Acute Antibody-Mediated Rejection in Liver Transplant Recipients with Autoimmune Liver Disease: A Clinical and Pathologic Study of 4 Cases
Abstract
1. Introduction
2. Patients and Methods
3. Histopathology
4. HLA Antibodies Detection
5. Immunosuppression Management
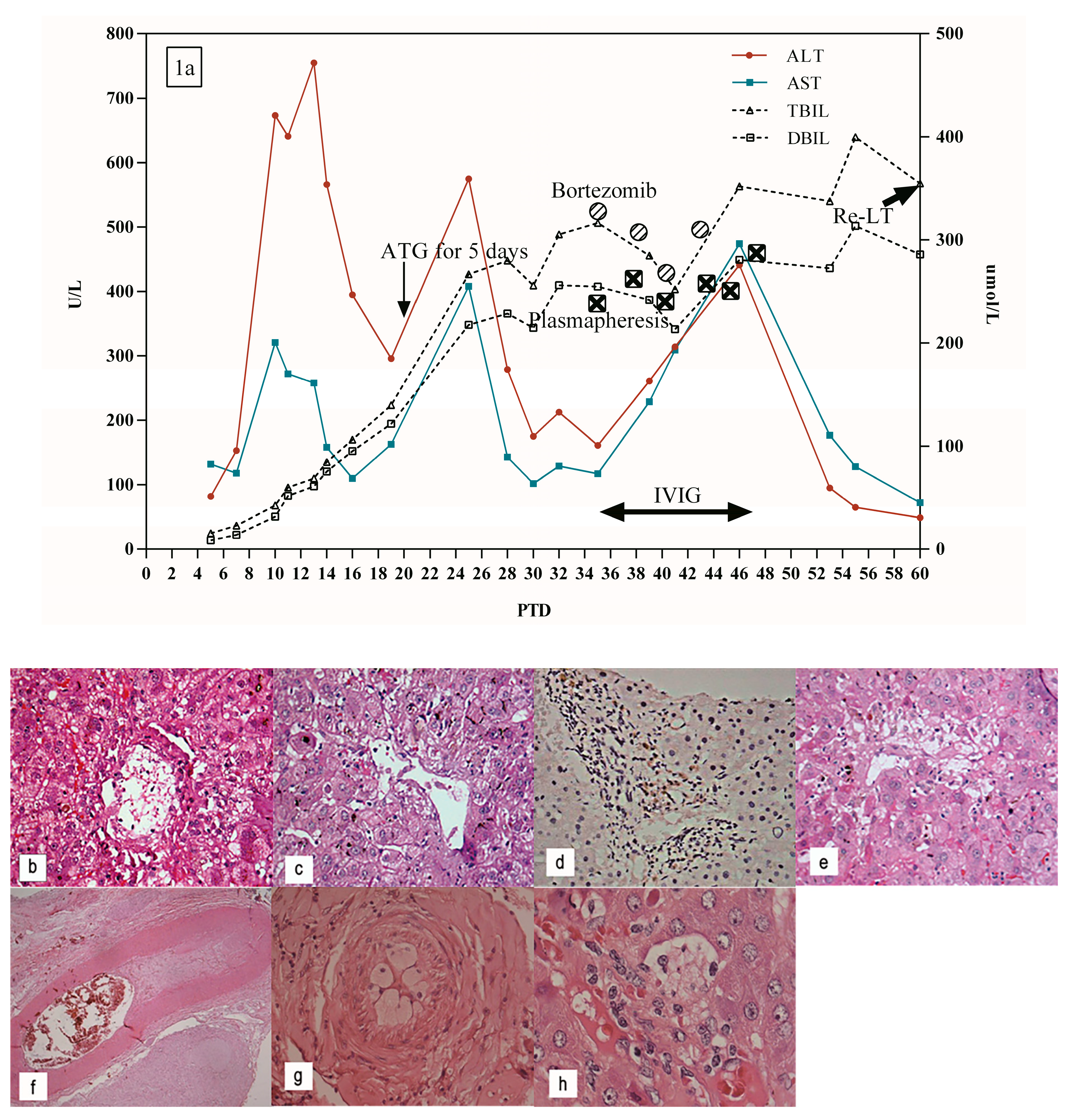
5.1. Patient 1
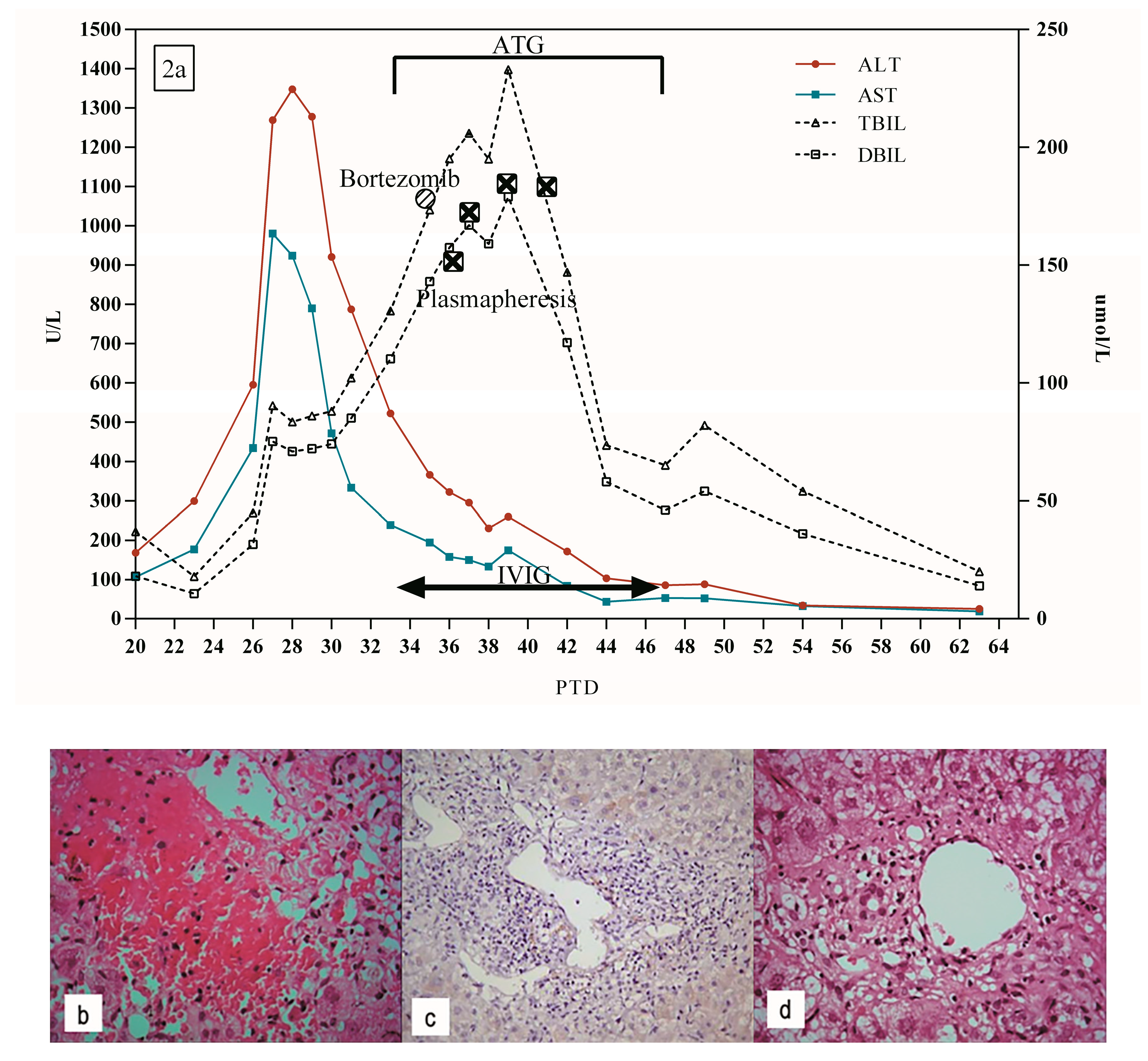
5.2. Patient 2
5.3. Patient 3
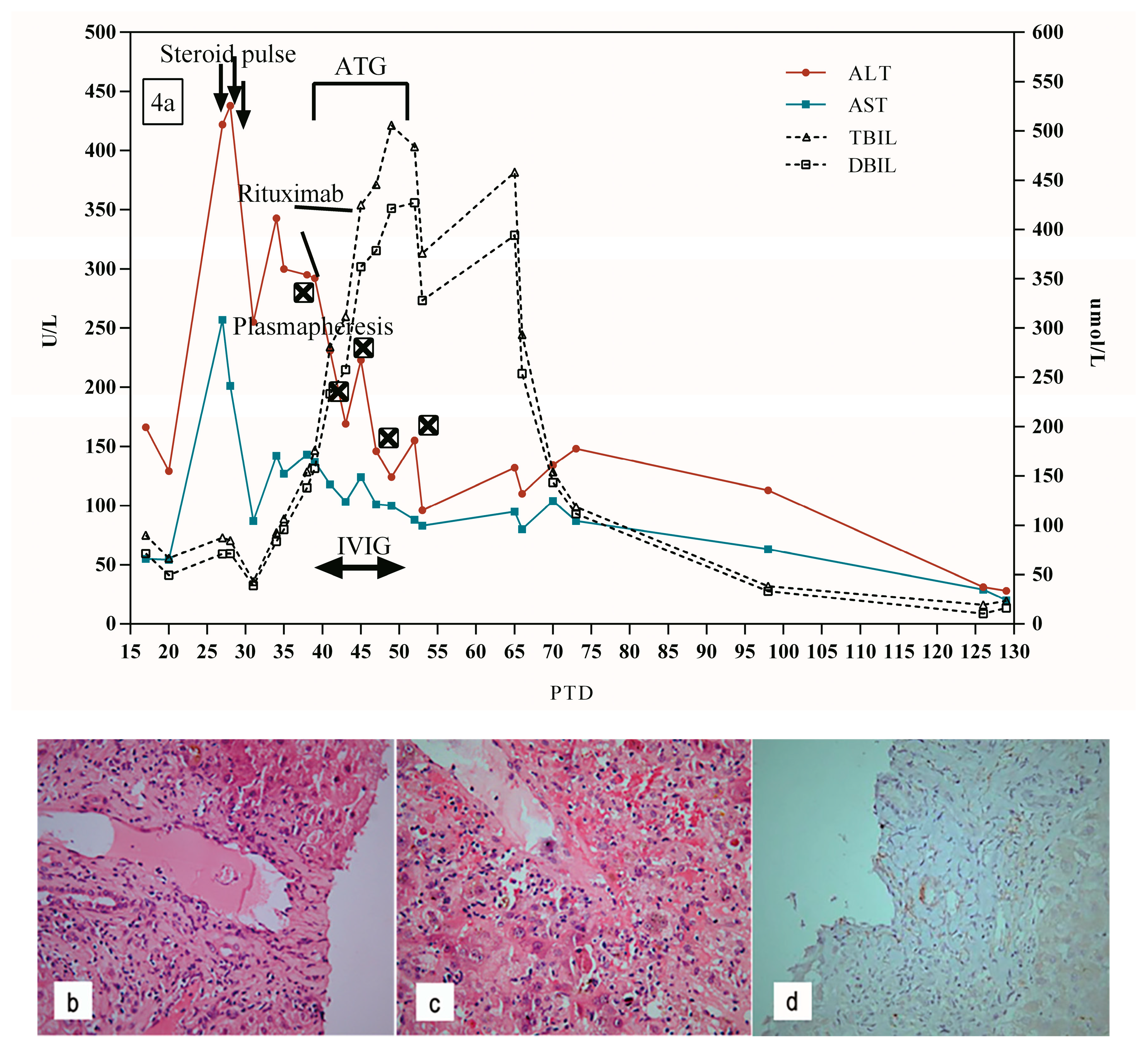
5.4. Patient 4
6. Results
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Edmunds, C.; Ekong, U.D. Autoimmune Liver Disease Post-Liver Transplantation: A Summary and Proposed Areas for Future Research. Transplantation 2016, 100, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Floreani, A.; De Martin, S.; Secchi, M.F.; Cazzagon, N. Extrahepatic autoimmunity in autoimmune liver disease. Eur. J. Intern. Med. 2019, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Kono, H.; Leung, P.S.C.; Gershwin, M.E. Recurrence of disease following organ transplantation in autoimmune liver disease and systemic lupus erythematosus. Cell. Immunol. 2020, 347, 104021. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, J.; Goldberg, D.; Smith, A.R.; Mansfield, S.A.; Gillespie, B.W.; Merion, R.M.; Lok, A.S.; Levy, G.; Kulik, L.; Abecassis, M.; et al. Acute Rejection Increases Risk of Graft Failure and Death in Recent Liver Transplant Recipients. Clin. Gastroenterol. Hepatol. 2017, 15, 584–593.e2. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, R.H.; Demetris, A.J.; Belle, S.H.; Seaberg, E.C.; Lake, J.R.; Zetterman, R.K.; Everhart, J.; Detre, K.M. Acute hepatic allograft rejection: Incidence, risk factors, and impact on outcome. Hepatology 1998, 28, 638–645. [Google Scholar] [CrossRef]
- Tannuri, A.C.; Lima, F.; Mello, E.S.; Tanigawa, R.Y.; Tannuri, U. Prognostic factors for the evolution and reversibility of chronic rejection in pediatric liver transplantation. Clinics (Sao Paulo) 2016, 71, 216–220. [Google Scholar] [CrossRef]
- Charlton, M.; Levitsky, J.; Aqel, B.; OʼGrady, J.; Hemibach, J.; Rinella, M.; Fung, J.; Ghabril, M.; Thomason, R.; Burra, P.; et al. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation 2018, 102, 727–743. [Google Scholar] [CrossRef]
- Colvin, R.B. C4d in liver allografts: A sign of antibody-mediated rejection? Am. J. Transpl. 2006, 6, 447–448. [Google Scholar] [CrossRef]
- Kerkar, N.; Lakhole, A. Pediatric liver transplantation: A North American perspective. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Paterno, F.; Shiller, M.; Tillery, G.; O’Leary, J.G.; Susskind, B.; Trotter, J.; Klintmalm, G.B. Bortezomib for acute antibody-mediated rejection in liver transplantation. Am. J. Transpl. 2012, 12, 2526–2531. [Google Scholar] [CrossRef]
- Wozniak, L.J.; Naini, B.V.; Hickey, M.J.; Bhattacharyya, S.; Reed, E.F.; Busuttil, R.W.; Farmer, D.G.; Vargas, J.H.; Venick, R.S.; McDiarmid, S.V. Acute antibody-mediated rejection in ABO-compatible pediatric liver transplant recipients: Case series and review of the literature. Pediatr. Transplant. 2017, 21, e12791. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.T.; Fiel, M.I.; Schiano, T.D. Antibody-mediated rejection of the liver allograft: An update and a clinico-pathological perspective. J. Hepatol. 2021, 75, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Shindoh, J.; Akamatsu, N.; Tanaka, T.; Kaneko, J.; Tamura, S.; Sakamoto, Y.; Hasegawa, K.; Sugawara, Y.; Makuuchi, M.; Kokudo, N. Risk factors for acute liver allograft rejection and their influences on treatment outcomes of rescue therapy in living donor liver transplantation. Clin. Transpl. 2016, 30, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.K.; Jones, O.D.; Vanatta, J.M.; Kamal, F.; Kedia, S.K.; Jiang, Y.; Nair, S.P.; Eason, J.D. Outcomes of Liver Transplant Recipients With Autoimmune Liver Disease Using Long-Term Dual Immunosuppression Regimen Without Corticosteroid. Transplant. Direct 2017, 3, e178. [Google Scholar] [CrossRef]
- Demetris, A.J.; Bellamy, C.; Hubscher, S.G.; O’Leary, J.; Randhawa, P.S.; Feng, S.; Neil, D.; Colvin, R.B.; McCaughan, G.; Fung, J.J.; et al. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am. J. Transplant. 2016, 16, 2816–2835. [Google Scholar] [CrossRef]
- Lunz, J.; Ruppert, K.M.; Cajaiba, M.M.; Isse, K.; Bentlejewski, C.A.; Minervini, M.; Nalesnik, M.A.; Randhawa, P.; Rubin, E.; Sasatomi, E.; et al. Re-examination of the lymphocytotoxic crossmatch in liver transplantation: Can C4d stains help in monitoring? Am. J. Transpl. 2012, 12, 171–182. [Google Scholar] [CrossRef]
- Tambur, A.R.; Herrera, N.D.; Haarberg, K.M.; Cusick, M.F.; Gordon, R.A.; Leventhal, J.R.; Friedewald, J.J.; Glotz, D. Assessing Antibody Strength: Comparison of MFI, C1q, and Titer Information. Am. J. Transpl. 2015, 15, 2421–2430. [Google Scholar] [CrossRef]
- Neil, D.A.H.; Bellamy, C.O.; Smith, M.; Haga, H.; Zen, Y.; Sebagh, M.; Ruppert, K.; Lunz, J.; Hübscher, S.G.; Demetris, A.J. Global quality assessment of liver allograft C4d staining during acute antibody-mediated rejection in formalin-fixed, paraffin-embedded tissue. Hum. Pathol. 2018, 73, 144–155. [Google Scholar] [CrossRef]
- Buis, C.I.; Geuken, E.; Visser, D.S.; Kuipers, F.; Haagsma, E.B.; Verkade, H.J.; Porte, R.J. Altered bile composition after liver transplantation is associated with the development of nonanastomotic biliary strictures. J. Hepatol. 2009, 50, 69–79. [Google Scholar] [CrossRef]
- Corbani, A.; Burroughs, A.K. Intrahepatic cholestasis after liver transplantation. Clin. Liver Dis. 2008, 12, 111–129. [Google Scholar] [CrossRef]
- Ge, X.; Uzunel, M.; Ericzon, B.G.; Sumitran-Holgersson, S. Biliary epithelial cell antibodies induce expression of toll-like receptors 2 and 3: A mechanism for post-liver transplantation cholangitis? Liver Transpl. 2005, 11, 911–921. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.G.; Michelle Shiller, S.; Bellamy, C.; Nalesnik, M.A.; Kaneku, H.; Jennings, L.W.; Isse, K.; Terasaki, P.I.; Klintmalm, G.B.; Demetris, A.J. Acute liver allograft antibody-mediated rejection: An inter-institutional study of significant histopathological features. Liver Transpl. 2014, 20, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chen, Z.H.; Chen, Z.S.; Zeng, F.J.; Ming, C.S.; Zhang, W.J.; Liu, B.; Gong, N.Q.; Jiang, J.P.; Wei, L.; et al. [Histopathological study of 268 hepatic allograft biopsies]. Zhonghua Yi Xue Za Zhi 2011, 91, 3401–3404. [Google Scholar] [PubMed]
- Ali, S.; Ormsby, A.; Shah, V.; Segovia, M.C.; Kantz, K.L.; Skorupski, S.; Eisenbrey, A.B.; Mahan, M.; Huang, M.A. Significance of complement split product C4d in ABO-compatible liver allograft: Diagnosing utility in acute antibody mediated rejection. Transpl. Immunol. 2012, 26, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Colvin, R.B.; Daha, M.R.; Drachenberg, C.B.; Haas, M.; Nickeleit, V.; Salmon, J.E.; Sis, B.; Zhao, M.H.; Bruijn, J.A.; et al. Pros and cons for C4d as a biomarker. Kidney Int. 2012, 81, 628–639. [Google Scholar] [CrossRef]
- Wozniak, L.J.; Hickey, M.J.; Venick, R.S.; Vargas, J.H.; Farmer, D.G.; Busuttil, R.W.; McDiarmid, S.V.; Reed, E.F. Donor-specific HLA Antibodies Are Associated With Late Allograft Dysfunction After Pediatric Liver Transplantation. Transplantation 2015, 99, 1416–1422. [Google Scholar] [CrossRef]
- Smith, J.D.; Banner, N.R.; Hamour, I.M.; Ozawa, M.; Goh, A.; Robinson, D.; Terasaki, P.I.; Rose, M.L. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am. J. Transpl. 2011, 11, 312–319. [Google Scholar] [CrossRef]
- Willicombe, M.; Brookes, P.; Sergeant, R.; Santos-Nunez, E.; Steggar, C.; Galliford, J.; McLean, A.; Cook, T.H.; Cairns, T.; Roufosse, C.; et al. De novo DQ donor-specific antibodies are associated with a significant risk of antibody-mediated rejection and transplant glomerulopathy. Transplantation 2012, 94, 172–177. [Google Scholar] [CrossRef]
- Harrington, C.R.; Yang, G.Y.; Levitsky, J. Advances in Rejection Management: Prevention and Treatment. Clin. Liver Dis. 2021, 25, 53–72. [Google Scholar] [CrossRef]
- Tajima, T.; Hata, K.; Okajima, H.; Nishikori, M.; Yasuchika, K.; Kusakabe, J.; Yoshizawa, A.; Fukumitsu, K.; Anazawa, T.; Tanaka, H.; et al. Bortezomib Against Refractory Antibody-Mediated Rejection After ABO-Incompatible Living-Donor Liver Transplantation: Dramatic Effect in Acute-Phase? Transpl. Direct 2019, 5, e491. [Google Scholar] [CrossRef]
- Del Bello, A.; Congy-Jolivet, N.; Muscari, F.; Lavayssière, L.; Esposito, L.; Cardeau-Desangles, I.; Guitard, J.; Dörr, G.; Suc, B.; Duffas, J.P.; et al. Prevalence, incidence and risk factors for donor-specific anti-HLA antibodies in maintenance liver transplant patients. Am. J. Transpl. 2014, 14, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, T.L.; Miezynska-Kurtycz, J.; Hodson, J.; Gunson, B.K.; Neuberger, J.; Milkiewicz, P.; Oo, Y.H. Longterm corticosteroid use after liver transplantation for autoimmune hepatitis is safe and associated with a lower incidence of recurrent disease. Liver Transpl. 2016, 22, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.H.; Wu, S.Y.; Ou, K.W.; Su, T.F.; Hsieh, C.B. Retransplant as Rescue Treatment for ABO-Compatible Living-Donor Liver Transplant Related Antibody-Mediated Rejection: A Case Report. Exp. Clin. Transpl. 2018, 16, 222–226. [Google Scholar]

| Patient | Age, Sex | Start of Graft Dysfunction | PRA Class I | PRA Class II | DSA Specificity | Treatment a | Notable Findings on Liver Histopathology | C4d Stain | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroids | Plasmapheresis | IVIG | Rituximab | ATG | Bortezomib | AMR Outcome | ||||||||
| 1 | 48 y, F | PTD 7 | 3.40% | 95.90% | DQ 7 | ✓ | ✓ | ✓ | ✓ | Re-LT, survival | PTD 20: portal inflammatory infiltrate, slight bile ductular reaction and hepatocyte swelling and necrosis around the central vein | - | ||
| PTD 35: the aggravation of bile duct cholestasis, severe hepatocyte necrosis around the central vein | ||||||||||||||
| PTD 60: further aggravation of central perivenulitis, bile duct cholestasis, and hepatocyte necrosis | ||||||||||||||
| PTD62: intrahepatic arterial endothelial edema, lumen stenosis, even occlusion, central perivenulitis, severe hepatocyte necrosis around the central vein in resected transplanted liver | ||||||||||||||
| 2 | 34 y, M | PTD 20 | 2.17% | 0.31% | DQ 2 | ✓ | ✓ | ✓ | ✓ | ✓ | Survival | PTD 30: lymphocyte infiltrates in the portal area, moderate to severe central perivenulitis, local hepatocyte hemorrhage and necrosis around central vein | - | |
| PTD 51: mild central perivenulitis | ||||||||||||||
| 3 | 44 y, F | PTD 27 | 98.56% | 84.05% | NT | ✓ | ✓ | ✓ | ✓ | Survival | PTD 33: mild central perivenulitis, portal inflammatory infiltration | - | ||
| 4 | 51 y, F | PTD 27 | 42.30% | 41.50% | DQ 7, DQ9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Survival | PTD 28: mild lymphocytic infiltrate in portal tract, ductular reaction, and scattered hepatocyte necrosis | - |
| PTD 39: central perivenulitis and hepatocyte necrosis around the central vein furtherly aggravated, and lymphocytic infiltrate in portal tract | ||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Guo, H.; Yang, B.; Zhao, Y.; Wei, L.; Chen, Z.; Chen, D. Acute Antibody-Mediated Rejection in Liver Transplant Recipients with Autoimmune Liver Disease: A Clinical and Pathologic Study of 4 Cases. J. Pers. Med. 2023, 13, 41. https://doi.org/10.3390/jpm13010041
Jiang H, Guo H, Yang B, Zhao Y, Wei L, Chen Z, Chen D. Acute Antibody-Mediated Rejection in Liver Transplant Recipients with Autoimmune Liver Disease: A Clinical and Pathologic Study of 4 Cases. Journal of Personalized Medicine. 2023; 13(1):41. https://doi.org/10.3390/jpm13010041
Chicago/Turabian StyleJiang, Hongmei, Hui Guo, Bo Yang, Yuanyuan Zhao, Lai Wei, Zhishui Chen, and Dong Chen. 2023. "Acute Antibody-Mediated Rejection in Liver Transplant Recipients with Autoimmune Liver Disease: A Clinical and Pathologic Study of 4 Cases" Journal of Personalized Medicine 13, no. 1: 41. https://doi.org/10.3390/jpm13010041
APA StyleJiang, H., Guo, H., Yang, B., Zhao, Y., Wei, L., Chen, Z., & Chen, D. (2023). Acute Antibody-Mediated Rejection in Liver Transplant Recipients with Autoimmune Liver Disease: A Clinical and Pathologic Study of 4 Cases. Journal of Personalized Medicine, 13(1), 41. https://doi.org/10.3390/jpm13010041






