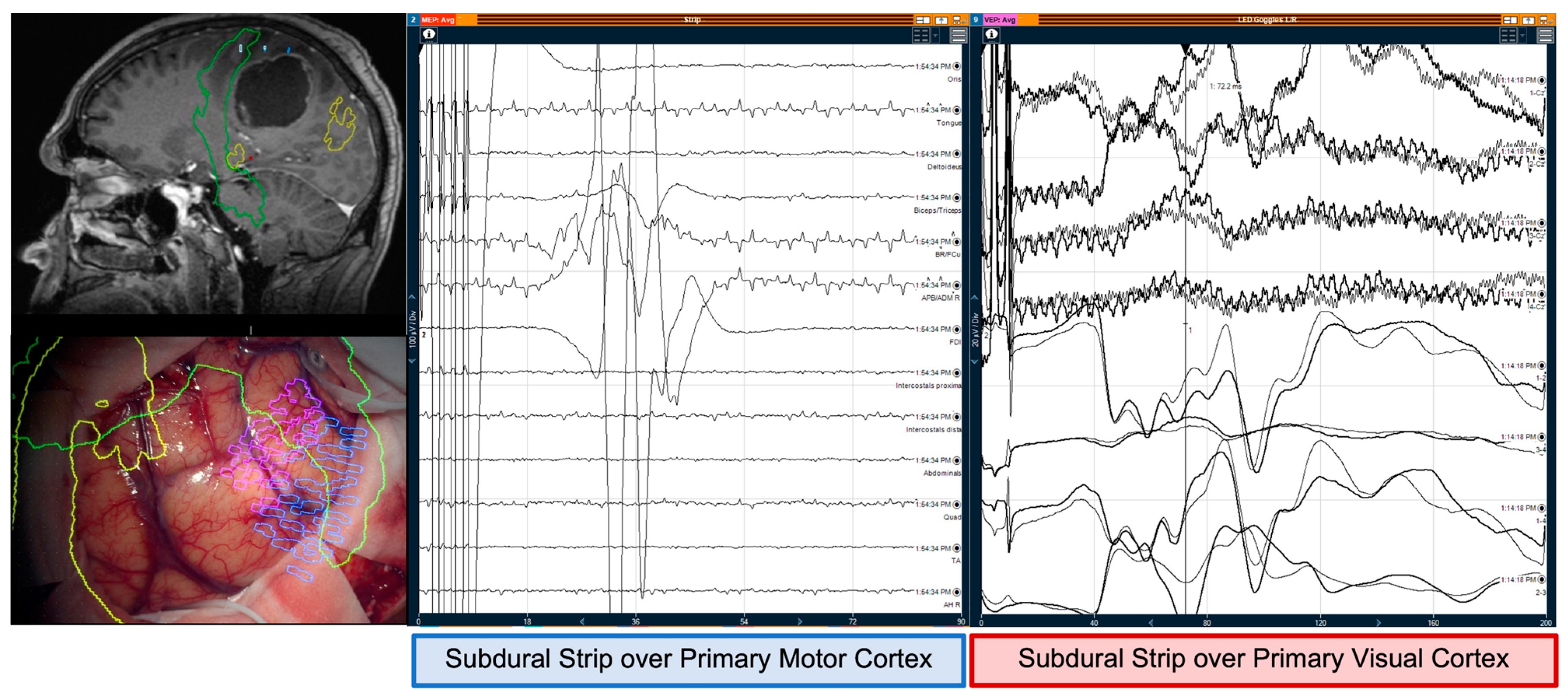
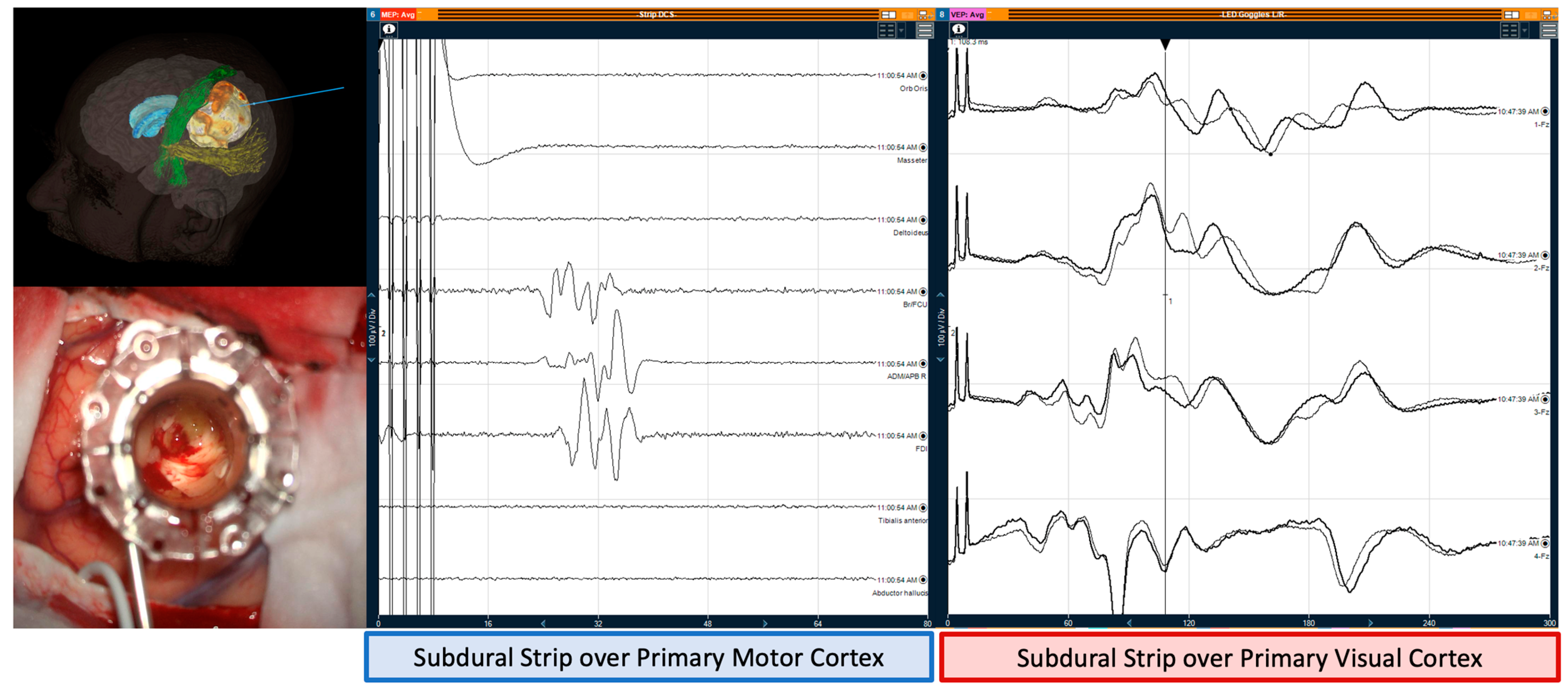
3.1. Monitoring and Mapping the CST
3.2. Monitoring and Mapping Optic Radiations
3.3. Tractography
3.4. Navigated Transcranial Magnetic Stimulation (nTMS)
3.5. Surgical Adjuncts
3.6. Schematic Illustrative Figure
3.7. Statistical Analysis
4. Results
4.1. Patient Characteristics
4.2. Intraoperative Neuromonitoring
4.3. Clinical Outcome
4.4. Predictive Factors for Clinical Outcome
4.5. Complications
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bzdok, D.; Hartwigsen, G.; Reid, A.; Laird, A.R.; Fox, P.T.; Eickhoff, S.B. Left inferior parietal lobe engagement in social cognition and language. Neurosci. Biobehav. Rev. 2016, 68, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, J.P.; Ghimire, P.; Brogna, C.; Furlanetti, L.; Patel, S.; Gullan, R.; Ashkan, K.; Bhangoo, R.; Vergani, F. Pre- and Intraoperative Mapping for Tumors in the Primary Motor Cortex: Decision-Making Process in Surgical Resection. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2021, 82, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Nachev, P. Space and the parietal cortex. Trends Cogn. Sci. 2007, 11, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Freund, H.J. Somatosensory and motor disturbances in patients with parietal lobe lesions. Adv. Neurol. 2003, 93, 179–193. [Google Scholar]
- Bisley, J.W.; Goldberg, M.E. Attention, Intention, and Priority in the Parietal Lobe. Annu. Rev. Neurosci. 2010, 33, 1–21. [Google Scholar] [CrossRef]
- Rolland, A.; Herbet, G.; Duffau, H. Awake Surgery for Gliomas within the Right Inferior Parietal Lobule: New Insights into the Functional Connectivity Gained from Stimulation Mapping and Surgical Implications. World Neurosurg. 2018, 112, e393–e406. [Google Scholar] [CrossRef]
- Hejrati, N.; Spieler, D.; Samuel, R.; Regli, L.; Weyerbrock, A.; Surbeck, W. Conscious Experience and Psychological Consequences of Awake Craniotomy. World Neurosurg. 2019, 129, e381–e386. [Google Scholar] [CrossRef]
- Nossek, E.; Matot, I.; Shahar, T.; Barzilai, O.; Rapoport, Y.; Gonen, T.; Sela, G.; Korn, A.; Hayat, D.; Ram, Z. Failed awake craniotomy: A retrospective analysis in 424 patients undergoing craniotomy for brain tumor. J. Neurosurg. 2013, 118, 243–249. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Li, J.; Lau, D.; Molinaro, A.M.; Perry, D.W.; Meng, L.; Berger, M.S. Awake craniotomy to maximize glioma resection: Methods and technical nuances over a 27-year period. J. Neurosurg. 2015, 123, 325–339. [Google Scholar] [CrossRef]
- Yamamoto, S.; Masaki, H.; Kamata, K.; Nomura, M.; Ozaki, M. A case of failed awake craniotomy due to progressive intraoperative hyponatremia. JA Clin. Rep. 2018, 4, 40. [Google Scholar] [CrossRef]
- Matsuda, A.; Mizota, T.; Tanaka, T.; Segawa, H.; Fukada, K. Difficult ventilation requiring emergency endotracheal intubation during awake craniotomy managed by laryngeal mask airway. Masui. Jpn. J. Anesthesiol. 2016, 65, 380–383. [Google Scholar]
- Rossi, M.; Nibali, M.C.; Viganò, L.; Puglisi, G.; Howells, H.; Gay, L.; Sciortino, T.; Leonetti, A.; Riva, M.; Fornia, L.; et al. Resection of tumors within the primary motor cortex using high-frequency stimulation: Oncological and functional efficiency of this versatile approach based on clinical conditions. J. Neurosurg. 2020, 133, 642–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winston, G.P.; Yogarajah, M.; Symms, M.R.; McEvoy, A.W.; Micallef, C.; Duncan, J.S. Diffusion tensor imaging tractography to visualize the relationship of the optic radiation to epileptogenic lesions prior to neurosurgery. Epilepsia 2011, 52, 1430–1438. [Google Scholar] [CrossRef]
- Guideline 5: Guidelines for Standard Electrode Position Nomenclature. J. Clin. Neurophysiol. 2006, 23, 107–110. [CrossRef]
- Odom, J.V.; Bach, M.; Brigell, M.; Holder, G.E.; McCulloch, D.L.; Mizota, A.; Tormene, A.P. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc. Ophthalmol. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Schucht, P.; SeiDel, K.; BecK, J.; MureK, M.; Jilch, A.; Wiest, R.; Fung, C.; Raabe, A. Intraoperative monopolar mapping during 5-ALA–guided resections of glioblastomas adjacent to motor eloquent areas: Evaluation of resection rates and neurological outcome. Neurosurg. Focus. 2014, 37, E16. [Google Scholar] [CrossRef]
- Seidel, K.; Beck, J.; Stieglitz, L.; Schucht, P.; Raabe, A. The warning-sign hierarchy between quantitative subcortical motor mapping and continuous motor evoked potential monitoring during resection of supratentorial brain tumors. J. Neurosurg. 2013, 118, 287–296. [Google Scholar] [CrossRef]
- Rosenstock, T.; Grittner, U.; Acker, G.; Schwarzer, V.; Kulchytska, N.; Vajkoczy, P.; Picht, T. Risk stratification in motor area–related glioma surgery based on navigated transcranial magnetic stimulation data. J. Neurosurg. 2017, 126, 1227–1237. [Google Scholar] [CrossRef]
- Neuloh, G. Time to revisit VEP monitoring? Acta Neurochir. 2010, 152, 649–650. [Google Scholar] [CrossRef]
- Kodama, K.; Goto, T.; Sato, A.; Sakai, K.; Tanaka, Y.; Hongo, K. Standard and limitation of intraoperative monitoring of the visual evoked potential. Acta Neurochir. 2010, 152, 643–648. [Google Scholar] [CrossRef]
- Sarubbo, S.; De Benedictis, A.; Milani, P.; Paradiso, B.; Barbareschi, M.; Rozzanigo, U.; Colarusso, E.; Tugnoli, V.; Farneti, M.; Granieri, E.; et al. The course and the anatomo-functional relationships of the optic radiation: A combined study with ‘post mortem’ dissections and ‘in vivo’ direct electrical mapping. J. Anat. 2015, 226, 47–59. [Google Scholar] [CrossRef]
- Verst, S.M.; de Melo, M.N.; Caivano, A.S.; Fonseca, U.S.; Mathias, L.R., Jr.; Alves, T.V. Awake surgery versus VEP in tumors of visual pathway: Case report. Interdiscip. Neurosurg. 2020, 20, 100675. [Google Scholar] [CrossRef]
- Chung, S.B.; Park, C.W.; Seo, D.W.; Kong, D.S.; Park, S.K. Intraoperative visual evoked potential has no association with postoperative visual outcomes in transsphenoidal surgery. Acta Neurochir. 2012, 154, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.; Fonseca, M.; Garrotes, M.; Lima, M.R.; Mendonça, M.; Pereira, M.; Lourenço, M.; Oliveira, E.; Lavrador, J.P. Corpus Callosum and Neglect Syndrome: Clinical Findings after Meningioma Removal and Anatomical Review. J. Neurosci. Rural Pract. 2017, 08, 101–106. [Google Scholar] [CrossRef]
- Husar, P.; Berkes, S.; Götze, A.; Henning, G.; Plagwitz, K.U. Improving SNR (signal to noise ratio) in multichannel EEG recording. Biomed. Tech. Biomed. Eng. 2002, 47, 566–569. [Google Scholar] [CrossRef]
- Torres, C.V.; Pastor, J.; Rocío, E.; Sola, R.G. Continuous monitoring of cortical visual evoked potentials by means of subdural electrodes in surgery on the posterior optic pathway. A case report and review of the literature. Revista de Neurología 2012, 55, 343–348. [Google Scholar] [CrossRef]
- Berro, D.H.; Herbet, G.; Duffau, H. New insights into the anatomo-functional architecture of the right sagittal stratum and its surrounding pathways: An axonal electrostimulation mapping study. Brain Struct. Funct. 2021, 226, 425–441. [Google Scholar] [CrossRef]
- Sollmann, N.; Zhang, H.; Kelm, A.; Schröder, A.; Meyer, B.; Pitkänen, M.; Julkunen, P.; Krieg, S.M. Paired-pulse navigated TMS is more effective than single-pulse navigated TMS for mapping upper extremity muscles in brain tumor patients. Clin. Neurophysiol. 2020, 131, 2887–2898. [Google Scholar] [CrossRef]
- Lavrador, J.P.; Gioti, I.; Hoppe, S.; Jung, J.; Patel, S.; Gullan, R.; Ashkan, K.; Bhangoo, R.; Vergani, F. Altered Motor Excitability in Patients with Diffuse Gliomas Involving Motor Eloquent Areas: The Impact of Tumor Grading. Neurosurgery 2020, 88, 183–192. [Google Scholar] [CrossRef]
- Lavrador, J.P.; Gioti, I.; Hoppe, S.; Jung, J.; Patel, S.; Gullan, R.; Ashkan, K.; Bhangoo, R.; Vergani, F. In Reply: Altered Motor Excitability in Patients with Diffuse Gliomas Involving Motor Eloquent Areas: The Impact of Tumor Grading. Neurosurgery 2021, 88, E304–E305. [Google Scholar] [CrossRef]
- Fountas, K.N.; Smith, J.R. Subdural Electrode-Associated Complications: A 20-Year Experience. Stereotact. Funct. Neurosurg. 2007, 85, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Joswig, H.; Lau, J.C.; Abdallat, M.; Parrent, A.G.; MacDougall, K.W.; McLachlan, R.S.; Burneo, J.G.; Steven, D.A. Stereoelectroencephalography Versus Subdural Strip Electrode Implantations: Feasibility, Complications, and Outcomes in 500 Intracranial Monitoring Cases for Drug-Resistant Epilepsy. Neurosurgery 2020, 87, E23–E30. [Google Scholar] [CrossRef] [PubMed]
- Galantucci, S.; Tartaglia, M.C.; Wilson, S.M.; Henry, M.L.; Filippi, M.; Agosta, F.; Dronkers, N.F.; Henry, R.G.; Ogar, J.M.; Miller, B.L.; et al. White matter damage in primary progressive aphasias: A diffusion tensor tractography study. Brain 2011, 134, 3011–3029. [Google Scholar] [CrossRef]


| Patient | Age Sex | Diagnosis | Use of US | Extent of Resection | Pre-Op Vision | Post-Op Vision | Pre-Op Motor Function | Post-Op Motor Function | Craniotomy Area (cm2) | Pre-Op Distance to CST (mm) | Post-Op Distance to CST (mm) | Pre-Op Distance to OR (mm) | Post-Op Distance to OR (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 M | Glioblastoma | Y | GTR | Hemianopia | No change | - | - | 15.9 | 15 | 0 | 0 | 0 |
| 2 | 73 M | Glioblastoma | N | GTR | Hemianopia | No change | - | - | 30.4 | 9.6 | 11.5 | 1 | 0 |
| 3 | 23 M | Pilocytic astrocytoma | Y | GTR | - | - | - | - | 14.1 | 1 | 18.7 | 0 | 6.7 |
| 4 | 69 M | Glioblastoma * | N | GTR | Hemianopia | No change | - | Left hemiparesis | 48.8 | 0 | 0 | No OR | No OR |
| 5 | 46 M | Glioblastoma | N | GTR | Hemianopia | No change | - | - | 45.0 | NA | NA | NA | NA |
| 6 | 47 F | Glioblastoma | N | GTR | Hemianopia | No change | - | - | 68.0 | 0 | 0 | No OR | No OR |
| 7 | 66 M | Glioblastoma | N | GTR | - | - | - | - | 18.6 | 0 | 4.1 | 0 | 2 |
| 8 | 35 F | Glioblastoma | N | STR | - | - | - | - | 15.0 | 1.5 | 3.9 | 1 | 2 |
| 9 | 55 F | Metastasis | N | GTR | Hemianopia | No change | - | - | 7.6 | 18.1 | 22.6 | 0 | 1.9 |
| 10 | 59 M | Glioblastoma | Y | GTR | Hemianopia | No change | Left hemiparesis | Improvement—no deficit | 22.5 | 5.2 | 3.7 | 1.3 | 0.3 |
| 11 | 66 F | Glioblastoma | Y | GTR | - | - | - | - | 14.8 | 2 | 1.3 | 0 | 1.1 |
| 12 | 54 M | Glioblastoma * | N | GTR | Hemianopia | No change | Left foot paresis | Improvement—no deficit | 91.9 | NA | NA | NA | NA |
| 13 | 57 M | Glioblastoma | N | STR | - | - | R hemiparesis (4/5) | Improvement—mild weakness (4+/5) | 9.9 | 0.7 | 22.5 | 0.6 | 16.9 |
| 14 | 71 M | Glioblastoma | Y | GTR | - | - | - | - | 35.3 | 1 | 0.8 | 2.2 | 8.3 |
| 15 | 49 M | Glioblastoma | Y | STR | Hemianopia | No change | Right hemiparesis | No change | 42.4 | 0 | 2.5 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajashekar, D.; Lavrador, J.P.; Ghimire, P.; Keeble, H.; Harris, L.; Pereira, N.; Patel, S.; Beyh, A.; Gullan, R.; Ashkan, K.; et al. Simultaneous Motor and Visual Intraoperative Neuromonitoring in Asleep Parietal Lobe Surgery: Dual Strip Technique. J. Pers. Med. 2022, 12, 1478. https://doi.org/10.3390/jpm12091478
Rajashekar D, Lavrador JP, Ghimire P, Keeble H, Harris L, Pereira N, Patel S, Beyh A, Gullan R, Ashkan K, et al. Simultaneous Motor and Visual Intraoperative Neuromonitoring in Asleep Parietal Lobe Surgery: Dual Strip Technique. Journal of Personalized Medicine. 2022; 12(9):1478. https://doi.org/10.3390/jpm12091478
Chicago/Turabian StyleRajashekar, Devika, Jose Pedro Lavrador, Prajwal Ghimire, Hannah Keeble, Lauren Harris, Noemia Pereira, Sabina Patel, Ahmad Beyh, Richard Gullan, Keyoumars Ashkan, and et al. 2022. "Simultaneous Motor and Visual Intraoperative Neuromonitoring in Asleep Parietal Lobe Surgery: Dual Strip Technique" Journal of Personalized Medicine 12, no. 9: 1478. https://doi.org/10.3390/jpm12091478
APA StyleRajashekar, D., Lavrador, J. P., Ghimire, P., Keeble, H., Harris, L., Pereira, N., Patel, S., Beyh, A., Gullan, R., Ashkan, K., Bhangoo, R., & Vergani, F. (2022). Simultaneous Motor and Visual Intraoperative Neuromonitoring in Asleep Parietal Lobe Surgery: Dual Strip Technique. Journal of Personalized Medicine, 12(9), 1478. https://doi.org/10.3390/jpm12091478