Management of Patients with Type V Hyperlipoproteinemia: An Uncommon Phenotype of Dyslipidemia with Chylomicronemia and Severe Hypertriglyceridemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Lipoprotein Electrophoresis (LPEP)
2.3. Identification of Each FLL Phenotype by apoB Algorithm and LPEP
2.4. Data Collection
2.5. Study Outcomes
2.6. Statistical Methods
3. Results
3.1. Baseline Demographic Characteristics of Patients
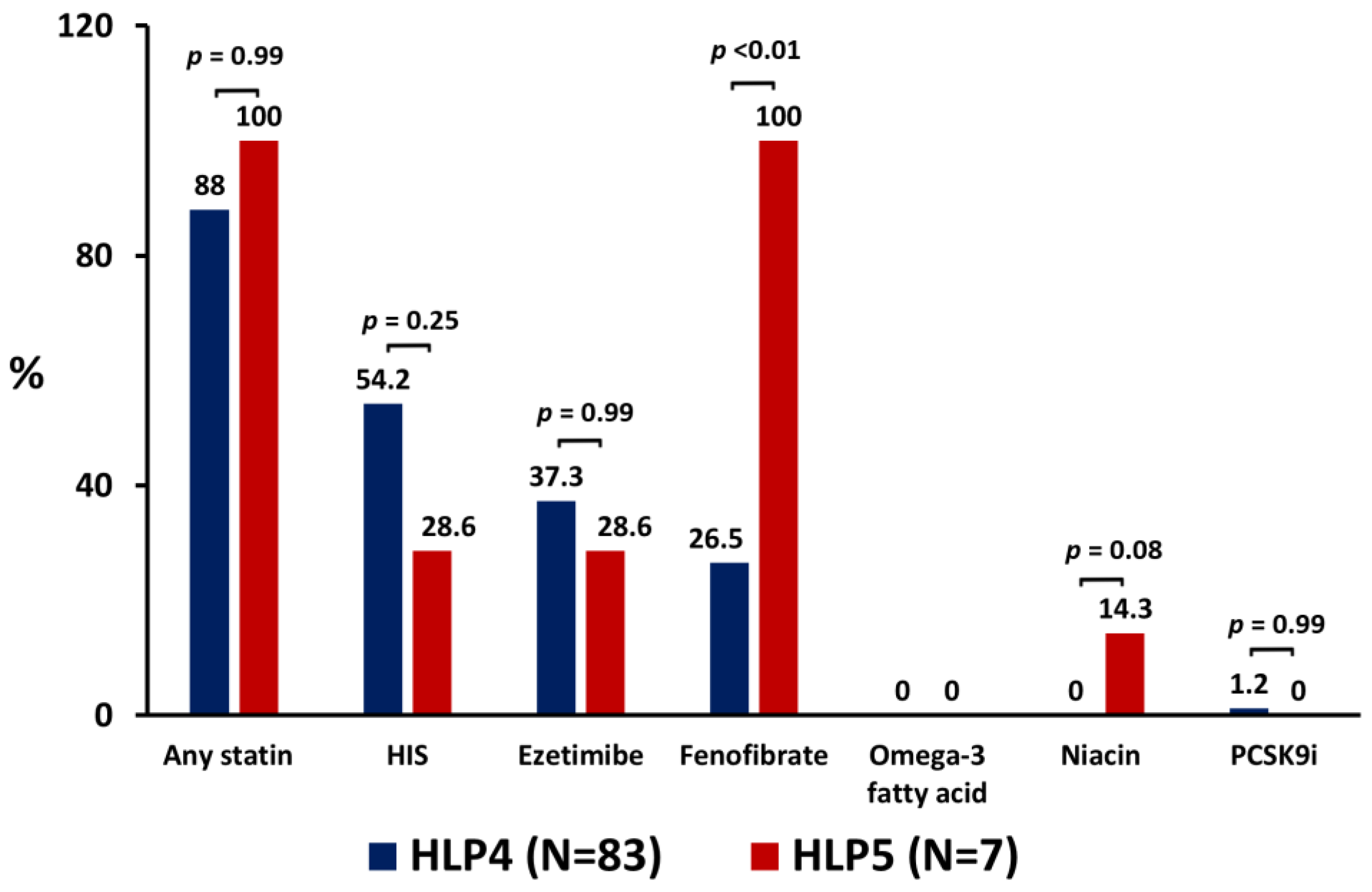
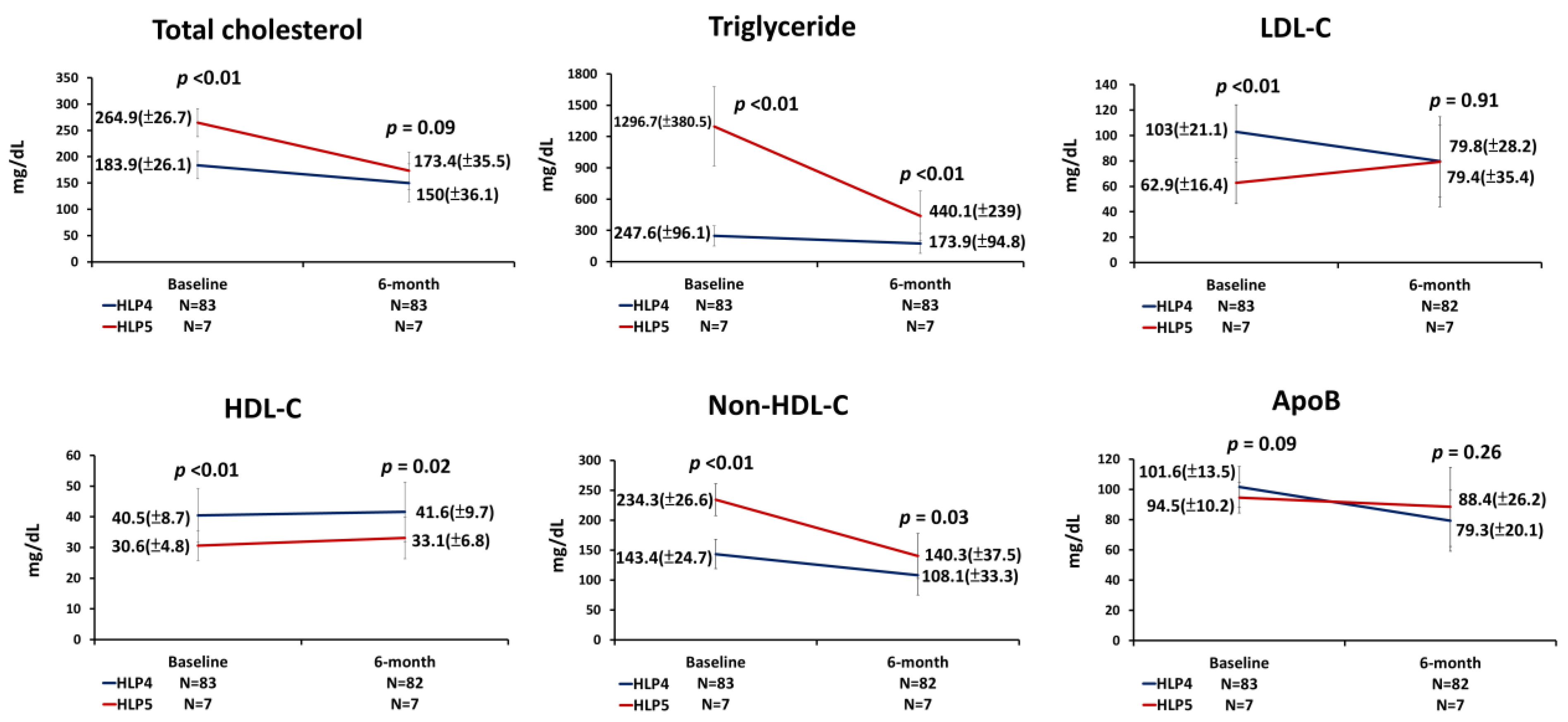
3.2. The Use of LLT and Lipid Parameters at the 6-Month Follow-Up
3.3. Percentages of Change in Lipid Profiles at the 6-Month Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fredrickson, D.S.; Levy, R.I.; Lees, R.S. Fat transport in lipoproteins--an integrated approach to mechanisms and disorders. N. Engl. J. Med. 1967, 276, 215–225. [Google Scholar] [CrossRef]
- Beaumont, J.L.; Carlson, L.A.; Cooper, G.R.; Fejfar, Z.; Fredrickson, D.S.; Strasser, T. Classification of hyperlipidaemias and hyperlipoproteinaemias. Bull. World Health Organ. 1970, 43, 891–915. [Google Scholar] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sniderman, A.; Couture, P.; de Graaf, J. Diagnosis and treatment of apolipoprotein B dyslipoproteinemias. Nat. Rev. Endocrinol. 2010, 6, 335–346. [Google Scholar] [CrossRef]
- Sathiyakumar, V.; Pallazola, V.A.; Park, J.; Vakil, R.M.; Toth, P.P.; Lazo-Elizondo, M.; Quispe, R.; Guallar, E.; Banach, M.; Blumenthal, R.S.; et al. Modern prevalence of the Fredrickson-Levy-Lees dyslipidemias: Findings from the Very Large Database of Lipids and National Health and Nutrition Examination Survey. Arch. Med. Sci. 2019, 16, 1279–1287. [Google Scholar] [CrossRef]
- Phillips, N.R.; Waters, D.; Havel, R.J. Plasma lipoproteins and progression of coronary artery disease evaluated by angiography and clinical events. Circulation 1993, 88, 2762–2770. [Google Scholar] [CrossRef] [Green Version]
- Nordestgaard, B.G.; Tybjaerg-Hansen, A. IDL, VLDL, chylomicrons and atherosclerosis. Eur. J. Epidemiol. 1992, 8 (Suppl. 1), 92–98. [Google Scholar] [CrossRef]
- Miller, M.; Cannon, C.P.; Murphy, S.A.; Qin, J.; Ray, K.K.; Braunwald, E.; PROVE IT-TIMI 22 Investigators. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J. Am. Coll. Cardiol. 2008, 51, 724–730. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; Wareham, N.; Bingham, S.; Boekholdt, S.M.; Khaw, K.T.; Gudnason, V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007, 115, 450–458. [Google Scholar] [CrossRef]
- Jørgensen, A.B.; Frikke-Schmidt, R.; West, A.S.; Grande, P.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur. Heart J. 2013, 34, 1826–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, M.; Varbo, A.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Low nonfasting triglycerides and reduced all-cause mortality: A mendelian randomization study. Clin. Chem. 2014, 60, 737–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.F.; Chuang, J.Y.; Hsiao, C.C.; Zeng, Y.H.; Chang, P.J.; Lu, T.Y.; Sun, F.J.; Lin, Y.S.; Chen, Y.H.; Yeh, H.I. Improvement of Goal Attainment of Low-Density Lipoprotein Cholesterol in High-Risk Patients by Individualized Target Value Reminding Approach. Int. J. Gerontol. 2021, 15, 354–360. [Google Scholar]
- Miwa, K. Low density lipoprotein particles are small in patients with coronary vasospasm. Int. J. Cardiol. 2003, 87, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Morris, P.B.; Agarwala, A.; Ballantyne, C.M.; Birtcher, K.K.; Kris-Etherton, P.M.; Ladden-Stirling, A.B.; Miller, M.; Orringer, C.E.; Stone, N.J. 2021 ACC Expert Consensus Decision Pathway on the Management of ASCVD Risk Reduction in Patients with Persistent Hypertriglyceridemia: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 78, 960–993. [Google Scholar] [CrossRef]
- Brahm, A.; Hegele, R.A. Hypertriglyceridemia. Nutrients 2013, 5, 981–1001. [Google Scholar] [CrossRef] [Green Version]
- Hegele, R.A.; Ban, M.R.; Hsueh, N.; Kennedy, B.A.; Cao, H.; Zou, G.Y.; Anand, S.; Yusuf, S.; Huff, M.W.; Wang, J. A polygenic basis for four classical Fredrickson hyperlipoproteinemia phenotypes that are characterized by hypertriglyceridemia. Hum. Mol. Genet. 2009, 18, 4189–4194. [Google Scholar] [CrossRef] [Green Version]
- Borén, J.; Watts, G.F.; Adiels, M.; Söderlund, S.; Chan, D.C.; Hakkarainen, A.; Lundbom, N.; Matikainen, N.; Kahri, J.; Vergès, B.; et al. Kinetic and Related Determinants of Plasma Triglyceride Concentration in Abdominal Obesity: Multicenter Tracer Kinetic Study. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2218–2224. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, H.N.; Packard, C.J.; Chapman, M.J.; Borén, J.; Aguilar-Salinas, C.A.; Averna, M.; Ference, B.A.; Gaudet, D.; Hegele, R.A.; Kersten, S.; et al. Triglyceride-rich lipoproteins and their remnants: Metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021, 42, 4791–4806. [Google Scholar] [CrossRef]
- Chapman, M.J.; Orsoni, A.; Tan, R.; Mellett, N.A.; Nguyen, A.; Robillard, P.; Giral, P.; Thérond, P.; Meikle, P.J. LDL subclass lipidomics in atherogenic dyslipidemia: Effect of statin therapy on bioactive lipids and dense LDL. J. Lipid Res. 2020, 61, 911–932. [Google Scholar] [CrossRef] [Green Version]
- Valdivielso, P.; Ramírez-Bueno, A.; Ewald, N. Current knowledge of hypertriglyceridemic pancreatitis. Eur. J. Intern. Med. 2014, 25, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.; Makadia, S.; Sutherland, A.; Miller, M. Optimizing Non-Pharmacologic Management of Hypertriglyceridemia. Arch. Med. Res. 2017, 48, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. REDUCE-IT Investigators. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- ACCORD Study Group; Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R., 3rd; Leiter, L.A.; Linz, P.; Friedewald, W.T.; Buse, J.B.; Gerstein, H.C.; et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar]
- Keech, A.; Simes, R.J.; Barter, P.; Best, J.; Scott, R.; Taskinen, M.R.; Forder, P.; Pillai, A.; Davis, T.; Glasziou, P.; et al. FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005, 366, 1849–1861. [Google Scholar]
- Komiya, I.; Yamamoto, A.; Sunakawa, S.; Wakugami, T. Pemafibrate decreases triglycerides and small, dense LDL, but increases LDL-C depending on baseline triglycerides and LDL-C in type 2 diabetes patients with hypertriglyceridemia: An observational study. Lipids Health Dis. 2021, 20, 17. [Google Scholar] [CrossRef]
- Schoonjans, K.; Staels, B.; Auwerx, J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim. Et Biophys. Acta 1996, 1302, 93–109. [Google Scholar] [CrossRef]
- Araki, E.; Yamashita, S.; Arai, H.; Yokote, K.; Satoh, J.; Inoguchi, T.; Nakamura, J.; Maegawa, H.; Yoshioka, N.; Tanizawa, Y.; et al. Effects of Pemafibrate, a Novel Selective PPARα Modulator, on Lipid and Glucose Metabolism in Patients with Type 2 Diabetes and Hypertriglyceridemia: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial. Diabetes Care 2018, 41, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Witztum, J.L.; Gaudet, D.; Freedman, S.D.; Alexander, V.J.; Digenio, A.; Williams, K.R.; Yang, Q.; Hughes, S.G.; Geary, R.S.; Arca, M.; et al. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N. Engl. J. Med. 2019, 381, 531–542. [Google Scholar] [CrossRef]
- Tardif, J.C.; Karwatowska-Prokopczuk, E.; Amour, E.S.; Ballantyne, C.M.; Shapiro, M.D.; Moriarty, P.M.; Baum, S.J.; Hurh, E.; Bartlett, V.J.; Kingsbury, J.; et al. Apolipoprotein C-III reduction in subjects with moderate hypertriglyceridaemia and at high cardiovascular risk. Eur. Heart J. 2022, 43, 1401–1412. [Google Scholar] [CrossRef] [PubMed]

| Total N = 90 | HLP4 N = 83 | HLP5 N = 7 | p * | ||
|---|---|---|---|---|---|
| Age, mean (SD) | 56.2 (10.8) | 57.4 (10.0) | 41.6 (10.5) | <0.01 | |
| Male, n (%) | 64 (71.1) | 57 (68.7) | 7 (100.0) | 0.10 | |
| BMI (Kg/m2) | 27.5 (3.8) | 27.2 (3.7) | 31.4 (2.2) | <0.01 | |
| Current smoker, n (%) | 30 (33.3) | 27 (32.5) | 3 (42.9) | 0.68 | |
| Alcohol drinking, n (%) | 6 (6.7) | 5 (6.1) | 1 (14.3) | 0.39 | |
| Medical history | Hypertension | 57 (63.3) | 56 (67.5) | 1 (14.3) | <0.01 |
| Diabetes | 51 (56.7) | 49 (59.0) | 2 (28.6) | 0.23 | |
| Ischemic stroke | 3 (3.3) | 3 (3.6) | 0 (0.0) | 0.99 | |
| CAD | 35 (38.9) | 34 (41.0) | 1 (14.3) | 0.24 | |
| PAD | 5 (5.6) | 5 (6.0) | 0 (0.0) | 0.99 | |
| History of pancreatitis | 1 (1.1) | 0 (0.0) | 1 (14.3) | 0.08 | |
| Prescribed medications | Antiplatelets | 41 (45.6) | 40 (48.2) | 1 (14.3) | 0.12 |
| ACEI/ARB | 60 (66.7) | 56 (67.5) | 4 (57.1) | 0.68 | |
| Beta-blocker | 52 (57.8) | 49 (59.0) | 3 (42.9) | 0.45 | |
| Any statin | 52 (57.8) | 51 (61.4) | 1 (14.3) | 0.04 | |
| HIS | 16 (17.8) | 16 (19.3) | 0 (0) | 0.35 | |
| Ezetimibe | 7 (7.8) | 7 (8.4) | 0 (0.0) | 0.99 | |
| PCSK9 inhibitor | 0 (0) | 0 (0.0) | 0 (0.0) | - | |
| Fibrate | 11 (12.2) | 11 (13.3) | 0 (0.0) | 0.59 | |
| Omega-3 fatty acid | 0 (0) | 0 (0) | 0 (0) | - | |
| Niacin | 0 (0) | 0 (0.0) | 0 (0.0) | - | |
| Laboratory data | Fasting glucose (mg/dL) | 125.5 (35.5) | 124.9 (34.5) | 133.6 (48.9) | 0.54 |
| HbA1c (%) | 6.7 (1.2) | 6.7 (1.3) | 6.6 (1.3) | 0.75 | |
| Cr (mg/dL) | 1.1 (0.8) | 1.1 (0.8) | 0.9 (0.1) | 0.16 | |
| eGFR (ml/min) | 79.2 (27.7) | 77.4 (27.9) | 100.5 (14.8) | 0.01 | |
| TC (mg/dL) | 190.2 (34) | 183.9 (26.1) | 264.9 (26.7) | <0.01 | |
| TG (mg/dL) | 329.2 (313.2) | 247.6 (96.1) | 1296.7 (380.5) | <0.01 | |
| HDL-C (mg/dL) | 39.7 (8.9) | 40.5 (8.7) | 30.6 (4.8) | <0.01 | |
| Non-HDL-C (mg/dL) | 150.5 (34.8) | 143.4 (24.7) | 234.3 (26.6) | <0.01 | |
| LDL-C (mg/dL) | 99.9 (23.4) | 103.0 (21.1) | 62.9 (16.4) | <0.01 | |
| ApoB (mg/dL) | 101.0 (13.4) | 101.6 (13.5) | 94.5 (10.2) | 0.09 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-H.; Lin, D.-Y.; Tsai, C.-L.; Liang, C.-H.; Yu, Y.-T.; Hsieh, Y.-L.; Chuang, J.-Y.; Chen, Y.-H.; Yeh, H.-I.; Lin, C.-F. Management of Patients with Type V Hyperlipoproteinemia: An Uncommon Phenotype of Dyslipidemia with Chylomicronemia and Severe Hypertriglyceridemia. J. Pers. Med. 2023, 13, 68. https://doi.org/10.3390/jpm13010068
Chang Y-H, Lin D-Y, Tsai C-L, Liang C-H, Yu Y-T, Hsieh Y-L, Chuang J-Y, Chen Y-H, Yeh H-I, Lin C-F. Management of Patients with Type V Hyperlipoproteinemia: An Uncommon Phenotype of Dyslipidemia with Chylomicronemia and Severe Hypertriglyceridemia. Journal of Personalized Medicine. 2023; 13(1):68. https://doi.org/10.3390/jpm13010068
Chicago/Turabian StyleChang, Ya-Hui, Dai-Yi Lin, Chia-Ling Tsai, Chih-Hung Liang, Yu-Ting Yu, Yi-Lin Hsieh, Jen-Yu Chuang, Yi-Han Chen, Hung-I Yeh, and Chao-Feng Lin. 2023. "Management of Patients with Type V Hyperlipoproteinemia: An Uncommon Phenotype of Dyslipidemia with Chylomicronemia and Severe Hypertriglyceridemia" Journal of Personalized Medicine 13, no. 1: 68. https://doi.org/10.3390/jpm13010068
APA StyleChang, Y.-H., Lin, D.-Y., Tsai, C.-L., Liang, C.-H., Yu, Y.-T., Hsieh, Y.-L., Chuang, J.-Y., Chen, Y.-H., Yeh, H.-I., & Lin, C.-F. (2023). Management of Patients with Type V Hyperlipoproteinemia: An Uncommon Phenotype of Dyslipidemia with Chylomicronemia and Severe Hypertriglyceridemia. Journal of Personalized Medicine, 13(1), 68. https://doi.org/10.3390/jpm13010068






