1. Introduction
The challenge of neuro-oncology surgery is finding the balance between maximal safe resection and function preservation [
1]. This is enabled by integrating pre-operative surgical mapping (cortical and subcortical), with intraoperative mapping and monitoring, to delineate functional resection boundaries [
2].
The parietal lobe has a role in language (mainly dominant hemisphere) [
1], spatial awareness [
3], motor cognition [
4], and attention [
5]. For these reasons, awake parietal surgery is increasingly performed [
6] if possible. Large tumours with mass effect, decreased consciousness, poor cooperation, comorbidities, neuropsychological factors, and patient preference are contraindications [
7]. Lack of intraoperative communication (due to dysphasia and antiepileptics), seizures, emotional intolerance, airway management, and electrolyte imbalance are factors associated with failed awake craniotomy, that correlate with poorer outcomes (language and length of stay) [
8,
9,
10,
11]. Failed awake surgery is related to a lesser extent of resection [
8].
Motor and visual functions are at risk during parietal lobe surgery. The corticospinal tract (CST) extending from the primary motor cortex to the corona radiata and internal capsule anteriorly, and the optic radiations (ORs) extending from the lateral geniculate body via the stratum sagittale to the primary visual cortex laterally and inferiorly in an antero-posterior direction, are significant subcortical functional boundaries. Monitoring and mapping of motor functions improves surgical outcomes [
12].
This study assesses the feasibility of a dual-strip method to obtain direct cortical stimulation, to gain continuous, real-time cortical monitoring and subcortical mapping of motor and visual pathways simultaneously. We propose this method to identify the motor and visual functions as onco-functional limits for parietal surgery in patient’s ineligible for awake surgery.
3. Intraoperative Neuromonitoring
3.1. Monitoring and Mapping the CST
After intubation of the patient, electrodes were placed in muscle groups of the hemibody contralateral to the tumour (face—
orbicularis oris and tongue; upper limb—
deltoid,
brachioradialis,
flexor carpi ulnaris,
first dorsal interossei,
abductor digiti minimi,
abductor pollicis brevis; lower limb—quadriceps,
tibialis anterior and
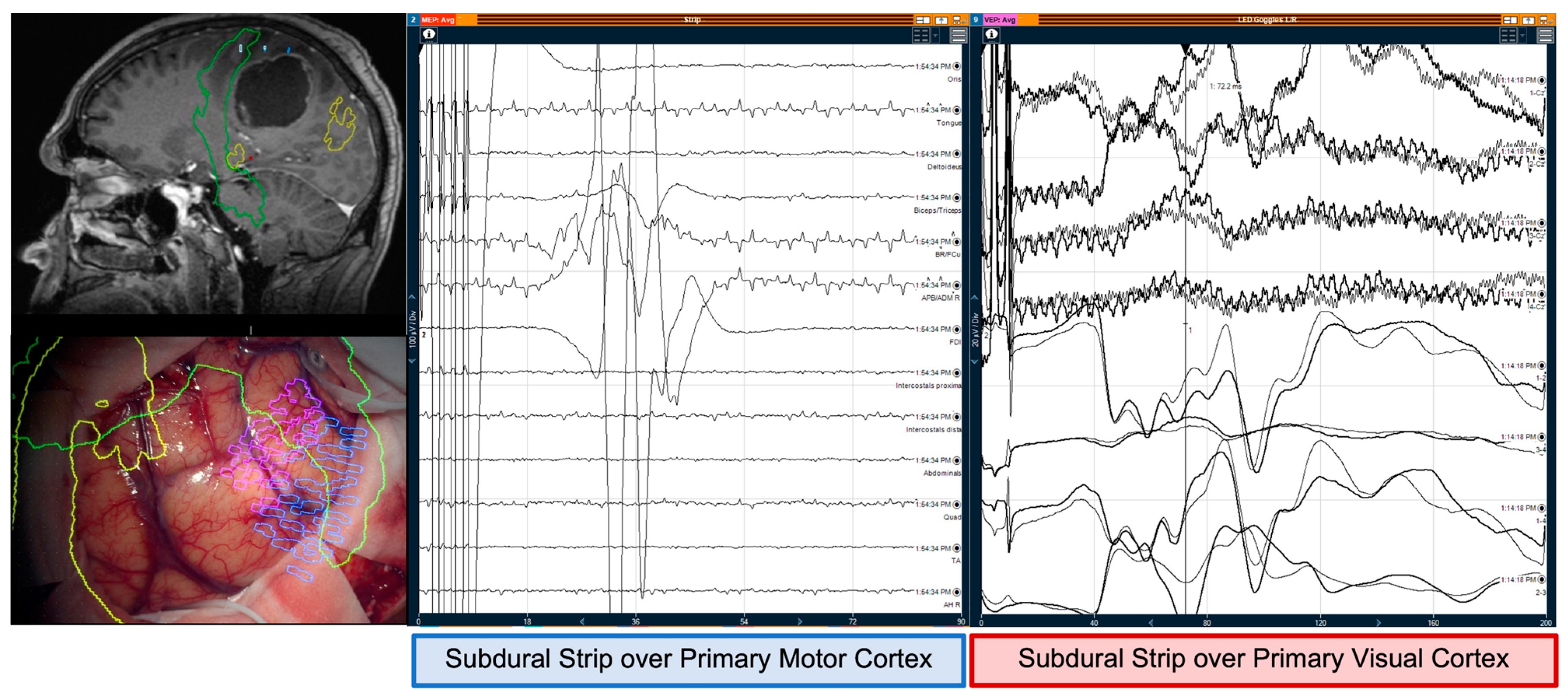
abductor hallucis), to detect motor responses. Bilateral electrodes were placed in the extremities to act as controls. Scalp corkscrew electrodes facilitated intermittent transcranial monitoring of motor evoked potentials (MEPs), and somatosensory evoked potentials (SSEPs). A subdural strip electrode was placed over the motor cortex, identified anatomically, with tractography of the CST and motor nTMS preoperative mapping, using neuro-navigation. One functional area was targeted—most commonly the area of the upper limb given its wider representation—as the strip was used for monitoring only (no tumour was infiltrating the primary motor cortex) and the elected functional area was considered a surrogate for the whole CST when it was approached at the level of the corona radiata or internal capsule. This facilitated continuous direct cortical stimulation (DCS), monitoring the stability of MEPs, and the integrity of the CST. Constant-current technology delivered high frequency stimulation using the train-of-5 anodal square pulse technique comprised of 500 µs pulse width, inter-stimulus-interval of 2–4 ms, and 1–25 mA was utilized for the cortical mapping and monitoring with the strip electrode and monopolar probe; a similar technique with cathodal pulses mapped the CST subcortically with a monopolar suction probe (Inomed Medizintechnik GmbH, Emmendingen, Germany) (
Supplementary Materials).
3.2. Monitoring and Mapping Optic Radiations
To record Visual Evoked Potentials (VEPs), the eyes were stimulated using light-emitting diode (LED) goggles (Inomed Medizintechnik GmbH, Dublin, Ireland) at 14,000–20,000 Ix. Corkscrew electrodes placed over the bilateral occipital lobes and referenced to electrodes placed over the mastoids and cranial midline, obtained intermittent VEPs from scalp recordings (SR) [
14]. To obtain VEPs from continuous direct cortical recordings (DCR), a second subdural strip electrode was placed over the visual cortex (calcarine fissure) and referenced to a corkscrew placed on the mastoid or the cranial midline. After gaining access to the midline, the strip was placed perpendicular to the calcarine fissure, and its position was confirmed under direct vision or with neuro-navigation and/or ultrasound. This monitored and mapped optic radiations. Whilst monitoring VEPs, a phase reversal of the VEPs were identified across the calcarine fissure. A ball tip bipolar fork probe with 8 mm between the poles was used to stimulate subcortical tissue intra-operatively with a biphasic pulse form, 1–4 Hz, and current dependent on proximity to the optic radiations (max 20 mA). P2 and N3 peaks (International Society for Clinical Electrophysiology of Vision (ISCEV) standards) were measured at baseline, and during debulking [
15] (
Supplementary Materials).
For both motor and visual mapping and monitoring, changes in the amplitude and latency of the waves acted as warning signs for the subdural strips to be repositioned. Only after movement of the strip was ruled out and optimal position verified according to preoperative mapping techniques (tractography and nTMS) and anatomical landmarks (hand knob and calcarine sulcus), the changes in the amplitude and latency of the waves were considered true monitoring warning signs.
3.3. Tractography
Pre-operatively, diffusion tensor imaging (DTI) and T1-post-gadolinium MRI scans were uploaded to StealthViz® (Medtronic) to delineate the CST and optic radiations. The closest distance (millimeters) between tract and tumour was calculated (zero if direct contact). Post-operative MRI was merged with the pre-operative tractography, and two people independently measured the closest distance of each tract to the resection cavity, using the mean.
In order to visualise the ipsilateral CST, a region of interest (ROI) was placed over the pre-central gyrus and a second ROI on the midbrain. The FA start value of 0.18 was used along with a maximal directional change of 45°. With knowledge of the tract anatomy, manual dissection was used to remove spurious tracts. For the ipsilateral optic radiations, an ROI was placed over the lateral geniculate body and a second ROI over the visual cortex. Once again, the FA start value of 0.18 was used, with a maximal directional change of 60°. Fibres that were not following the anatomical pathway for these tracts were dissected appropriately.
3.4. Navigated Transcranial Magnetic Stimulation (nTMS)
nTMS uses a high-precision coil, neuronavigation, and appropriate software to deliver biphasic magnetic stimulation to the cortex. A single-pulse nTMS applied to the primary motor cortex, generates a muscle output that is recorded via continuous EMG.
nTMS was performed as a non-invasive adjunct for preoperative motor mapping. A T1 weighted post contrast MRI sequence for each patient was uploaded onto the Nexstim© (Helsinki, Finland) TMS hardware to enable accurate mapping of the motor cortex and collection of data on the resting motor threshold (RMT), latency, amplitude, interhemispheric resting motor threshold ratio (iRMTr), and the cortical excitability score (CES).
Continuous electromyography was used to monitor motor evoked potentials (MEPs) of the abductor policis brevis (APB), first dorsal interossus (FDI), and the abductor digiti minimi (ADM) in both upper limbs, as well as the tibialis anterior (TA) and extensor hallucis longus (EHL) in both lower limbs. Single pulse stimulation was applied at 1 hz to both hemispheres at rest to identify the motor areas and ascertain the RMTs. Positive muscle responses were defined as MEPs greater than 50 µV. Once determined, a final motor map was generated over the hemisphere of interest at 105% of the RMT.
The iRMTr was calculated as a ratio of the RMTs between the limbs in both hemispheres and was considered to be pathological if there was a difference of more than 10%. The Cortical Excitability Score (CES) was calculated and defined as the number of pathological iRMTs recorded: 0 (no pathological iRMTr present); 1 (only one pathological iRMTr present, either for the upper or lower limb); and 2 (when both upper and lower limb demonstrated a pathological iRMTr) (
Supplementary Materials).
The motor maps generated were exported as DICOM files and used for intraoperative augmented reality and navigation.
3.5. Surgical Adjuncts
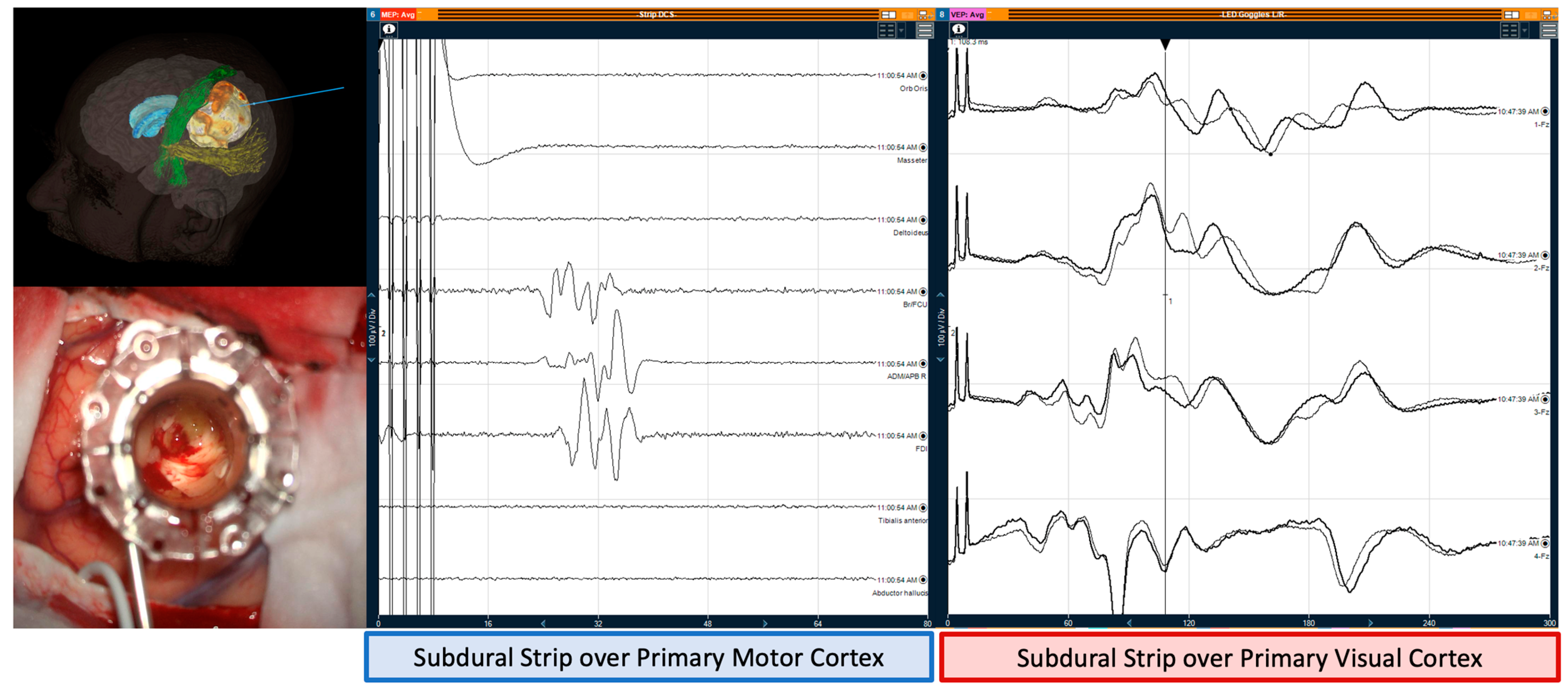
5-Aminolevulinic Acid was used in suspected high-grade gliomas using the BLUE 400 florescence filter (KINEVO® 900, Zeiss, Oberkochen, Germany). Intraoperative Ultrasound (MyLab™Eight Ultrasound, Esaote, Genoa, Italy) determined the position of the subdural strip directed to the calcarine sulcus. Tubular-retractor systems (BrainPath®, NICO, Indianapolis, IN, USA and The ViewSite™ Brain Access System, Vycor Medical, Boca Raton, FL, USA) accessed deep lesions, allowing minimally invasive parafascicular surgery (MIPS).
3.6. Schematic Illustrative Figure
Pre-processed diffusion-weighted MRI data were retrieved for a young adult male from the Human Connectome Project (
www.humanconnectome.org (accessed on 8 June 2022)). Diffusion tensor modelling and tractography were computed in StarTrack (
www.mr-startrack.com (accessed on 2 July 2022)) according to the following criteria: minimum fractional anisotropy threshold = 0.2; step size = 0.5 mm; maximum angle threshold = 30°. The optic radiations and corticospinal tract were manually dissected in TrackVis (
www.trackvis.org (accessed on 3 July 2022)). The CST was dissected using an axial waypoint ROI at the level of the pons and another to intersect terminations in sensorimotor cortex. The optic radiation was dissected using a termination ROI at the level of the lateral geniculate nucleus and a coronal waypoint ROI at the level of the occipital lobe. The dissected tracts were aligned to the MNI152 brain template for final display within the brain surface in SurfIce (
www.nitrc.org/projects/surfice (accessed on 1 July 2022)). The locations of each patient’s response points for the CST and OR were manually estimated on the MNI152 T1-weighted image, and the resulting coordinates were imported into the final display for illustrative purposes (
Supplementary Materials).
3.7. Statistical Analysis
STATA 13.1© (StataCorp, College Station, TX, USA) was used. Linear regression assessed the lesion-to-tract distance (LTD), cavity-to-tract distance (CTD), intraoperative distance to CST, and iRMTrs in the motor and visual outcomes. Ordered logistic regression assessed the CES in motor outcome. p < 0.05 was considered statistically significant.
4. Results
4.1. Patient Characteristics
Fifteen patients (11 males, 4 females) with parietal lesions were included, with a median age of 57 (range 23–77). Thirteen underwent primary resection, and two for recurrence. Ultrasound was used in six. Two underwent MIPS. Six lesions were located within the superior parietal lobule, five inferior parietal lobule, and three in both. One patient had a lesion in the transition between the corona radiata and the internal capsule with no cortical expression. Glioblastoma (GBM) was diagnosed in 13 (including two recurrences), with nine MGMT methylated and two isocitrate dehydrogenase-1 (IDH-1) mutant. One had a breast metastasis, and one a pilocytic astrocytoma (
Table 1).
4.2. Intraoperative Neuromonitoring
The size of the craniotomy was not related to the need for inserting a subdural strip electrode. The average craniotomy size was 32.0 cm
2 with a standard deviation of 23.9 (
Table 1). The average duration of monitoring was 218 min (range 160–302).
All patients had stable VEPs and MEPs at the beginning of the monitoring (once the strips were placed over primary motor and visual cortices). No complications were verified during the placement of the strip electrodes, in particular bleeding. Moreover, no complications related with continuous monitoring (in particular, seizures) occurred. The mean cortical resting motor threshold with monopolar high-frequency stimulation was 7.5 ± 1.23 mA. Continuous subcortical CST stimulation was performed during the resection, and the mean minimal amplitude of stimulation at end of resection was 6.4 mA (min 3 mA; max 12 mA). Transcranial and continuous MEPs from DCS remained stable. Abnormal iRMTr for the upper limbs was potentially related with longer intraoperative distance to the CST, although not significant (p = 0.054). LTD and iRMTrLL were not significantly related with the intraoperative distance to the CST.
The optic radiations were mapped subcortically in 13 patients, with a mean intensity of 12 mA ± 2.3 mA. Six patients (5 with previous hemianopia and one with no visual deficit) had deterioration of the VEPs superior to 50% in both amplitude and latency of the evoked potentials. Five had persistent post-operative hemianopia—which correlated with intraoperative transgression of the optic radiations as per preoperative tractography—and one had no visual changes—optic radiations preserved according to preoperative tractography despite the abovementioned reduction in the VEPs (
Figure 1).
In MIPS cases, there was no change in both MEPs and VEPs before, during, and after the insertion of retractor (
Figure 2).
4.3. Clinical Outcome
GTR was achieved in 80% (n = 12).
Eleven patients had no motor deficit pre- or post-operatively. Four had a pre-operative contralateral hemiparesis, with two resolving post-operatively. One patient’s weakness improved from Medical Research Council (MRC) grade 3 to grade 4, and one had no change. One had a new contralateral hemiparesis (3/5), which resolved at 3-months.
Nine patients had persistence of pre-operative contralateral homonymous hemianopia. Six had no visual deficits pre- or post-operatively.
Five developed de novo visual-spatial neglect which they recover at 3-months. There was no surgery related mortality (at 30 days). Nine were discharged home and six (two motor deficit, four de novo visual-spatial neglect) to a step-down neuro-rehabilitation unit for 2 weeks for perioperative intensive rehabilitation before proceeding with ambulatory oncological treatment. At 2 weeks after hospital discharge, all patients were ambulatory at home. All included patients proceed on having oncological treatment after surgery.
4.4. Predictive Factors for Clinical Outcome
nTMS was completed for motor movements in seven patients. The pre-operative findings were confirmed intraoperatively with directly cortical stimulation. 6/7 patients had abnormal cortical excitability—six with abnormal interhemispheric RMT ratio (two CES of 1, four CES of 2).
13 patients had pre-operative tractography (two not possible due to clinical urgency). Pre-operatively, the mean distance of the CST to the tumour was 4.2 mm (range 0–18.1 mm), with four tumours in direct contact (
Figure 3). Post-operatively, the mean distance of the resection cavity to the CST was 7 mm (range 0–22.6 mm). Three had the CST in direct contact with the cavity. Pre-operatively, the mean distance of the tumour to the optic radiations was 0.5 mm. Six had optic radiations in direct contact with the lesion, and two tumours had obliterated the optic radiations, correlating with hemianopia pre-operatively. Post-operatively, the mean distance between radiations and the cavity was 3.4 mm (range 0–16.9 mm). Four had tracts in contact with, or within, the resection cavity. The increase in distance is explained by incomplete resection in three, and location of cystic components in two; with significant distortion without invasion of the CST that reverted upon cyst drainage.
An inverse correlation between the iRMTr and the motor outcome function was statistically significant (
p = 0.013). No other cortical excitability measures were significant. When the LTD is compared with the motor and visual outcomes, there was no statistical significance (motor—
p = 0.877; visual—
p = 0.585). The CTD was not related with motor outcome (
p = 0.211) but was related to visual outcome (
p = 0.041)—longer distance in patients with no visual deficit. 5/6 with postoperative hemianopia had CTD ≤ 1 mm, and all with visual deficit had a CTD < 2 mm (only 1/7 with CTD < 2 mm had no postoperative deficit) (
Figure 3 and
Figure 4).
The impact of the intraoperative minimal distance to the CST and the postoperative motor function was not statistically significant (mean minimal positive subcortical stimulation: post-operative motor deterioration—6 mA; post-operative stable neurology—6.8 ± 0.73; post-operative improvement of motor function—5.5 ± 1.5, p = 0.694).
4.5. Complications
One patient had meningitis, making a good recovery.
5. Discussion
This study demonstrates the safety and feasibility of continuous, simultaneous monitoring and mapping of motor and visual function during parietal surgery using two subdural strip electrodes. This was reproduced in highly eloquent motor and visual tumours considering the abnormal motor cortical excitability. To the best of our knowledge, this is the first study that uses subdural strip electrodes to monitor and map MEPs and VEPs simultaneously. The use of integrated neuro-navigation, allowed for strip electrodes to be placed along unexposed cortical surfaces, alleviating the need for large craniotomies.
The use of subdural strip electrodes for recording of MEPs is a well-established technique for location of motor cortex [
16,
17]. Monitoring is a useful predictor of deficits, but its value is limited, as signal alterations can be irreversible in 40% [
17]. We maintained stable recordings of MEPs in fourteen patients throughout surgery, correlating with functional outcomes. The patient with a de novo transitory hemiparesis had redo surgery for a GBM, with a lesion-to-CST distance of 0 mm (infiltration of the CST) and abnormal cortical excitability; with a high-risk motor eloquent lesion according to pre-operative motor risk stratification scores [
2,
18].
The most recognized method of continuous monitoring of VEPs is via transcranial recording with corkscrew scalp electrodes. Whilst these have shown good results, there is dispute about their correlation with post-operative deficits [
19,
20,
21,
22,
23], due to low spatial resolution, and the effect of anesthetic agents and brain manipulation on signal reproducibility [
24]. Subdural strip electrodes use DCRs to enable cortical and subcortical mapping, to improve the accuracy of signals, and signal to noise ratio [
25]. Subdural electrode recordings achieved adequate spatial resolution and intensity of response [
26]. Nevertheless, the close relationship between the OR and the inferior fronto-occipital fasciculus (IFOF) in the stratum sagittale as well as their occipital terminations must be acknowledged. Recently, it has been proposed a dual system organization of the stratum sagittale with a core and a peripheral system where both OR and IFOF below to the same core system [
27].
The current stimulation methods may not have the specificity required to distinguish between the stimulation of these two tracts. Nevertheless, the injury of one tract may increase the risk of injury of other tracts in the same system. Therefore, a positive subcortical-cortical evoked potential should be considered significant. Moreover, if it is elicited in the same place where it was recorded during flashlight stimulation, that would increase the probability of involvement of the OR.
In this study, patients with previous visual field deficits did not recover after surgery even though we were able to preserve the visual fields in patients with no previous visual deficit. We believe this is related with the infiltrative and aggressive nature of the tumours in most of the included patients (13/15 patients had a diagnosis of WHO Grade 4 Glioblastoma) allied to the fact that patients consented for resection of visual eloquent tumour if the optic radiations were demonstrably infiltrated at the time of surgery (either microscopic or due to 5-ALA positive tissue). This is the main reason why this study is not focused on the potential for preservation of the visual fields with this technique but instead the feasibility and the correlation of the intraoperative findings with the clinical outcomes.
Abnormal cortical excitability for the lower limbs, as shown by the iRMTrLL, and the distance from the surgical cavity to the optic radiation as per preoperative tractography, as shown by CTD < 2 mm, proved significant. The LTD and CTD analysis did not correlate with the motor outcome. Even though different thresholds are reported in the literature (LTD < 8 mm or LTD < 12 mm) [
18,
28], those are consistently reviewed intraoperatively by the IONM data, particularly the stability of the continuous MEPs from the subdural strip and the CTD. The intraoperative distance to the CST, calculated with the 1 mm = 1 mA rule, did not correlate with motor outcome. This is explained by the integration of pre-operative and intraoperative mapping data to minimize motor deficits [
2]. There is a suggestion of a longer intraoperative distance to the CST in patients where the iRMTr for the upper limb was abnormal. This reflects the understanding of abnormal cortical excitability of the motor cortex as an initial step for motor injury, which requires a more cautious resection towards the M1-CST complex [
18,
29,
30]. For visual outcome, the significance of the CTD supports a need for better intraoperative mapping techniques, though the subdural strip proved to be reliable and predictive of outcome, as a deterioration of the recordings related to postoperative deficit.
Two disadvantages of subdural strip electrodes were the potential for the strips to become displaced (following large debulking), and difficulty accessing the midline in lateral and/or inferior tumours. Feedback from the neurophysiologist, and securing the electrodes prior to debulking, can mitigate this risk.
The study of complications associated with subdural strip electrode placement has been done largely in the context of epilepsy surgery. Two studies report the rates of subdural haematomas being low which is in-keeping with our experience (
n = 0). Fountas et al., report a combined rate of bleeding for strip and grid electrodes of 1.1%, whilst Joswig et al., report rates of 1.4% for subdural strip electrodes alone [
31,
32].
Limitations include the fact that this is a single-centre study with a small sample. This study proves the feasibility and describes the pre- and intra-operative technique. To minimize the subjectivity of tract dissections and distances assessed, these were performed independently by two people. The technique used to assess the CTD was previously validated in the literature [
13]. We are aware that this method is dependent on co-registration, and it is not new diffusion data for de novo postoperative tractography. Nevertheless, immediate postoperative diffusion data is affected by blood degradation products and hemostatic materials that can impair a reliable tractography of both CST and OR and a delayed MRI could be affected by tumour progression, which justified the chosen technique. This technique does not allow for mapping and monitoring of visual-spatial neglect function. This function was not specifically considered in this study due to the lack of reproducibility of the cortico-cortical evoked potentials in the superior longitudinal fasciculus system and the limitations of the DTI for reconstruction of this system. Also, regardless the incidence of postoperative visuo-spatial neglect in this series (5/15), all patients were ambulatory at home 2 weeks after surgery [
33].
Despite the above limitations, this study provides an integrated model with preoperative and intraoperative assessment of patients that are not eligible for or refused awake craniotomy for intra-axial non-dominant parietal lesions. Even though the more reliable technique that allows holistic patient-centred mapping and monitoring cannot be applied, we believe that an asleep multi-functional approach should be considered. This study shows that this dual-strip technique is safe and with reliable results that correlate with clinical outcomes.