Swept-Source OCT Angiography Features in Patients after Macular Hole Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ophthalmic Examination
2.3. Subgroup Analysis
2.4. Surgical Procedures
2.5. OCT Image Acquisition
2.6. OCTA Image Acquisition
2.7. OCTA Image Analysis
2.8. Statistical Analysis
3. Results
3.1. Overall Clinical Characteristics
3.2. Changes in OCT and OCTA Parameters after MH Surgery
3.3. Comparison of OCT and OCTA Parameters between the Unaffected Eye and MH at Six Months Postoperatively
3.3.1. MH Eye in the Nearly Recovered Group versus the Unaffected Eye
3.3.2. MH Eye in the Partially Recovered Group versus the Unaffected Eye
3.4. Correlation Analyses of the OCT and OCTA Parameters and BCVA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duker, J.S.; Kaiser, P.K.; Binder, S.; de Smet, M.D.; Gaudric, A.; Reichel, E.; Sadda, S.R.; Sebag, J.; Spaide, R.F.; Stalmans, P. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 2013, 120, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.L., Jr. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology 2000, 107, 1939–1948. [Google Scholar] [CrossRef]
- Lois, N.; Burr, J.; Norrie, J.; Vale, L.; Cook, J.; McDonald, A.; Boachie, C.; Ternent, L.; McPherson, G. Full-Thickness Macular Hole and Internal Limiting Membrane Peeling Study (FILMS) Group. Internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole: A pragmatic randomized controlled trial. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1586–1592. [Google Scholar] [CrossRef]
- Bae, K.; Kang, S.W.; Kim, J.H.; Kim, S.J.; Kim, J.M.; Yoon, J.M. Extent of Internal Limiting Membrane Peeling and its Impact on Macular Hole Surgery Outcomes: A Randomized Trial. Am. J. Ophthalmol. 2016, 169, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.R.; Wong, J.; Belliveau, M.; Rayat, J.; Gale, J. Anatomical and visual outcomes of macular hole surgery with short-duration 3-day face-down positioning. Retina 2012, 32, 506–510. [Google Scholar] [CrossRef]
- Yamamoto, K.; Hori, S. Long-term outcome of vitrectomy combined with internal limiting membrane peeling for idiopathic macular holes. Nippon Ganka Gakkai Zasshi 2011, 115, 20–26. [Google Scholar] [PubMed]
- Michalewska, Z.; Michalewski, J.; Nawrocki, J. Continuous changes in macular morphology after macular hole closure visualized with spectral optical coherence tomography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Hangai, M.; Larson, E.; Ogino, N. Progressive changes of regional macular thickness after macular hole surgery with internal limiting membrane peeling. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4491–4497. [Google Scholar] [CrossRef]
- Nukada, K.; Hangai, M.; Ooto, S.; Yoshikawa, M.; Yoshimura, N. Tomographic features of macula after successful macular hole surgery. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2417–2428. [Google Scholar] [CrossRef]
- Ruiz-Moreno, J.M.; Staicu, C.; Piñero, D.P.; Montero, J.; Lugo, F.; Amat, P. Optical coherence tomography predictive factors for macular hole surgery outcome. Br. J. Ophthalmol. 2008, 92, 640–644. [Google Scholar] [CrossRef]
- Villate, N.; Lee, J.E.; Venkatraman, A.; Smiddy, W.E. Photoreceptor layer features in eyes with closed macular holes: Optical coherence tomography findings and correlation with visual outcomes. Am. J. Ophthalmol. 2005, 139, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Christensen, U.C.; Krøyer, K.; Sander, B.; Larsen, M.; la Cour, M. Prognostic significance of delayed structural recovery after macular hole surgery. Ophthalmology 2009, 116, 2430–2436. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Wang, Y.; Li, X.X. Morphologic and functional evaluation before and after successful macular hole surgery using spectral-domain optical coherence tomography combined with microperimetry. Retina 2012, 32, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Yamamoto, S.; Arai, M.; Arai, E.; Sugawara, T.; Mitamura, Y.; Mizunoya, S. Correlation of visual recovery and presence of photoreceptor inner/outer segment junction in optical coherence images after successful macular hole repair. Retina 2008, 28, 453–458. [Google Scholar] [CrossRef]
- Zeng, J.; Li, J.; Liu, R.; Chen, X.; Pan, J.; Tang, S.; Ding, X. Choroidal thickness in both eyes of patients with unilateral idiopathic macular hole. Ophthalmology 2012, 119, 2328–2333. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, M.; Wu, Y.; Lu, B.; Li, T.; Zhao, J.; Wang, F.; Sun, X. Choroidal thickness in unilateral idiopathic macular hole: A Cross-Sectional Study and Meta-Analysis. Retina 2017, 37, 60–69. [Google Scholar] [CrossRef]
- Reibaldi, M.; Boscia, F.; Avitabile, T.; Uva, M.G.; Russo, V.; Zagari, M.; Bonfiglio, V.; Reibaldi, A.; Longo, A. Enhanced depth imaging optical coherence tomography of the choroid in idiopathic macular hole: A cross-sectional prospective study. Am. J. Ophthalmol. 2011, 151, 112–117. [Google Scholar] [CrossRef]
- Itoh, Y.; Inoue, M.; Rii, T.; Hiraoka, T.; Hirakata, A. Correlation between length of foveal cone outer segment tips line defect and visual acuity after macular hole closure. Ophthalmology 2012, 119, 1438–1446. [Google Scholar] [CrossRef]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express 2012, 20, 4710–4725. [Google Scholar] [CrossRef]
- Teng, Y.; Yu, M.; Wang, Y.; Liu, X.; You, Q.; Liu, W. OCT angiography quantifying choriocapillary circulation in idiopathic macular hole before and after surgery. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 893–902. [Google Scholar] [CrossRef]
- Ahn, J.; Yoo, G.; Kim, J.T.; Kim, S.W.; Oh, J. Choriocapillaris layer imaging with swept-source optical coherence tomography angiography in lamellar and full-thickness macular hole. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Theodossiadis, P.G.; Grigoropoulos, V.G.; Theodossiadis, G.P. The significance of the external limiting membrane in the recovery of photoreceptor layer after successful macular hole closure: A study by spectral domain optical coherence tomography. Ophthalmologica 2011, 225, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Liu, K.; Alibhai, A.Y.; Gendelman, I.; Braun, P.X.; Ishibazawa, A.; Sorour, O.; Duker, J.S.; Waheed, N.K. Impact of Binarization Thresholding and Brightness/Contrast Adjustment Methodology on Optical Coherence Tomography Angiography Image Quantification. Am. J. Ophthalmol. 2019, 205, 54–65. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, F.; Motulsky, E.H.; Gregori, G.; Chu, Z.; Chen, C.L.; Li, C.; De Sisternes, L.; Durbin, M.; Rosenfeld, P.J.; et al. A Novel Strategy for Quantifying Choriocapillaris Flow Voids Using Swept-Source OCT Angiography. Investig. Ophthalmol. Vis. Sci. 2018, 59, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Sabry, D.; El-Kannishy, A.; Kamel, R.; Abou Samra, W. Correlation Between En Face Optical Coherence Tomography Defects of the Inner Retinal Layers and Ganglion Cell Inner Plexiform Layer Analysis After Internal Limiting Membrane Peeling for Idiopathic Full-Thickness Macular Hole. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT444–OCT450. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Yamamoto, S.; Kimoto, R.; Oshitari, T.; Sato, E. Reduction of thickness of ganglion cell complex after internal limiting membrane peeling during vitrectomy for idiopathic macular hole. Eye 2012, 26, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Sato, A.; Fukui, E. Asymmetrical thickness of parafoveal retina around surgically closed macular hole. Br. J. Ophthalmol. 2010, 94, 1545–1546. [Google Scholar] [CrossRef] [PubMed]
- Sabater, A.L.; Velázquez-Villoria, Á.; Zapata, M.A.; Figueroa, M.S.; Suárez-Leoz, M.; Arrevola, L.; Teijeiro, M.Á.; García-Layana, A. Evaluation of macular retinal ganglion cell-inner plexiform layer thickness after vitrectomy with internal limiting membrane peeling for idiopathic macular holes. BioMed Res. Int. 2014, 2014, 458631. [Google Scholar] [CrossRef]
- Takamura, Y.; Tomomatsu, T.; Matsumura, T.; Arimura, S.; Gozawa, M.; Takihara, Y.; Inatani, M. Correlation between central retinal thickness after successful macular hole surgery and visual outcome. Jpn. J. Ophthalmol. 2015, 59, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Ezra, E.; Wells, J.A.; Gray, R.H.; Kinsella, F.M.; Orr, G.M.; Grego, J.; Arden, G.B.; Gregor, Z.J. Incidence of idiopathic full-thickness macular holes in fellow eyes. A 5-year prospective natural history study. Ophthalmology 1998, 105, 353–359. [Google Scholar] [CrossRef]
- Karkhaneh, R.; Nikbakht, M.; Bazvand, F.; Oskouei, A.K.; Ghasemi, H.; Ghassemi, F. Choroidal thickness in idiopathic macular hole. J. Curr. Ophthalmol. 2016, 29, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Furukawa, M.; Suetsugu, T.; Ogino, N. Foveal avascular zone area after internal limiting membrane peeling for epiretinal membrane and macular hole compared with that of fellow eyes and healthy controls. Retina 2018, 38, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Kakisu, M.; Nizawa, T.; Oshitari, T.; Yamamoto, S. Superficial foveal avascular zone determined by optical coherence tomography angiography before and after macular hole surgery. Retina 2017, 37, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Wilczyński, T.; Heinke, A.; Niedzielska-Krycia, A.; Jorg, D.; Michalska-Małecka, K. Optical coherence tomography angiography features in patients with idiopathic full-thickness macular hole, before and after surgical treatment. Clin. Interv. Aging 2019, 14, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Inoue, M.; Kita, R.; Sano, M.; Orihara, T.; Itoh, Y.; Hirota, K.; Koto, T.; Hirakata, A. Changes in the size of the foveal avascular zone after vitrectomy with internal limiting membrane peeling for a macular hole. Jpn. J. Ophthalmol. 2017, 61, 465–471. [Google Scholar] [CrossRef]
- Samara, W.A.; Say, E.A.; Khoo, C.T.; Higgins, T.P.; Magrath, G.; Ferenczy, S.; Shields, C.L. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina 2015, 35, 2188–2195. [Google Scholar] [CrossRef]
- Park, S.H.; Cho, H.; Hwang, S.J.; Jeon, B.; Seong, M.; Yeom, H.; Kang, M.H.; Lim, H.W.; Shin, Y.U. Changes in the Retinal Microvasculature Measured Using Optical Coherence Tomography Angiography According to Age. J. Clin. Med. 2020, 9, 883. [Google Scholar] [CrossRef]
- Hong, E.H.; Cho, H.; Kim, D.R.; Kang, M.H.; Shin, Y.U.; Seong, M. Changes in Retinal Vessel and Retinal Layer Thickness After Vitrectomy in Retinal Detachment via Swept-Source OCT Angiography. Investig. Ophthalmol. Vis. Sci. 2020, 61, 35. [Google Scholar] [CrossRef]
- Nesper, P.L.; Soetikno, B.T.; Fawzi, A.A. Choriocapillaris Nonperfusion is Associated With Poor Visual Acuity in Eyes With Reticular Pseudodrusen. Am. J. Ophthalmol. 2017, 174, 42–55. [Google Scholar] [CrossRef]
- Yun, C.; Ahn, J.; Kim, M.; Kim, J.T.; Hwang, S.Y.; Kim, S.W.; Oh, J. Characteristics of retinal vessels in surgically closed macular hole: An optical coherence tomography angiography study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1923–1934. [Google Scholar] [CrossRef]
- Wangsa-Wirawan, N.D.; Linsenmeier, R.A. Retinal oxygen: Fundamental and clinical aspects. Arch. Ophthalmol. 2003, 121, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Kur, J.; Newman, E.A.; Chan-Ling, T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res. 2012, 31, 377–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
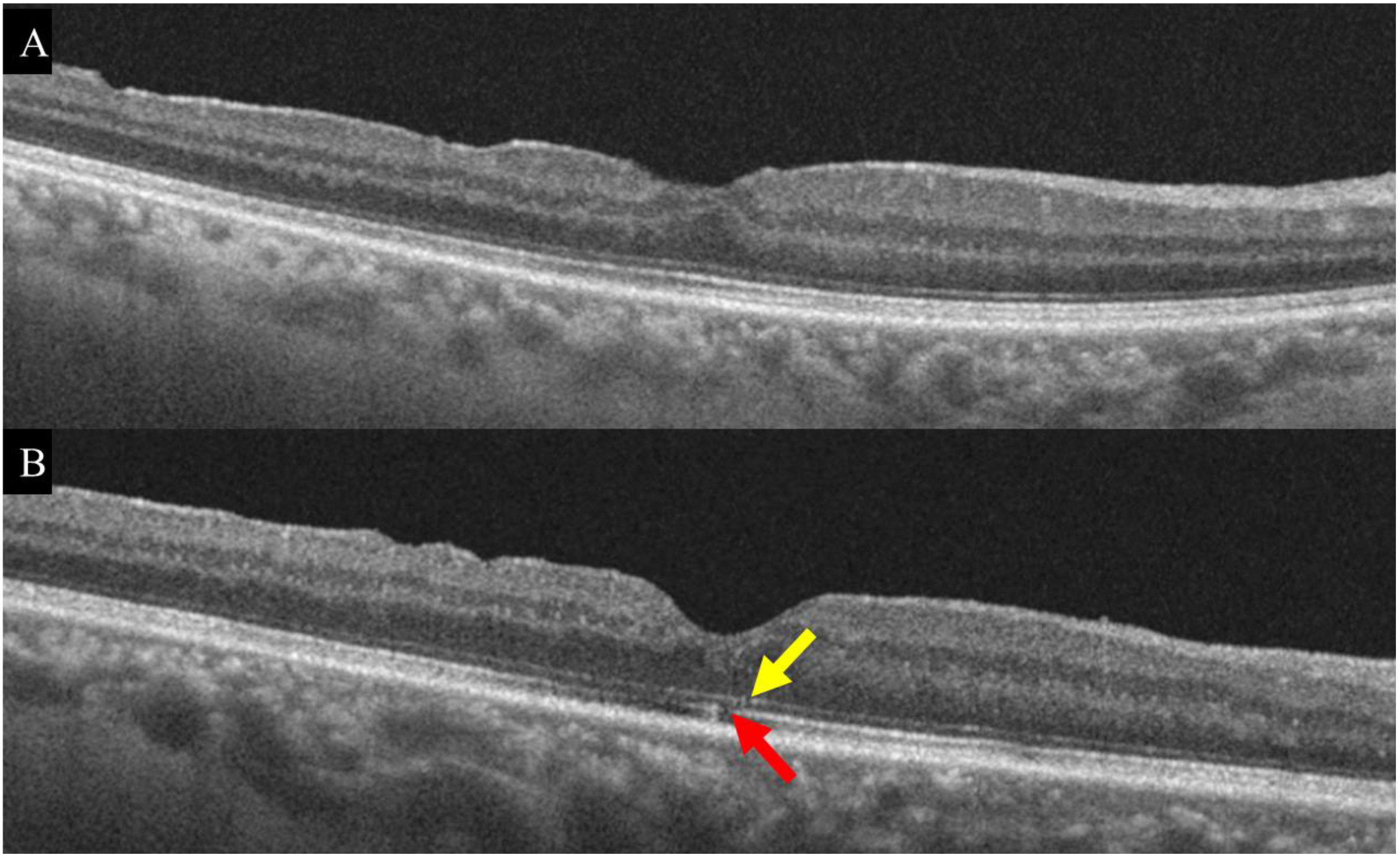





| Characteristics | Total | Outer Retina Status Six Months after Surgery | ||
|---|---|---|---|---|
| Nearly Recovered | Partially Recovered | p-Value | ||
| Numbers of patients | 30 | 12 | 18 | |
| Age (years) | 61.83 ± 10.81 | 62.90 ± 4.70 | 61.17 ± 13.36 | 0.808 |
| Gender (male) | 15 | 6 | 9 | 0.092 |
| Laterality (right) | 15 | 6 | 9 | 0.162 |
| Axial length (mm) | 23.48 ± 1.26 | 23.39 ± 0.79 | 23.53 ± 1.48 | 0.555 |
| Baseline BCVA (logMAR) | 0.61 ± 0.43 | 0.44 ± 0.22 | 0.72 ± 0.50 | 0.16 |
| Postoperative BCVA (logMAR) (6 months) | 0.39 ± 0.39 | 0.15 ± 0.26 | 0.54 ± 0.38 | 0.005 |
| Minimum linear diameter (MLD) of the macular hole (μm) | 305.40 ± 118.30 | 308.25 ± 101.98 | 301.54 ± 91.58 | 0.653 |
| Variables | Baseline | Three Months after Surgery | Six Months after Surgery | p-Value | |||
|---|---|---|---|---|---|---|---|
| Base vs. 3m | Base vs. 6m | 3m vs. 6m | |||||
| BCVA (logMAR) | 0.61 ± 0.43 | 0.4 ± 0.37 | 0.39 ± 0.39 | 0.001 | 0.001 | 0.453 | |
| Retina thickness (μm) | Center | 331.88 ± 92.69 | 253.91 ± 49.40 | 249.03 ± 60.28 | 0.003 | 0.002 | 0.624 |
| Parafovea | 335.78 ± 38.08 | 306.38 ± 26.23 | 306.31 ± 26.87 | 0.001 | 0.001 | 0.508 | |
| RNFL thickness (μm) | Center | 50.80 ± 52.57 | 17.86 ± 9.62 | 16.41 ± 6.77 | <0.001 | <0.001 | 0.838 |
| Parafovea | 45.74 ± 18.54 | 29.72 ± 3.66 | 28.53 ± 3.37 | <0.001 | <0.001 | 0.476 | |
| GC-IPL thickness (μm) | Center | 67.16 ± 25.57 | 55.47 ± 12.48 | 56.58 ± 11.25 | 0.012 | 0.011 | 0.143 |
| Parafovea | 84.83 ± 11.33 | 70.22 ± 10.25 | 69.32 ± 12.79 | 0.015 | 0.018 | 0.215 | |
| Choroid thickness (μm) | Center | 211.23 ± 67.75 | 206.39 ± 85.18 | 192.96 ± 73.17 | 0.765 | 0.049 | 0.289 |
| Parafovea | 189.90 ± 68.58 | 198.53 ± 78.88 | 182.22 ± 59.54 | 0.477 | 0.085 | 0.136 | |
| EZ-RPE thickness (μm) | Center | n/a | 32.08 ± 12.16 | 34.13 ± 13.57 | n/a | n/a | 0.346 |
| FAZ area (mm2) | 0.402 ± 0.08 | 0.265 ± 0.09 | 0.251 ± 0.007 | <0.001 | <0.001 | 0.612 | |
| SCP VD (%) | Center | 22.77 ± 3.47 | 29.43 ± 5.18 | 30.12 ± 5.60 | 0.004 | 0.003 | 0.173 |
| Total | 47.63 ± 2.27 | 47.22 ± 1.78 | 47.06 ± 1.82 | 0.232 | 0.943 | 0.463 | |
| DCP VD (%) | Center | 24.17 ± 3.22 | 33.50 ± 6.95 | 32.07 ± 8.57 | 0.011 | 0.227 | 0.6 |
| Total | 52.64 ± 4.30 | 52.96 ± 2.68 | 51.66 ± 1.95 | 0.957 | 0.943 | 0.28 | |
| Choriocapillaris flow void (%) | Center | 50.07 ± 3.35 | 48.18 ± 3.44 | 48.05 ± 3.88 | 0.027 | 0.007 | 0.146 |
| Total | 54.25 ± 1.04 | 53.18 ± 1.09 | 52.36 ± 1.05 | 0.81 | 0.031 | 0.117 | |
| OCT Parameters (μm) | Area | Nearly Recovered Group | Partially Recovered Group | |||||
|---|---|---|---|---|---|---|---|---|
| Operated Eye | Unaffected Eye | p-Value * | Operated Eye | Unaffected Eye | p-Value ** | p-Value *** | ||
| Retinal thickness | Center | 286.63 ± 25.77 | 224.80 ± 26.57 | 0.005 | 226.056 ± 64.19 | 219.43 ± 18.51 | 0.307 | 0.001 |
| Parafovea | 307.06 ± 15.18 | 299.57 ± 8.38 | 0.047 | 305.86 ± 32.45 | 305.18 ± 14.90 | 0.061 | 0.642 | |
| RNFL thickness | Center | 19.81 ± 5.56 | 5.30 ± 3.16 | 0.005 | 14.33 ± 6.73 | 5.18 ± 3.95 | 0.001 | 0.035 |
| Parafovea | 28.02 ± 3.57 | 26.45 ± 4.34 | 0.074 | 28.85 ± 3.31 | 27.20 ± 3.94 | 0.01 | 0.34 | |
| GCIPL thickness | Center | 72.27 ± 13.83 | 40.70 ± 10.41 | 0.005 | 55.06 ± 22.63 | 37.68 ± 5.91 | 0.001 | 0.011 |
| Parafovea | 82.20 ± 10.81 | 86.32 ± 12.65 | 0.683 | 80.79 ± 14.14 | 87.35 ± 7.21 | 0.222 | 0.982 | |
| EZ-RPE thickness | Center | 41.54 ± 5.75 | 49.10 ± 3.24 | 0.008 | 29.61 ± 15.06 | 49.00 ± 2.98 | 0.001 | 0.039 |
| Choroidal thickness | Center | 215.45 ± 60.17 | 223.90± 71.07 | 0.333 | 179.22 ± 78.49 | 194.31 ± 43.67 | 0.776 | 0.22 |
| Parafovea | 205.11 ± 61.77 | 216.50 ± 59.40 | 0.139 | 168.24 ± 55.21 | 190.93 ± 51.10 | 0.379 | 0.22 | |
| OCT Parameters | Area | Nearly Recovered Group | Partially Recovered Group | |||||
|---|---|---|---|---|---|---|---|---|
| Operated Eye | Unaffected Eye | p-Value * | Operated Eye | Unaffected Eye | p-Value ** | p-Value *** | ||
| SCP VD (%) | Center | 35.27 ± 2.32 | 25.17 ± 4.47 | 0.005 | 27.04 ± 5.21 | 23.21 ± 3.86 | 0.015 | 0.122 |
| Total | 47.43 ± 1.40 | 47.07 ± 1.57 | 0.386 | 46.82 ± 2.05 | 47.02 ± 2.07 | 0.424 | 0.611 | |
| DCP VD (%) | Center | 34.20 ± 6.75 | 22.29 ± 6.40 | 0.005 | 31.88 ± 6.20 | 24.77 ± 6.14 | 0.026 | 0.164 |
| Total | 51.14 ± 1.87 | 50.57 ± 2.04 | 0.444 | 51.99 ± 1.99 | 52.07 ± 2.81 | 0.374 | 0.43 | |
| Choriocapillaris flow void (%) | Center | 48.81 ± 2.90 | 49.43 ± 2.62 | 0.386 | 47.78 ± 3.26 | 49.13 ± 5.76 | 0.374 | 0.311 |
| total | 52.27 ± 0.92 | 51.94 ± 0.44 | 0.169 | 54.11 ± 1.01 | 51.73 ± 1.15 | 0.022 | 0.638 | |
| FAZ area size (mm2) | 0.301 ± 0.964 | 0.47 2± 0.140 | 0.037 | 0.382 ± 0.132 | 0.496 ± 0.876 | 0.015 | 0.109 | |
| OCT Parameters | Area | Nearly Recovered Group | Partially Recovered Group | |||||
|---|---|---|---|---|---|---|---|---|
| Preop. | Postop 6mo. | p-Value * | Preop. | Postop 6mo. | p-Value ** | p-Value *** | ||
| SCP VD (%) | Center | 21.76 ± 2.79 | 35.27 ± 2.32 | 0.018 | 23.55 ± 4.38 | 27.04 ± 5.21 | 0.026 | 0.252 |
| Total | 46.23 ± 2.01 | 47.43 ± 1.40 | 0.042 | 45.72 ± 1.88 | 46.82 ± 2.05 | 0.345 | 0.131 | |
| DCP VD (%) | Center | 21.43 ± 7.11 | 34.20 ± 6.75 | 0.018 | 26.29 ± 13.47 | 31.88 ± 6.20 | 0.045 | 0.47 |
| Total | 51.16 ± 2.71 | 51.14 ± 1.87 | 0.999 | 50.03 ± 5.19 | 51.99 ± 1.99 | 0.463 | 0.408 | |
| Choriocapillaris flow void (%) | Center | 50.25 ± 5.95 | 48.81 ± 2.90 | 0.018 | 49.92 ± 9.94 | 47.78 ± 3.26 | 0.249 | 0.681 |
| total | 51.67 ± 0.92 | 52.27 ± 0.92 | 0.236 | 51.00 ± 1.12 | 54.11 ± 1.01 | 0.139 | 0.299 | |
| FAZ area size (mm2) | 0.542 ± 0.0155 | 0.301 ± 0.964 | 0.015 | 0.826 ± 0.130 | 0.382 ± 0.132 | 0.009 | 0.702 | |
| Thickness | Center | p-Value | Parafovea | p-Value |
|---|---|---|---|---|
| Retina | −0.413 | 0.086 | −0.405 | 0.064 |
| RNFL | −0.093 | 0.65 | 0.386 | 0.104 |
| GC-IPL | −0.315 | 0.117 | −0.233 | 0.252 |
| Choroid | −0.116 | 0.574 | −0.144 | 0.481 |
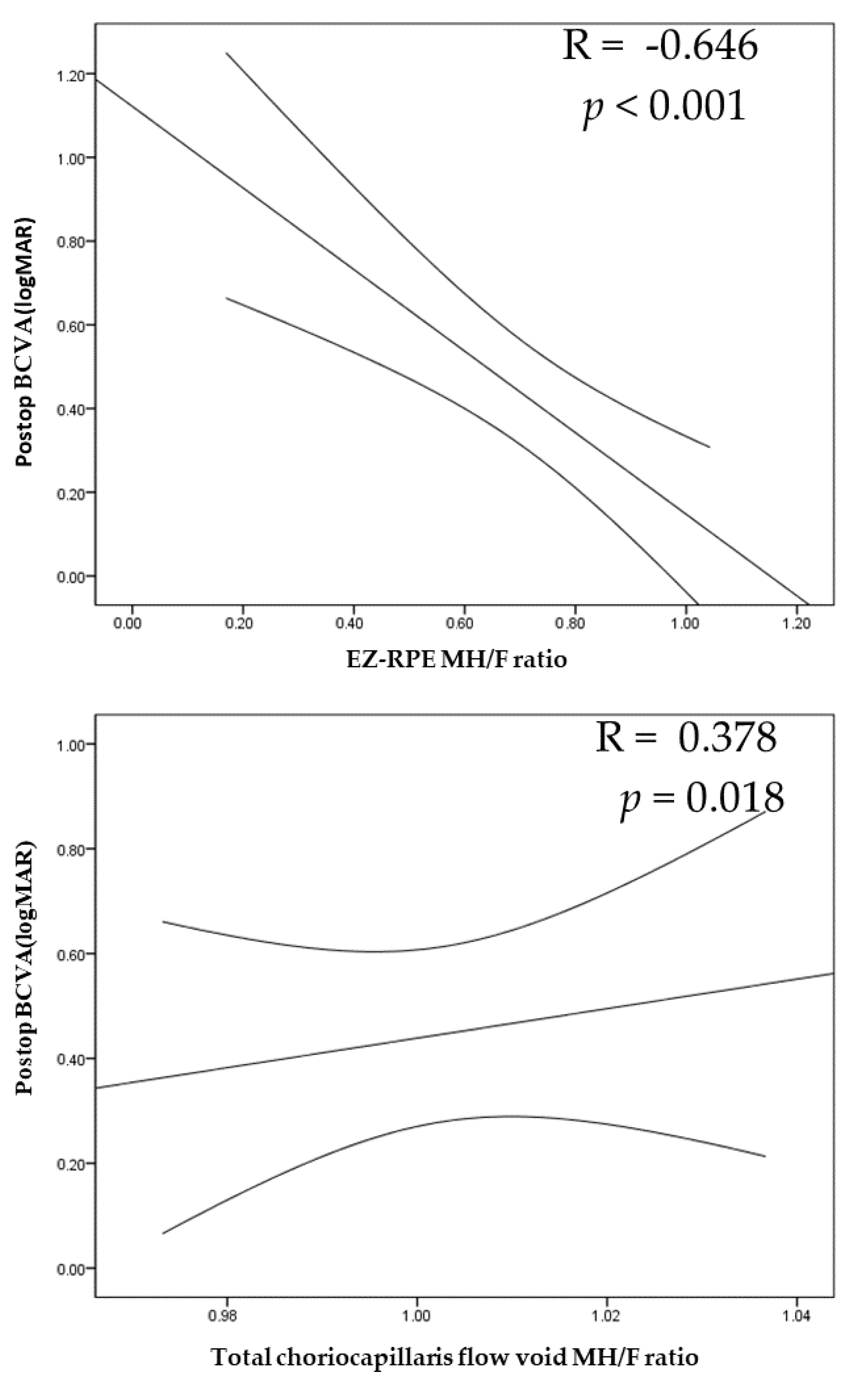
| EZ-RPE | −0.646 | <0.001 | ||
| Center | p-value | Total | p-value | |
| SCP VD | −0.289 | 0.191 | 0.032 | 0.889 |
| DCP VD | −0.002 | 0.94 | −0.111 | 0.622 |
| Choriocapillaris flow void | −0.029 | 0.974 | 0.378 | 0.018 |
| FAZ area | 0.143 | 0.536 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Kang, M.-H.; Seong, M.; Cho, H.; Shin, Y.-U. Swept-Source OCT Angiography Features in Patients after Macular Hole Surgery. J. Pers. Med. 2022, 12, 1493. https://doi.org/10.3390/jpm12091493
Hwang S, Kang M-H, Seong M, Cho H, Shin Y-U. Swept-Source OCT Angiography Features in Patients after Macular Hole Surgery. Journal of Personalized Medicine. 2022; 12(9):1493. https://doi.org/10.3390/jpm12091493
Chicago/Turabian StyleHwang, Sunjin, Min-Ho Kang, Mincheol Seong, Heeyoon Cho, and Yong-Un Shin. 2022. "Swept-Source OCT Angiography Features in Patients after Macular Hole Surgery" Journal of Personalized Medicine 12, no. 9: 1493. https://doi.org/10.3390/jpm12091493
APA StyleHwang, S., Kang, M.-H., Seong, M., Cho, H., & Shin, Y.-U. (2022). Swept-Source OCT Angiography Features in Patients after Macular Hole Surgery. Journal of Personalized Medicine, 12(9), 1493. https://doi.org/10.3390/jpm12091493







