Open-Source Hardware May Address the Shortage in Medical Devices for Patients with Low-Income and Chronic Respiratory Diseases in Low-Resource Countries
Abstract
:1. Introduction
2. The Open-Source Approach
3. Low-Cost, Open-Source Devices for Respiratory Diseases
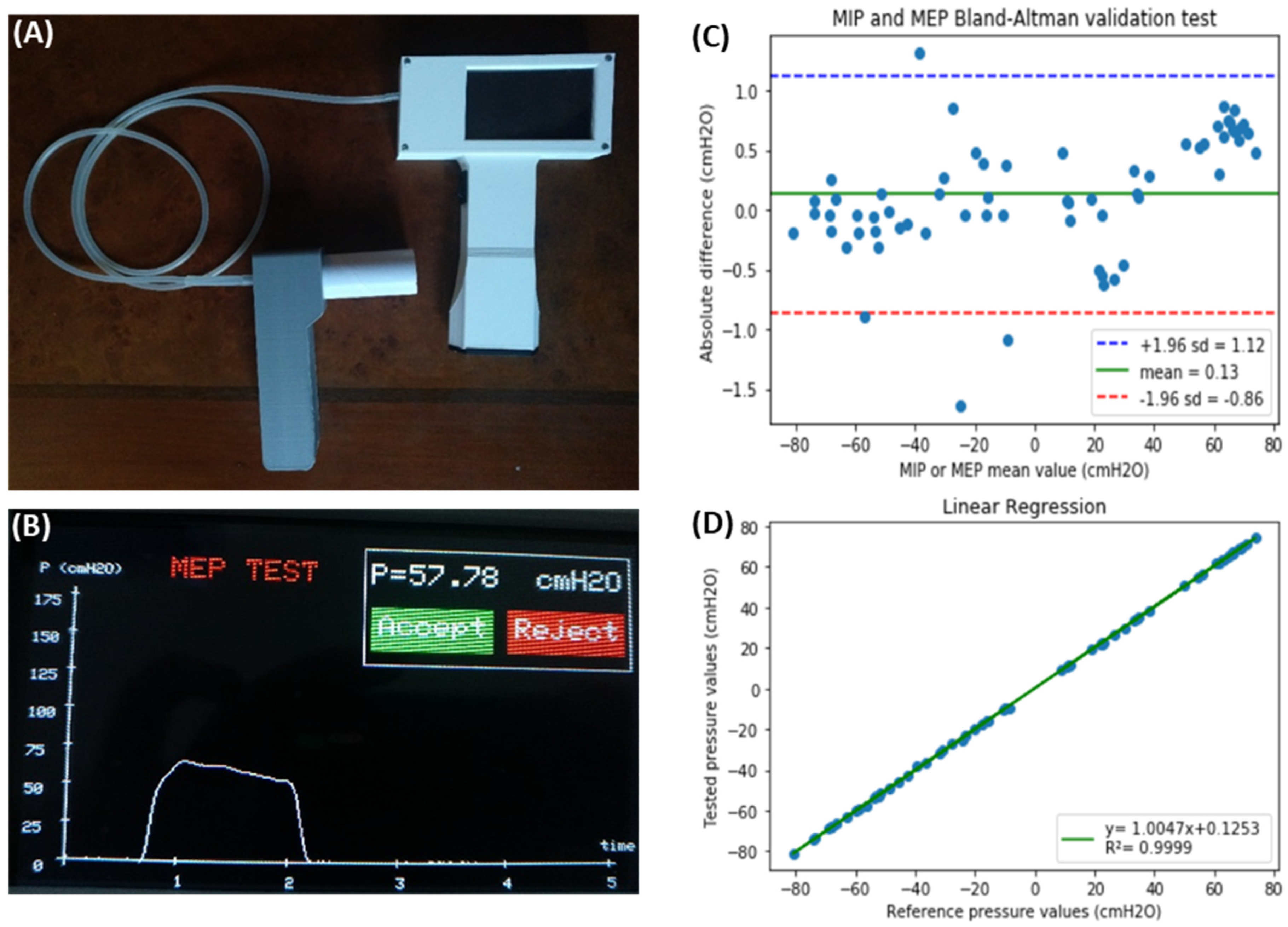
3.1. Device to Measure Maximal Inspiratory and Expiratory Pressures
3.2. Device to Measure the Tidal Volume Delivered by Mechanical Ventilators
3.3. Construction and Calibration of Accurate Pneumotachographs
3.4. Pediatric Continuous Positive Airway Pressure (CPAP)
3.5. Non-Invasive Pressure Support Ventilator
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Ayres, J.G.; Forsberg, B.; Annesi-Maesano, I.; Dey, R.; Ebi, K.L.; Helms, P.J.; Medina-Ramón, M.; Windt, M.; Forastiere, F. Environment and Health Committee of the European Respiratory Society. Climate change and respiratory disease: European Respiratory Society position statemen. Eur. Respir. J. 2009, 34, 295–302. [Google Scholar] [CrossRef]
- Annesi-Maesano, I.; Forastiere, F.; Balmes, J.; Garcia, E.; Harkema, J.; Holgate, S.; Kelly, F.; Khreis, H.; Hoffmann, B.; Maesano, C.N.; et al. The clear and persistent impact of air pollution on chronic respiratory diseases: A call for interventions. Eur. Respir. J. 2021, 57, 2002981. [Google Scholar] [CrossRef] [PubMed]
- Mandelzweig, K.; Leligdowicz, A.; Murthy, S.; Lalitha, R.; Fowler, R.A.; Adhikari, N.K.J. Non-invasive ventilation in children and adults in low- and low-middle income countries: A systematic review and meta-analysis. J. Crit. Care 2018, 47, 310–319. Available online: https://linkinghub.elseviecom/retrieve/pii/S0883944117302472 (accessed on 13 August 2022). [CrossRef] [PubMed]
- Murthy, S.; Leligdowicz, A.; Adhikari, N.K.J. Intensive Care Unit Capacity in Low-Income Countries: A Systematic Review. PLoS ONE 2015, 10, e0116949. [Google Scholar] [CrossRef] [PubMed]
- Howie, S.R.C.; Hill, S.E.; Peel, D.; Sanneh, M.; Njie, M.; Hill, P.C.; Mulholland, K.; Adegbola, R.A. Beyond good intentions: Lessons on equipment donation from an African hospital. Bull. World Health Organ. 2008, 86, 52–56. [Google Scholar] [CrossRef]
- Mackintosh, M.; Tibandebage, P.; Njeru, M.K.; Kungu, J.K.; Israel, C.; Mujinja, P.G. Rethinking health sector procurement as developmental linkages in East Africa. Soc. Sci. Med. 2018, 200, 182–189. [Google Scholar] [CrossRef]
- Ramadurai, K.W.; Bhatia, S.K. Frugal Medical Technologies and Adaptive Solutions: Field-Based Applications. In Reimagining Innovation in Humanitarian Medicine; Springer Cham: Cham, Switzerland, 2018; pp. 49–73. [Google Scholar] [CrossRef]
- Tran, V.T.; Ravaud, P. Frugal innovation in medicine for low resource settings. BMC Med. 2016, 7, 102. [Google Scholar] [CrossRef]
- WHO. WHO Compendium of Innovative Health Technologies for Low-Resource Settings 2021. COVID-19 and other Health Priorities. Available online: https://www.who.int/publications/i/item/9789240032507 (accessed on 13 August 2022).
- Garmendia, O.; Rodríguez-Lazaro, M.A.; Otero, J.; Phan, P.; Stoyanova, A.; Dinh-Xuan, A.T.; Gozal, D.; Navajas, D.; Montserrat, J.M.; Farré, R. Low-cost, easy-to-build noninvasive pressure support ventilator for under-resourced regions: Open source hardware description, performance and feasibility testing. Eur. Respir. J. 2020, 55, 2000846. [Google Scholar] [CrossRef] [Green Version]
- Open Hardware Definition (English). Available online: https://www.oshwa.org/definition/ (accessed on 20 September 2021).
- Lakhani, K.R.; von Hippel, E. How Open Source Software Works: “Free” User-to-User Assistanc in Produktentwicklung Mit Virtuellen Communities: Kundenwünsche Erfahren und Innovationen Realisieren; Herstatt, C., Sander, J.G., Eds.; Gabler Verlag: Wiesbaden, Germany, 2004; pp. 303–339. ISBN 978-3-322-84540-5. [Google Scholar]
- Zeitlyn, D. Gift Economies in the Development of Open Source Software: Anthropological Reflections. Res. Policy 2003, 32, 1287–1291. [Google Scholar] [CrossRef]
- Raymond, E. The Cathedral and the Bazaar. Know. Technol. Pol. 1999, 12, 23–49. [Google Scholar] [CrossRef]
- Herstatt, C.; Ehls, D. Open Source Innovation: The Phenomenon, Participant’s Behaviour, Business Implications; Routledge: Abindgdon, UK, 2015; ISBN 978-1-317-62425-7. [Google Scholar]
- Comino, S.; Manenti, F.M.; Parisi, M.L. From Planning to Mature: On the Success of Open Source Projects. Res. Policy 2007, 36, 1575–1586. [Google Scholar] [CrossRef]
- Lee, S.-Y.T.; Kim, H.-W.; Gupta, S. Measuring Open Source Software Success. Omega 2009, 37, 426–438. [Google Scholar] [CrossRef]
- Weber, S. The Success of Open Source; Harvard University Press: Cambridge, MA, USA, 2004; ISBN 978-0-674-01292-9. [Google Scholar]
- Eclips IoT Developer Survey 2019 Results. Available online: https://ioeclipsorg/community/resources/iot-surveys/assets/iot-developer-survey-2019.pdf (accessed on 24 February 2022).
- IDC—Smartphone Market Share-Market Share. Available online: https://www.idc.com/promo/smartphone-market-share (accessed on 24 February 2022).
- Hiteshdawda. Realising the Value of Cloud Computing with Linux. Available online: https://www.rackspaccom/en-gb/blog/realising-the-value-of-cloud-computing-with-linux (accessed on 24 February 2022).
- Parloff, R. How Linux Conquered the Fortune 500|Fortune. Available online: https://fortuncom/2013/05/06/how-linux-conquered-the-fortune-500/ (accessed on 24 February 2022).
- Vaughan-Nichols, S. Supercomputers: All Linux, All the Time. Available online: https://www.zdnecom/article/supercomputers-all-linux-all-the-time/ (accessed on 24 February 2022).
- Pearce, J.M. Building Research Equipment with Free. Open-Source Hardwar. Sci. 2012, 337, 1303–1304. [Google Scholar]
- Pearce, J.M. Open-Source Lab: How to Build Your Own Hardware and Reduce Research Costs; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Sells, E.; Bailard, S.; Smith, Z.; Bowyer, A.; Olliver, V. RepRap: The Replicating Rapid Prototyper: Maximizing Customizability by Breeding the Means of Production. In Handbook of Research in Mass Customization and Personalization; World Scientific Publishing Company: Singapore, China, 2009; pp. 568–580. ISBN 978-981-4280-25-9. [Google Scholar]
- Jones, R.; Haufe, P.; Sells, E.; Iravani, P.; Olliver, V.; Palmer, C.; Bowyer, A. RepRap—The replicating rapid prototype. Robotica 2011, 29, 177–191. [Google Scholar] [CrossRef]
- Bowyer, A. 3D Printing and Humanity’s First Imperfect Replicato. 3D Prin. Addit. Manuf. 2014, 1, 4–5. [Google Scholar] [CrossRef]
- Oberloier, S.; Pearce, J.M. General Design Procedure for Free and Open-Source Hardware for Scientific Equipment. Designs 2018, 2, 2. [Google Scholar] [CrossRef]
- Rundle, G. A Revolution in the Making; Simon and Schuster: New York, NY, USA, 2014; ISBN 978-1-922213-48-8. [Google Scholar]
- Wittbrodt, B.T.; Glover, A.G.; Laureto, J.; Anzalone, G.C.; Oppliger, D.; Irwin, J.L.; Pearce, J.M. Life-cycle economic analysis of distributed manufacturing with open-source 3-D printers. Mechatronics 2013, 23, 713–726. [Google Scholar] [CrossRef]
- Petersen, E.E.; Pearce, J. Emergence of Home Manufacturing in the Developed World: Return on Investment for Open-Source 3-D Printers. Technologies 2017, 5, 7. [Google Scholar] [CrossRef]
- Pearce, J.; Qian, J.-Y. Economic Impact of DIY Home Manufacturing of Consumer Products with Low-Cost 3D Printing from Free and Open Source Designs. Eur. J. Soc. Impact Circ. Econ. 2022, 1–24. [Google Scholar] [CrossRef]
- Banzi, M.; Shiloh, M. Getting Started with Arduino: The Open Source Electronics Prototyping Platform; Maker Media Inc.: Sebastopol, CA, USA, 2014; ISBN 978-1-4493-6329-1. [Google Scholar]
- Gibney, E. ‘Open-Hardware’ Pioneers Push for Low-Cost Lab Kit. Nature 2016, 531, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Oellermann, M.; Jolles, J.W.; Ortiz, D.; Seabra, R.; Wenzel, T.; Wilson, H.; Tanner, R.L. Open Hardware in Science: The Benefits of Open Electronics. Integr. Comp. Biol. 2022, icac043. [Google Scholar] [CrossRef] [PubMed]
- Kondaveeti, H.K.; Kumaravelu, N.K.; Vanambathina, S.D.; Mathe, S.E.; Vappangi, S. A Systematic Literature Review on Prototyping with Arduino: Applications, Challenges, Advantages, and Limitations. Comput. Sci. Rev. 2021, 40, 100364. [Google Scholar] [CrossRef]
- Pearce, J.M. Economic Savings for Scientific Free and Open Source Technology: A Review. HardwareX 2020, 8, e00139. [Google Scholar] [CrossRef]
- Gibb, A. Building Open Source Hardware: DIY Manufacturing for Hackers and Makers; Pearson Education: London, UK, 2014. [Google Scholar]
- Powell, A. Democratizing production through open source knowledge: From open software to open hardware Media. Cult. Soc. 2012, 34, 691–708. [Google Scholar] [CrossRef]
- Pearce, J.M. Create, Share, and Save Money Using Open-Source Projects; McGraw-Hill Education: New York, NY, USA, 2021; ISBN 978-1-260-46176-3. [Google Scholar]
- Veselovská, L. Supply Chain Disruptions in the Context of Early Stages of the Global COVID-19 Outbreak. Probl. Perspect. Manag. 2020, 18, 490–500. [Google Scholar] [CrossRef]
- Chowdhury, P.; Paul, S.K.; Kaisar, S.; Moktadir, M.A. COVID-19 Pandemic Related Supply Chain Studies: A Systematic Review. Transp. Res. Part E Logist. Transp. Rev. 2021, 148, 102271. [Google Scholar] [CrossRef]
- Bowser, A.; Long, A.; Novak, A.; Parker, A.; Weinberg, M. Stitching Together a Solution: Lessons from the Open Source Hardware Response to COVID-19|Wilson Center. Available online: https://www.wilsoncenteorg/publication/stitching-together-solution-lessons-open-source-hardware-response-covid-19 (accessed on 11 August 2022).
- Pearce, J.M. Distributed Manufacturing of Open Source Medical Hardware for Pandemics. J. Manuf. Mater. Process. 2020, 4, 49. [Google Scholar] [CrossRef]
- Flanagan, S.T.; Ballard, D.H. 3D Printed Face Shields: A Community Response to the COVID-19 Global Pandemic. Acad. Radiol. 2020, 27, 905–906. [Google Scholar] [CrossRef]
- Ballard, D.H.; Jammalamadaka, U.; Meacham, K.W.; Hoegger, M.J.; Burke, B.A.; Morris, J.A.; Scott, A.R.; O’Connor, Z.; Gan, C.; Hu, J.; et al. Quantitative Fit Tested N95 Respirator-Alternatives Generated with CT Imaging and 3D Printing: A Response to Potential Shortages during the COVID-19 Pandemic. Acad. Radiol. 2021, 28, 158–165. [Google Scholar] [CrossRef]
- Skrzypczak, N.G.; Tanikella, N.G.; Pearce, J.M. Open Source High-Temperature RepRap for 3-D Printing Heat-Sterilizable PPE and Other Applications. HardwareX 2020, 8, e00130. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.; Henke-Adams, A.; Henke, D.M.; Kravitz, A.V.; Gay, H.A. Modified Full-Face Snorkel Mask as COVID-19 Personal Protective Equipment: Quantitative Results. HardwareX 2021, 9, e00185. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, B.R.; Pearce, J.M. Conversion of Self-Contained Breathing Apparatus Mask to Open Source Powered Air-Purifying Particulate Respirator for Fire Fighter COVID-19 Respons. HardwareX 2020, 8, e00129. [Google Scholar] [CrossRef] [PubMed]
- Gallup, N.; Pringle, A.M.; Oberloier, S.; Tanikella, N.G.; Pearce, J.M. Parametric Nasopharyngeal Swab for Sampling COVID-19 and Other Respiratory Viruses: Open Source Design, SLA 3-D Printing and UV Curing System. HardwareX 2020, 8, e00135. [Google Scholar] [CrossRef] [PubMed]
- Manoj, A.; Bhuyan, M.; Raj Banik, S.; Ravi Sankar, M. 3D Printing of Nasopharyngeal Swabs for COVID-19 Diagnose: Past and Current Trends. Mater. Today Proc. 2021, 44, 1361–1368. [Google Scholar] [CrossRef]
- Abuzairi, T.; Sumantri, N.; Irfan, A.; Mohamad, R.M. Infrared Thermometer on the Wall (IThermowall): An Open Source and 3-D Print Infrared Thermometer for Fever Screening. HardwareX 2021, 9, e00168. [Google Scholar] [CrossRef]
- Bentancor, M.; Fernández, S.; Viera, F.; Etcheverry, S.; Poradosú, C.; D’Angelo, P.; Montemuiño, H.; Mirazo, S.; Irigoyen, Á.; Sanabria, A.; et al. LUCIA: An Open Source Device for Disinfection of N95 Masks Using UV-C Radiatio. HardwareX 2021, 9, e00181. [Google Scholar] [CrossRef]
- Bentancor, M.; Vidal, S. Programmable and Low-Cost Ultraviolet Room Disinfection Devic. HardwareX 2018, 4, e00046. [Google Scholar] [CrossRef]
- Santhosh, R.; Yadav, S. Low Cost Multipurpose UV-C Sterilizer Box for Protection against COVID’19. In Proceedings of the 2021 International Conference on Artificial Intelligence and Smart Systems (ICAIS), Coimbatore, India, 25–27 March 2021; pp. 1495–1498. [Google Scholar]
- Pearce, J.M. A Review of Open Source Ventilators for COVID-19 and Future Pandemics. F1000Research 2020, 9, 218. [Google Scholar] [CrossRef]
- Aymerich, C.; Rodríguez-Lázaro, M.; Solana, G.; Farré, R.; Otero, J. Low-Cost Open-Source Device to Measure Maximal Inspiratory and Expiratory Pressures. Front. Physiol. 2021, 12, 719372. [Google Scholar] [CrossRef]
- Farré, R.; Artigas, A.; Torres, A.; Albaiceta, G.M.; Dinh-Xuan, A.T.; Gozal, D. A Simple Procedure to Measure the Tidal Volume Delivered by Mechanical Ventilators: A Tool for Bedside Verification and Quality Control. Arch. Bronconeumol. 2022; in press. [Google Scholar] [CrossRef]
- Farré, R.; Rodríguez-Lázaro, M.A.; Gozal, D.; Trias, G.; Solana, G.; Navajas, D.; Otero, J. Simple low-cost construction and calibration of accurate pneumotachographs for monitoring mechanical ventilation in low-resource settings. Fron. Med. 2022, 9, 938949. [Google Scholar] [CrossRef]
- Farré, R.; Trias, G.; Solana, G.; Ginovart, G.; Gozal, D.; Navajas, D. Novel Approach for Providing Pediatric Continuous Positive Airway Pressure Devices in Low-Income, Underresourced Regions. Am. J. Respir. Crit. Care Med. 2019, 199, 118–120. [Google Scholar] [CrossRef]
- Truog, R.D.; Mitchell, C.; Daley, C.Q. The Toughest Triage—Allocating Ventilators in a Pandemic. N. Eng. J. Med. 2020, 382, 1973–1975. [Google Scholar] [CrossRef]
- Nachiappan, N.; Koo, J.M.; Chockalingam, N.; Scott, T.E. A low-cost field ventilator: An urgent global need. Health Sci. Rep. 2021, 5, e349. [Google Scholar] [CrossRef]
- Ranger, B.J.; Mantzavinou, A. Design thinking in development engineering education: A case study on creating prosthetic and assistive technologies for the developing world. Dev. Eng. 2018, 3, 166–174. [Google Scholar] [CrossRef]
- Clifford, K.L.; Zaman, M.H. Engineering, global health, and inclusive innovation: Focus on partnership, system strengthening, and local impact for SDGs. Glob. Health Action 2016, 9, 30175. [Google Scholar] [CrossRef]
- Chagas, A.M. Haves and Have Nots Must Find a Better Way: The Case for Open Scientific Hardwar. PLoS Biol. 2018, 16, e3000014. [Google Scholar] [CrossRef]
- Pearce, J.M. Emerging Business Models for Open Source Hardwar. J. Open Hardw. 2017, 1, 2. [Google Scholar] [CrossRef]
- Richterich, A. When Open Source Design Is Vital: Critical Making of DIY Healthcare Equipment during the COVID-19 Pandemic. Health Sociol. Rev. 2020, 29, 158–167. [Google Scholar] [CrossRef]
- Lakshmi, U.; Hofmann, M.; Mack, K.; Hudson, S.E.; Mankoff, J.; Arriaga, R. Medical Maker Response to COVID-19: Distributed Manufacturing Infrastructure for Stopgap Protective Equipmen. In Proceedings of the 2021 CHI Conference on Human Factors in Computing Systems, Yokohama, Japan, 8–13 May 2021; Association for Computing Machinery: New York, NY, USA, 2021; pp. 1–13. [Google Scholar]
- Sullivan, C.E. Nasal Positive Airway Pressure and Sleep Apnea. Reflections on an Experimental Method That Became a Therapy. Am. J. Respir. Crit. Care Med. 2018, 198, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.B. Peltzman Revisited: Quantifying 21st Century Opportunity Costs of FDA Regulation. 2021. Available online: https://https://bfi.uchicago.edu/working-paper/peltzman-revisited-quantifying-21st-century-opportunity-costs-of-fda-regulation/ (accessed on 13 August 2022).
- Grabowski, H.G.; Guha, R.; Salgado, M. Regulatory and Cost Barriers Are Likely to Limit Biosimilar Development and Expected Savings in the Near Future. Health Aff. 2014, 33, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Bergsland, J.; Elle, O.J.; Fosse, E. Barriers to Medical Device Innovation. Med. Devices 2014, 7, 205–209. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Clinical Trials in Human Medicine. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/clinical-trials-human-medicines (accessed on 13 August 2022).
- U.S. Food and Drug Administration. Clinical Trials and Human Subject Protection. Available online: https://www.fda.gov/science-research/science-and-research-special-topics/clinical-trials-and-human-subject-protection (accessed on 13 August 2022).
- U.S. Food and Drug Administration. Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices during the Coronavirus Disease 2019 (COVID-19) Public Health Emergency—Guidance for Industry and Food and Drug Administration Staff; FDA: Silver Spring, MD, USA, 2020. [Google Scholar]
- U.S. Food and Drug Administration. The Emergency Use Authorization; FDA: Silver Spring, MD, USA, 2020. [Google Scholar]
- CSMS #42124872—Information for Filing Personal Protective Equipment and Medical Devices during COVID-19. Available online: https://contengovdelivery.com/accounts/USDHSCBP/bulletins/282c648 (accessed on 3 April 2020).
- Burnside, M.J.; Lewis, D.M.; Crocket, H.R.; Meier, R.A.; Williman, J.A.; Sanders, O.J.; Jefferies, C.A.; Faherty, A.M.; Paul, R.G.; Lever, C.S.; et al. Open-Source Automated Insulin Delivery in Type 1 Diabetes. N. Engl. J. Med. 2022, 387, 869–881. [Google Scholar] [CrossRef]





| Item | Sub-Item | Conventional Industry Market | Open-Source Local Production |
|---|---|---|---|
| Technological complexity of the device | Very high | YES | DIFFICULT * |
| Low–medium | YES | YES | |
| Cost and availability for most patients | Cost | EXPENSIVE | CHEAP |
| Availability | LOW | HIGH | |
| Health provider perspective | Local servicing/repair | DIFFICULT | EASY |
| Adaptability to local needs | DIFFICULT | EASY | |
| Local industry promotion | LOW | HIGH | |
| Requiring team-building initiative | NO | YES | |
| Regulations and safety requirements | International standards (FDA, CE) | YES | DIFFICULT |
| Local approval | NO | YES |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farré, R.; Gozal, D.; Nguyen, V.-N.; Pearce, J.M.; Dinh-Xuan, A.T. Open-Source Hardware May Address the Shortage in Medical Devices for Patients with Low-Income and Chronic Respiratory Diseases in Low-Resource Countries. J. Pers. Med. 2022, 12, 1498. https://doi.org/10.3390/jpm12091498
Farré R, Gozal D, Nguyen V-N, Pearce JM, Dinh-Xuan AT. Open-Source Hardware May Address the Shortage in Medical Devices for Patients with Low-Income and Chronic Respiratory Diseases in Low-Resource Countries. Journal of Personalized Medicine. 2022; 12(9):1498. https://doi.org/10.3390/jpm12091498
Chicago/Turabian StyleFarré, Ramon, David Gozal, Viet-Nhung Nguyen, Joshua M. Pearce, and Anh Tuan Dinh-Xuan. 2022. "Open-Source Hardware May Address the Shortage in Medical Devices for Patients with Low-Income and Chronic Respiratory Diseases in Low-Resource Countries" Journal of Personalized Medicine 12, no. 9: 1498. https://doi.org/10.3390/jpm12091498
APA StyleFarré, R., Gozal, D., Nguyen, V.-N., Pearce, J. M., & Dinh-Xuan, A. T. (2022). Open-Source Hardware May Address the Shortage in Medical Devices for Patients with Low-Income and Chronic Respiratory Diseases in Low-Resource Countries. Journal of Personalized Medicine, 12(9), 1498. https://doi.org/10.3390/jpm12091498









