Open Innovation as the Catalyst in the Personalized Medicine to Personalized Digital Medicine Transition
Abstract
:1. Introduction
- -
- Care continuity: the delocalization of healthcare services encounters new strategies of RPM (e.g., virtual assistance or technological support) that need to be integrated with the traditional one.
- -
- Business continuity: the valorization of off-the-shelf products (e.g., clinical expertise, scientific knowledge) should be conducted in a sustainable and ethical way (in terms of costs, protection of intellectual property and privacy, etc.).
- -
- Innovation continuity: the deployment of effective clinical and digital solutions must adapt to rapid changes and new healthcare challenges.
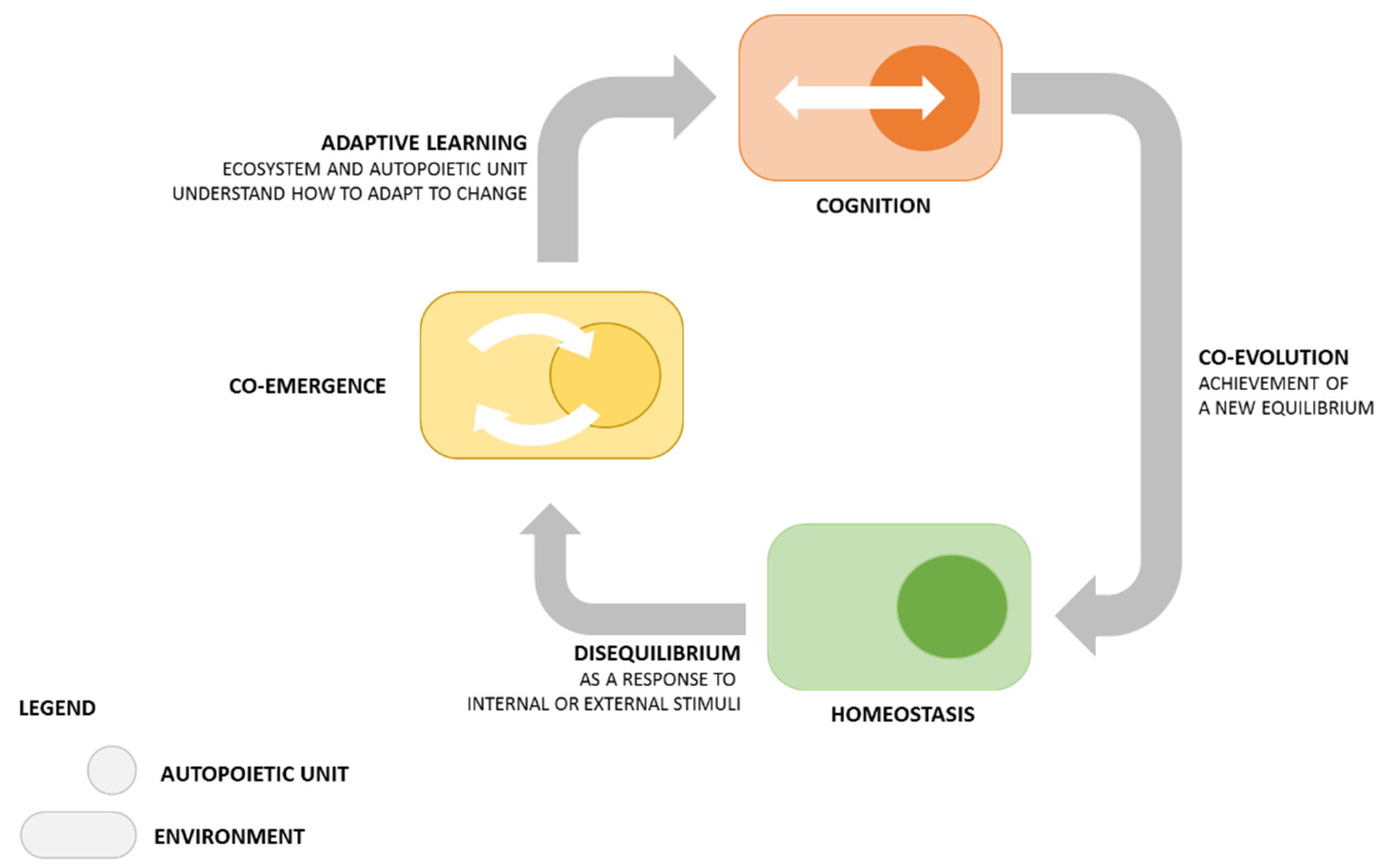
2. Framework: From Biology to Open Innovation
2.1. How Knowledge Is Generated
2.2. Why Opening Is Fundamental for Research
- -
- Knowledge integration: by internalizing the external resources they access with their efficiency and width (the more specialized they are, the less flexible they are).
- -
- Knowledge transfer: by constantly innovating their internal resources through specific tools that enable cross-fertilization of knowledge between organizations.
- -
- Outbound: new ideas generated in the organization are disseminated outside, to other organizations and other environments (from inside to outside).
- -
- Inbound: knowledge within the organization is enriched with the integration of external resources (from outside to inside).
- -
- Combined: a balance is created between the two previous archetypes, in alliance with partners.
2.3. From Theory to Practice
3. Methodology
- Scouting of an internal or external idea to valorize.
- Identification of the potential industrial/academic partner/s (matchmaking).
- Creation of a protected environment for the brokerage of intellectual property (non-disclosure agreements).
- Definition of the co-creation and co-development projects (cross-fertilization).
- Implementation of the co-creation and co-development project.
- Creation of a go-to-market strategy shared with the partner(s).
3.1. Integrating a New Paradigm within the Hospital Traditional Philosophy
- -
- in theories and practices supporting individual and collective mental models (paradigm);
- -
- in the biomedical field because new forms of knowledge are transferred and integrated in it with original solutions and ideas (position);
- -
- in the ways, methodologies, and pathways towards which every form of knowledge is generated (process); and
- -
- in the outcomes of personalized research, prevention, and treatment that, in this sense, differ from traditional medicine (product).
3.2. Integrating New Resources, Skills, and Ideas without Losing Organizational Identity
- -
- Clarify the ethical principles of the organization and its value network.
- -
- Define business priorities (value chain, end users, and stakeholders).
- -
- Maintain the identity of the hospital while allowing for soft integrations.
4. Results
- -
- The researcher metamorphosis (micro level); change happened within the core value of the organization; people and paradigm.
- -
- The organizational metamorphosis (meso level): individual change was translated into organizational practices and processes, leading the hospital to a new competitive position in the market.
- -
- The market metamorphosis (macro level): new digital and technological solutions have been implemented for PM to anticipate disease manifestation/progression/recurrence (e.g., predictive models).
4.1. The Researcher Metamorphosis
- -
- What skills does the researcher already have?
- -
- What are the new skills the researcher needs to acquire?
- -
- to recognize implicit and explicit know-how;
- -
- to create literacy (also when the researcher is highly educated and skilled); and
- -
- to train the researcher’s soft skills.
- Modeling: The unit performed the action to be learned (e.g., a first contact interview with an industry) while the researcher observed it.
- Coaching: The researcher is introduced to the subject/skill and assisted by the unit that provided feedback on what has been done.
- Scaffolding: The researcher learned to perform the task under the guidance of the unit.
- Fading: The unit continued to accompany the researcher allowing her/him to act independently and only providing support, if needed.
4.2. The Organizational Metamorphosis
- -
- Preparedness (paradigm-position): what are the dynamic capabilities* of the organization? Is it ready to deal with change and openness?
- -
- Readiness (process-product): what infrastructure (tangible** and intangible*** assets) does the organization have? Is it ready to support the innovation process?
- a.
- The creation of a value network that respects the organizational identity, including ethical and scientific impact.
- b.
- The valorization of internal tangible** (e.g., facilities, instruments, technologies) and intangible*** (e.g., know-how, experience, expertise) assets. For example, we gave value to the expertise of our researchers in the conduction of clinical trials, as well as to the presence of the G-STeP with, in the case of digital medicine, the Gemelli Generator research center, specifically dedicated to data sciences.
- c.
- The habilitation of its dynamic capabilities, which consist in the ability to absorb, adapt, and build internal and external resources to rapidly respond to the changing environment [43].
4.3. The Market Metamorphosis
5. Discussion
- The study of -omic information (e.g., genomics, proteomics, metabolomics, etc.) analyzed by the discipline of bioinformatics to retrieve -omic patterns (for example, to identify a gene alteration and consequently find a personalized target therapy for that alteration) [44].
- The study of the clinical phenotype combined with holistic information (e.g., lifestyle, psychology, sociology, which go under the definition of RWD) analyzed by systems medicine and accelerated with the discipline of artificial intelligence to find a clinical pattern associated to a behavioral one, to predict and model their evolution into a clinical outcome and/or behavioral change [45].
- -
- Strengthening the ethical and regulatory framework on data protection, privacy compliance and consent to treatment [50].
- -
- Expanding collaboration among different research centers and healthcare providers to share data [51].
- -
- Certifying/validating the reliability of data sources [52].
- -
- Implement a transparent and secure supply chain for collection, simplification, and conversion of raw to refined data for their actionability in clinical decision support systems that are useful for patients and physicians and, at large, for healthcare operators [53].
- -
- Implement an evidence-based supply chain on training, verification, and validation of algorithms in compliance with national and international laws and regulations [54].
6. Beyond Hype
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cesario, A.; D’Oria, M.; Bove, F.; Privitera, G.; Boškoski, I.; Pedicino, D.; Boldrini, L.; Erra, C.; Loreti, C.; Liuzzo, G.; et al. Personalized clinical phenotyping through systems medicine and artificial intelligence. J. Pers. Med. 2021, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Khatab, Z.; Yousef, G.M. Disruptive innovations in the clinical laboratory: Catching the wave of precision diagnostics. Crit. Rev. Clin. Lab. Sci. 2021, 58, 546–562. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.; Routray, S.; Ahmad, S.; Waris, M.M. Internet of Medical Things (IoMT)-based smart healthcare system: Trends and progress. Comput. Intell. Neurosci. 2022, 16, 7218113. [Google Scholar] [CrossRef] [PubMed]
- Digital Medicine Society. Defining Digital Medicine. 2022. Available online: https://www.dimesociety.org/about-us/defining-digital-medicine/ (accessed on 5 August 2022).
- Shillington, A.; Ganjuli, A.; Clewell, J. The impact of patient support programs on adherence, clinical, humanistic, and economic patient outcomes: A targeted systematic review. Patient Prefer. Adherence 2016, 10, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, O.R.; Dearing, C.; Jagers, D.; Shaw, M.J.; Raffan, F.; Jones, A.; Taggart, R.; Sinclair, T.; Anderson, T.; Ritchie, A.G. Virtual health care for community management of patients with COVID-19 in Australia: Observational cohort study. J. Med. Internet Res. 2021, 23, e21064. [Google Scholar] [CrossRef] [PubMed]
- Bruynseels, K.; Santoni de Sio, F.; van den Hoven, J. Digital Twins in Health Care: Ethical implications of an emerging engi-neering paradigm. Front. Genet. 2018, 13, 31. [Google Scholar] [CrossRef]
- Viceconti, M.; Pappalardo, F.; Rodriguez, B.; Horner, M.; Bischoff, J.; Tshinanu, F.M. In silico trials: Verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods 2021, 185, 120–127. [Google Scholar] [CrossRef]
- Van Norman, G.A. Decentralized clinical trials: The future of medical product development? JACC Basic Transl. Sci. 2021, 6, 384–387. [Google Scholar] [CrossRef]
- Gussoni, G. Digital therapeutics: An opportunity for Italy, and beyond. Tendenze Nuove 2021, 4, 3–8. [Google Scholar]
- Maugeri-Saccà, M.; De Maria, R. Translating basic research in cancer patient care. Ann. Ist. Super Sanita 2011, 47, 64–71. [Google Scholar] [CrossRef]
- Schein, E. La Consulenza di Processo; Raffaello Cortina: Milan, Italy, 2001. [Google Scholar]
- Nonaka, I.; Takeuchi, H. The Knowledge-Creating Company; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Argyris, C.; Schön, D. Apprendimento Organizzativo; Guerini e Associati: Milan, Italy, 1998. [Google Scholar]
- Argote, L.; Ingram, P. Knowledge transfer: A basis for competitive advantage in firms. Organ. Behav. Hum. Decis. Process. 2000, 82, 150–169. [Google Scholar] [CrossRef] [Green Version]
- Maturana, H.; Varela, F. The Tree of Knowledge: The Biological Roots of Human Understanding; Shambhala Publications Inc.: Boston, MA, USA, 1987. [Google Scholar]
- Luisi, P.L. Autopoiesis: A review and a reappraisal. Naturwissenschaften 2003, 90, 49–59. [Google Scholar] [CrossRef]
- McFarland, D.; Gomez, C. Organizational Analysis; Stanford University Press: Stanford, CA, USA, 2016. [Google Scholar]
- Donald, M. Origins of the Modern Mind: Three Stages in the Evolution of Culture and Cognition; Harvard University Press: Boston, MA, USA, 1991. [Google Scholar]
- Christensen, C. The Innovator’s Dilemma; Harvard Business School Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Orsenigo, A. Cambiamenti organizzativi. Animazione Soc. 1997, 12, 39–52. [Google Scholar]
- Chesbrough, H. Open Innovation: The New Imperative for Creating and Profiting from Technology; Harvard Business School Press: Oxford, UK, 2003. [Google Scholar]
- Chesbrough, H. Open Innovation: Researching a New Paradigm; Harvard Business School Press: Oxford, UK, 2006. [Google Scholar]
- Gassman, O.; Enkel, E. Towards a Theory of Open Innovation: Three Core Process Archetypes. In Proceedings of the RADMA Conference, Lisbon, Portugal; 2004; pp. 1–18. Available online: https://www.alexandria.unisg.ch/274/1/Gassmann_Enkel.pdf. (accessed on 4 August 2022).
- Zangrandi, A.; Fanelli, S.; Donelli, C.C.; Elefanti, M. (A Cura di) Crisis Management: La Gestione di un Ospedale Durante una Pandemia; EGEA: Milan, Italy, 2020. [Google Scholar]
- Liu, Z.; Shi, Y.; Yang, B. Open innovation in times of crisis: An overview of the healthcare sector in response to the COVID-19 Pandemic. J. Open Innov. Technol. Mark. Complex. 2022, 8, 21. [Google Scholar] [CrossRef]
- Verma, S.; Gustafsson, A. Investigating the emerging COVID-19 research trends in the field of business and man-agement: A bibliometric analysis approach. J. Bus. Res. 2020, 118, 253–261. [Google Scholar] [CrossRef]
- Chesbrough, H.W. Open innovation: A new paradigm for understanding industrial innovation. In Open Innovation: Researching a New Paradigm; Chesbrough, W., Vanhaverbeke, J.W., Eds.; Oxford University Press: Oxford, UK, 2006; pp. 1–12. [Google Scholar]
- Fondazione Policlinico Universitario Agostino Gemelli IRCCS (2022). Mission and Impact Report 2021. Available online: https://www.policlinicogemelli.it/wp-content/uploads-shared/mir2021/#page=31 (accessed on 4 August 2022).
- Garret-Jones, S.; Turpin, T.; Bellavista, J.; Hill, S. Using Basic Research: Assessing Connections between Basic Research and Socio-economic Objectives; National Board of Employment, Education and Training, Australian Government Publishing Service: Canberra, Australia, 1995.
- Scambia, G.; Valentini, V.; Cesario, A.; Parolini, O.; Nero, C. Asset a supporto della ricerca. In La Medicina Personalizzata fra Ricerca e Cura; Cesario, A., D’Oria, M., Scambia, G., Eds.; FrancoAngeli: Milan, Italy, 2021; pp. 163–168. [Google Scholar]
- European Commission. European Parliament Legislative Resolution of 17 April 168 2020 on the Proposal for a Regulation of the European Parliament and of the Council Amending Regulation(EU) 2017/745 on Medical Devices as Regards the Dates of Application of Certain of Its Provisions (COM(2020)0144—C9-0098/2020—2020/0060(COD). 2020. Available online: www.europarl-.europa.eu/doceo/document/TA-9-2020-0053_EN.html#title2 (accessed on 3 August 2022).
- Tidd, J.; Bessant, J.R. Managing Innovation. Integrating Technological, Market and Organizational Change; Wiley: New Delhi, India, 2016. [Google Scholar]
- Kichko, K. Personalized Medicine as Innovation. What Can Germany Learn from the USA; Springer Gabler: Wiesbaden, Germany, 2019. [Google Scholar] [CrossRef]
- Bogers, M. The open innovation paradox: Knowledge sharing and protection in R&D collaborations. Eur. J. Innov. Manag. 2011, 14, 93–117. [Google Scholar] [CrossRef]
- Tani, M.; Papaluca, O.; Sasso, P. The system thinking perspective in the open-innovation research: A systematic review. J. Open Innov. 2018, 4, 38. [Google Scholar] [CrossRef]
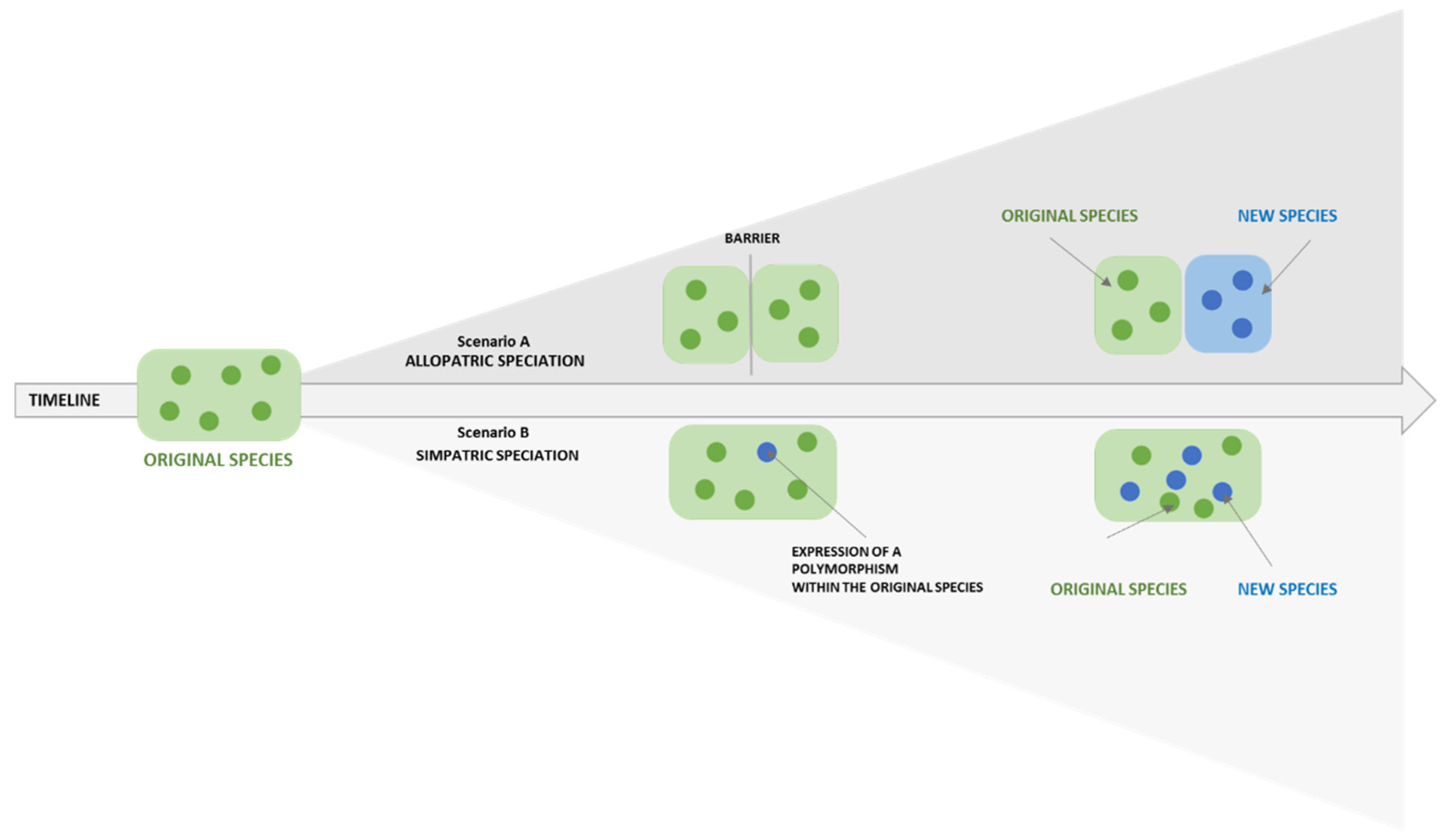
- BioNinja. Species and Speciations. 2022. Available online: http://old-ib.bioninja.com.au/options/option-d-evolution-2/d2-species-and-speciation.html (accessed on 4 August 2021).
- Jütte, W.; Wildemeersch, D. Editorial: Digital the new normal—Multiple challenges for the education and learning of adults. Eur. J. Res. Educ. Learn. Adults 2017, 8, 7–20. [Google Scholar] [CrossRef]
- Mezirow, J. Learning as Transformation; Jossey-Bass: San Francisco, CA, USA, 2000. [Google Scholar]
- Mezirow, J. Transformative Learning as Discourse; Sage Publications: Thousand Oaks, CA, USA, 2003. [Google Scholar]
- Hansman, C. Context-based adult learning. In New Directions for Adult and Continuing Education; Jossey-Bass: San Francisco, CA, USA, 2001; Volume 89, pp. 43–51. [Google Scholar]
- Nieuwenhuizen, C. Enterpreneurial Skills; JUTA: Cape Town, South Africa, 2008. [Google Scholar]
- Cordes-Berszinn, P. Dynamic Capabilities: How Organisational Structures Affect Knowledge Processes; Palgrave Macmillan: New York, NY, USA, 2013. [Google Scholar]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef]
- Sturmberg, J.; Carmel, M. Handbook of Systems and Complexity in Health; Springer: New York, NY, USA, 2013. [Google Scholar]
- Bousquet, J.; Anto, J.M.; Sterk, P.J.; Adcock, I.M.; Chung, K.F.; Roca, J.; Agusti, A.; Brightling, C.; Cambon-Thomsen, A.; Cesario, A.; et al. Systems medicine and integrated care to combat chronic noncommunicable diseases. Genome Med. 2011, 3, 43. [Google Scholar] [CrossRef]
- Bousquet, J.; Jorgensen, C.; Dauzat, M.; Cesario, A.; Camuzat, T.; Bourret, R.; Best, N.; Anto, J.; Abecassis, F.; Aubas, P.; et al. Systems medicine approaches for the definition of complex phenotypes in chronic diseases and ageing. From concept to implementation and policies. Curr. Pharm. Des. 2014, 20, 5928–5944. [Google Scholar] [CrossRef] [PubMed]
- Cesario, A.; Auffray, C.; Agusti, A.; Apolone, G.; Balling, R.; Barbanti, P.; Bellia, A.; Boccia, S.; Bousquet, J.; Cardaci, V.; et al. A Systems medicine clinical platform for understanding and managing non-communicable diseases. Curr. Pharm. Des. 2014, 20, 5945–5956. [Google Scholar] [CrossRef] [PubMed]
- Cesario, A.; D’Oria, M.; Calvani, R.; Picca, A.; Pietragalla, A.; Lorusso, D.; Daniele, G.; Lohmeyer, F.; Boldrini, L.; Valentini, V.; et al. The role of artificial intelligence in managing multimorbidity and cancer. J. Pers. Med. 2021, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, P.; Harri, P.; Prater, A.; Safdar, N.M. Protecting your patients’ interests in the era of big data, artificial intelligence, and predictive analytics. J. Am. Coll. Radiol. 2018, 15, 580–586. [Google Scholar] [CrossRef]
- Dewey, M.; Bosserdt, M.; Dodd, J.D.; Thun, S.; Kressel, H.Y. Clinical imaging research: Higher evidence, global collaboration, improved reporting, and data sharing are the grand challenges. Radiology 2019, 291, 547–552. [Google Scholar] [CrossRef]
- Househ, M.; Aldosari, B. The hazards of data mining in healthcare. Stud. Health Technol. Inform. 2017, 38, 80–83. [Google Scholar] [CrossRef]
- Cutiongco, M.F.A.; Jensen, B.S.; Reynolds, P.M.; Gadegaard, N. Predicting gene expression using morphological cell responses to nanotopography. Nat. Commun. 2020, 11, 1384. [Google Scholar] [CrossRef]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef]
- Pastorino, R.; Loreti, C.; Giovannini, S.; Ricciardi, W.; Padua, L.; Boccia, S. Challenges of Prevention for a Sustainable Personalized Medicine. J. Pers. Med. 2021, 11, 311. [Google Scholar] [CrossRef]
- Cesario, A.; Auffray, C.; Russo, P.; Hood, L. P4 Medicine Needs P4 Education. Curr. Pharm. Des. 2014, 20, 6071–6072. [Google Scholar] [CrossRef]

| Product | Short Description |
|---|---|
| Patient Support Program | Virtual programs through which care continuity can be provided to patients beyond the outpatient setting. By making use of multiple remote monitoring tools (apps, wearable devices, questionnaires, etc.) provided to the patient, healthcare professionals can check his/her health status in real time, be in constant contact with him/her, and intervene when appropriate [5]. AI algorithms can predict the risk of relapse (either physical or psychological), and they are specifically designed to monitor patients in the time distances between two clinical encounters (i.e., when he/she is not physically present in the hospital and may live outside the region). Patients are trained through virtual coaching sessions with dedicated health professionals (e.g., psychologist, nutritionist) in properly using the monitoring tools, and have access to apps/portals for educational materials on disease management. Those plug-ins are digital solutions aiming at meeting several needs in the patient journey and delivering innovative care models and/or identifying profiles of progression in the transition from health to disease. |
| Virtual Ward | Digitalized solutions for patient monitoring to facilitate real-time observation of certain parameters (clinical, psychological, etc.), to follow patients who cannot go to the hospital remotely [6]. For example, if a cancer patient is unable to travel from home (for example, due to an infection such as COVID-19), he/she can still be followed by doctors by checking his/her own biometric parameters (e.g., oxygen levels) after being adequately informed and trained for the correct use of the measuring tools. Information is delivered (via video call, call, or messaging) to health professionals who are in daily contact with the patient, offering telemedicine services and recalling him/her to the hospital for observation or treatment if necessary. Algorithms can support data processing to predict the future course of the patient’s condition and help the physician to make more personalized data-driven decisions. |
| Human Digital Twin | Computerized avatars that simulate the information of the patient, by connecting medical history (including family history, if available) with current illnesses and symptoms [7]. Human digital twins (HDTs) are created by mathematical and computational models to provide insightful mechanisms by regulating patient responses to treatment, and to generate virtual cohorts with practical applications in oncology (e.g., breast cancer, melanoma, brain cancer, lung cancer), infectious diseases (HIV, SARS-CoV2), metabolic diseases (diabetes) and cardiovascular diseases (e.g., coronary artery disease). HDTs are important for clinicians and patients because they give a comprehensive overview of the patient’s past and current clinical history, to develop a personalized plan and simulate predictions to anticipate disease onset or exacerbation. |
| In silico Clinical Trial | Reliable computational models of virtual trials, which can be used to study the effect of virtual treatments on virtual patients and predict the outcome of a clinical treatment in terms of safety and efficacy [8]. They can identify worst-case scenarios to optimize the safety of a preclinical trial. For example, machine learning (ML)/deep learning (DL) algorithms can be used in designing and simulating virtual trials—often customized on HDTs—to study the development or regulatory evaluation of a virtual drug, a device, or a therapeutic intervention. Studies with real patients may be reduced in favor of sophisticated simulations that predict, for example, the safety and efficacy of a treatment on a specific patient, or a subset of patients with similar clinical patophenotype. Another example regards the development/improvement of drugs and therapies to predict their outcomes (including the assessment of possible toxicity or the onset of adverse events) and personalize them according to each patient’s characteristics. These models can further facilitate greater understanding of molecules’ behavior (e.g., potential antimicrobial activity) by screening a large volume of molecules and virtually test them to identify antibacterial compounds structurally distant from known antibiotics. |
| Decentralized Clinical Trial | Studies that leverage “virtual” tools, such as sensory-based technologies, wearable medical devices, home visits, patient-driven virtual health care interfaces, and direct delivery of study drugs and materials to patients’ homes. In a fully decentralized clinical trial, subject recruitment, delivery and administration of study medication, and acquisition of trial outcomes data all proceed without involving in-person contact between the study team and the patient/subject [9]. Focused on patient-centricity, these trials allow patients to participate and improve compliance where constraints, such as socioeconomic factors, may occur by giving them more flexibility in ways to participate. |
| Digital Therapeutics | Evidence-based therapeutic interventions for the patient that a physician “prescribes” from a clinical dashboard (e.g., alerts for therapy management, progress tracking, health literacy, doctor jump-in) based on AI algorithms that are driven by high-quality software programs to prevent, manage, or treat a medical disorder or disease. They are used independently or in concert with medications, devices, or other therapies to optimize patient care and health outcomes [10]. The “active ingredient” corresponds to the component that shows an evidence-based therapeutic effect (e.g., motivational interview, psychoeducation, or similar tools elaborated on the experience of clinicians or from the literature). The “excipient” shapes the active ingredient to promote its intake; therefore it can be the user interface, specifically designed to maximize the efficacy of the active ingredient. |
| Results | Details | Total |
|---|---|---|
| Fundings | Fundings for industrial projects Contracts for industrial projects (FPG) | 497,000 € 4 |
| Fundings from European projects European projects awarded | 1,723,250 € 4 | |
| Funding for Innovation Total funds raised | 12,000,000 € 14,220,250 € | |
| Projects | Patient support programs
| 3 1 |
| AI-driven predictive models Investigator-driven clinical trials | 1 4 | |
| Publications * | Peer-review articles | 31 |
| Books | 3 | |
| Chapters Total impact factor Total citations | 14 91.6 points 71 | |
| Education | Training courses provided by the OI Unit
| 10 3 3 3 1 |
| Our OI Unit as a “case study” for Master thesis | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesario, A.; D’Oria, M.; Simone, I.; Patarnello, S.; Valentini, V.; Scambia, G. Open Innovation as the Catalyst in the Personalized Medicine to Personalized Digital Medicine Transition. J. Pers. Med. 2022, 12, 1500. https://doi.org/10.3390/jpm12091500
Cesario A, D’Oria M, Simone I, Patarnello S, Valentini V, Scambia G. Open Innovation as the Catalyst in the Personalized Medicine to Personalized Digital Medicine Transition. Journal of Personalized Medicine. 2022; 12(9):1500. https://doi.org/10.3390/jpm12091500
Chicago/Turabian StyleCesario, Alfredo, Marika D’Oria, Irene Simone, Stefano Patarnello, Vincenzo Valentini, and Giovanni Scambia. 2022. "Open Innovation as the Catalyst in the Personalized Medicine to Personalized Digital Medicine Transition" Journal of Personalized Medicine 12, no. 9: 1500. https://doi.org/10.3390/jpm12091500
APA StyleCesario, A., D’Oria, M., Simone, I., Patarnello, S., Valentini, V., & Scambia, G. (2022). Open Innovation as the Catalyst in the Personalized Medicine to Personalized Digital Medicine Transition. Journal of Personalized Medicine, 12(9), 1500. https://doi.org/10.3390/jpm12091500








