Abstract
Giant axonal neuropathy (GAN) is a pediatric, hereditary, neurodegenerative disorder that affects both the central and peripheral nervous systems. It is caused by mutations in the GAN gene, which codes for the gigaxonin protein. Gigaxonin plays a role in intermediate filament (IF) turnover hence loss of function of this protein leads to IF aggregates in various types of cells. These aggregates can lead to abnormal cellular function that manifests as a diverse set of symptoms in persons with GAN including nerve degeneration, cognitive issues, skin diseases, vision loss, and muscle weakness. GAN has no cure at this time. Currently, an adeno-associated virus (AAV) 9-mediated gene replacement therapy is being tested in a phase I clinical trial for the treatment of GAN. This review paper aims to provide an overview of giant axonal neuropathy and the current efforts at developing a treatment for this devastating disease.
1. Introduction
Giant axonal neuropathy (GAN, OMIM #256850) is a rare neuromuscular disease with an autosomal recessive mode of inheritance [1]. The disease presents as a prominent sensorimotor neuropathy in early childhood and commonly progresses to affect both the peripheral and central nervous systems (PNS and CNS) [2,3]. Most children become wheelchair-dependent in the second decade of life and typically die in the third decade from respiratory failure [4,5,6].
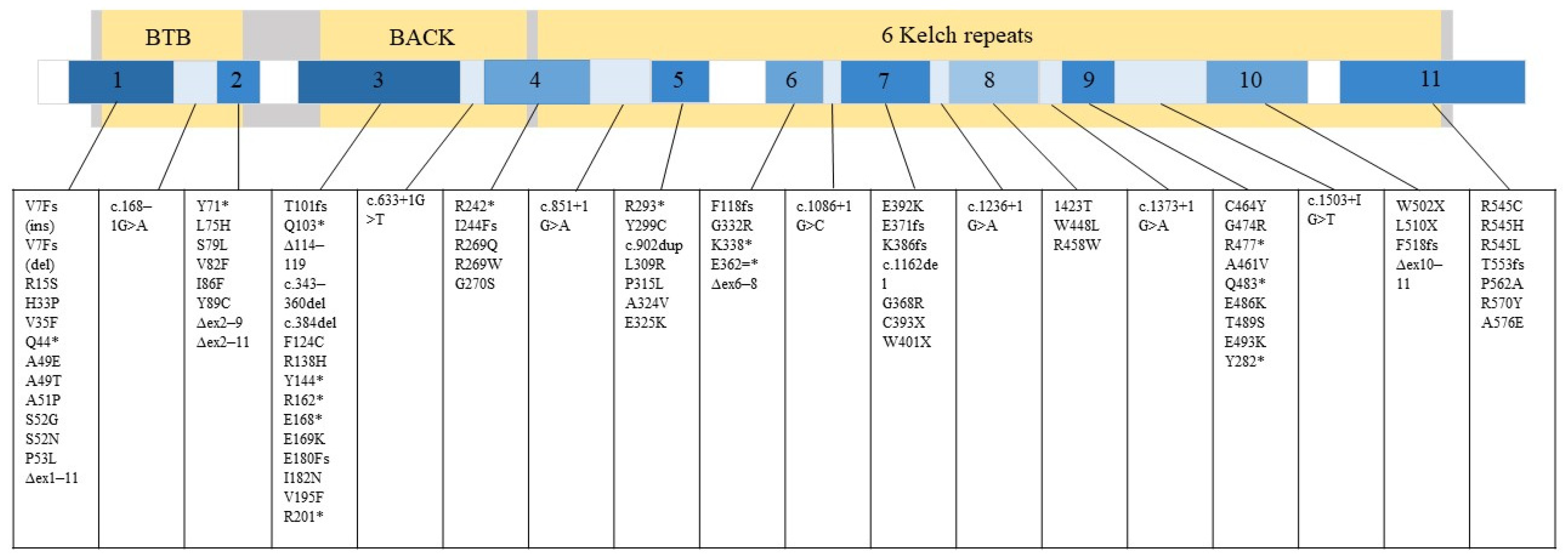
GAN is caused by a mutation in the GAN gene, which encodes a protein called gigaxonin [1]. Gigaxonin is a part of the ubiquitin-proteasome system and controls autophagosome production [7]. Specifically, gigaxonin plays an important role in the breakdown of neurofilament (NF) [8], where the cellular hallmark of GAN pathology is the formation of its large aggregates [2,3,5]. According to the Leiden Open Variation Database (LOVD) (updated 2022), National Center for Biotechnology Information (NCBI) (updated 2022), and the mutation summary reported by Lescouzères and Bomont, P. (2020), there are 89 variants that lead to manifestation of GAN (Figure 1) [5,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36].
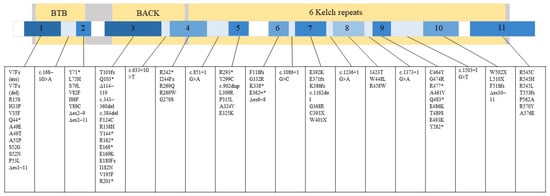
Figure 1.
89 variants associated with GAN that can occur in either exon or intron are listed. Gigaxonin is illustrated by the large grey rectangle and its domains in yellow. The smaller rectangle depicts the GAN gene with its exons numbered 1–11. The relative number of mutations that can occur in different gene regions is represented in a blue gradient. Darker blue equals more mutation. White equals no mutation reported in that region.
Presently there is no effective treatment for GAN. Only symptomatic management to slow the progression of the disease are in place [4]. A combination of speech, occupation, and physical therapy is used by physicians to enhance physical and cognitive development. As such, there is a need for a therapy that provides an effective yet safe treatment that addresses the primary cause of GAN.
Here, we summarize current understanding and genetic approaches that have been explored for the treatment of GAN. In addition, we will discuss the difficulties they may be facing on their way from preclinical studies to clinical translation and offer some future perspectives.
2. Clinical Heterogeneity in Persons with GAN
Since the first documented case in 1972, several dozens of patients with GAN have been reported, with a strong clinical heterogeneity and diverse prognosis [2,3,22]. The classic GAN typically manifests as an infantile to early-childhood onset neurodegenerative disorder with a decline in motor and sensory function. During infancy, clinical assessment usually reveals motor and sensory neuropathy with moderate axonal degeneration (Table 1) [2,3,22,23]. Progressive distal motor weakness is the initial symptom in all cases. Diffused muscle atrophy, most predominantly in distal muscles, flaccid, paralysis, severely decreased muscle strength, low muscle tone, and loss of reflexes (areflexia) may be observed as the disease advances (Table 2) [3,22,23,24,25,26,37,38]. Diagnosis of PNS degeneration in early infancy and sensorimotor pathway involvement in teens resemble more commonly inherited peripheral neuropathy called Charcot-Marie-Tooth (CMT) diseases [27]. Owing to similarities in clinical presentation between patients with GAN and CMT or Friedreich ataxia, CTM Disease Pediatric Scale and Friedreich Ataxia Rating Scale and Gross Motor Function measure may be implemented for physical examination [4,37]. Though, making PNS deterioration unique from some of the other peripheral neuropathies, GAN also leads to proximal motor weakness, evidenced by pectoralis chest-wall muscle wasting, winged scapula, and exhibition of myopathic or “waddling” gait disturbances and positive Gower’s sign during the attempt to achieve erect position, indicative of pelvic girdle and quadriceps muscle weakness [2,4,6,25,28]. Consistent with PNS clinical presentation, an electromyogram (EMG) demonstrates neuropathic changes indicative of multiple peripheral nerve damage with chronic denervation, mainly involving the sensorimotor branches of limbs [29]. Nerve conduction studies show markedly decreased to absent compound muscle action potentials and sensory nerve action potentials of the upper and lower limb, and prolonged motor and sensory nerve conduction velocities, even to the demyelinating range [23,25,26,29,39]. The electron microscopy of the sural nerve and skin/muscle fiber biopsy shows abnormally large axons in their paranodal junction and decreased myelinated axons (Table 1) [23,26,40] The general morphology of nerve fibers may be maintained, although lacks normally identifiable sidearms that extend from healthy NF from increased NF packing, which is critical for modulating NF spacing and managing the mechanical integrity of nerve cells [4,41]. The enlarged axons exhibit significantly decreased myelin sheath thickness and are filled with disorganized NF aggregates, distending axon and axoplasm, and widening periaxonal space [26,41]. This causes the pathological hallmark of GAN, a ‘giant’ axon. These axons also contain a reduced number of microtubules and other remaining axonal organelles such as mitochondria are pushed out into sub-axolemmal space [4,6,41]. The swelling can occur both in myelinated and unmyelinated axon fibers, often beginning at the node of Ranvier and having a segmental swelling appearance [2,4]. Some fibers can be found surrounded by basal lamina, which has an important role in regeneration and remyelination [22,42]. This agrees with EMG that shows partial reinnervation beside demyelinated or lesions fibers, especially in the patient with CMT-like presentation [22,29]. Not limited to the disorganization of NF, other types of disorganized intermediate filament (IF) accumulation can be found in various peripheral nerves and cells.

Table 1.
Summary of suggestive findings and clinical testing including peripheral nerve biopsy, electrophysiologic, and neuroimaging examinations available for diagnosis of giant axonal neuropathy.

Table 2.
General categorization of clinical phenotypes observed in individuals with classical giant axonal neuropathy. Multiple systems are often involved to reflect a certain symptom observed.
GAN also differs from other neuropathies due to CNS involvement including pyramidal signs, positive Babinski signs indicative of deficit in the upper motor neuron, and positive Romberg signs associated with dorsal column lesion (Table 2) [2,22]. Among these, cerebellar involvement is most extensive, including truncal ataxia, incoordination, fine movement impairment, tremor, dysmetria, nystagmus, and oculomotor apraxia [25,37,39]. Cranial nerve impairment is also described in patients with GAN, causing facial weakness, ptosis, and ophthalmoplegia, involving facial, oculomotor, trochlear, and abducens nerves [6,26,29,30]. Many patients have vision loss with optic atrophy, as well as dysarthria, dysphonia, dysphasia, and hearing impairment, reflecting the combination of both central and peripheral dysfunction [25,30]. Severe CNS involvement may include vertigo, intellectual disability, spasticity, seizures/epilepsy, and dementia (Table 2) [4,29,39,40]. Neuroimaging study demonstrates slowly progressive diffuse leukoencephalopathy with demyelination and atrophy, and signal abnormalities throughout cerebrum, cerebellum, and notably in spinal cord [22,30,41]. One of the early signs in disease is the signal abnormalities (increased T2 signal intensity) detected with magnetic resonance imaging (MRI) [25,26,39]. T2 hyperintense lesion involve internal capsule, globus pallidus of the basal ganglia, thalami, brainstem. and spinal cord, where frontoparietal and periventricular white matter and dentate nucleus of cerebellum affected most prominently [25,43]. T1 hyperintense lesion and decreased T1 signal at varying parts of the brain associated with brain atrophy may be also observed. Similarly, the fractional anisotropy value is low reflecting increased diffusivity due to intracellular water increase from axonal distension and demyelination [43]. Spinal cord atrophy in posterior column, medial lemniscus spinocerebellar tracts, and cortical spinal tracts, involving abnormality in inferior olivary, gracile and cuneate nuclei, and cerebellar peduncles along pathways are prominent [40,43]. As axonal transport is impaired, peripheral nerves, posterior columns, cerebellum and pyramidal tracts are most severely affected [4]. Besides axonal loss, loss of Purkinje cells and other neuronal cells are also reported [40]. Secondary demyelination may also be present due to nerve cell body loss [4,26]. In addition to IF aggregations in CNS that leads to aforementioned axonal demyelination and atrophy, there is also a prominent presence of Rosenthal fibers which is an astrocyte pathology described in Alexander disease [6,23,40]. The electroencephalogram (EEG) study further supports clinical signs. Evoked potentials show increased latency in auditory, visual, and somatosensory evoked response, suggesting lesion in brainstem and higher cortices [44]. EEG may also show epileptiform transients discharges in the form of spikes and sharp waves in patient with or without history of epilepsy (Table 1) [26,29]. GAN also alters brain metabolites. Major detectable brain metabolites with magnetic resonance spectroscopy (MRS) include the predominantly neuroaxonal compound N-acetylaspartate (NAA), the energy metabolites creatine and Cho compounds involved in membrane turnover, and osmolyte myoinositol (Ins) [43]. The Cho and Ins typically increase with respect to demyelination and glial proliferation while NAA decreases with axonal damage/loss [43]. While these typical GAN clinical findings are prevalent, individuals with genetically confirmed GAN can be affected more mildly [4,22]. Milder cases can be regarded as CMT-plus phenotype and is becoming increasingly recognized. The disease may present with slow onset and progression of peripheral neuropathy with minimal CNS involvement. [29,39,40,41].
GAN is also associated with gastrointestinal (GI) and systemic issues including constipation, reflux, regurgitation, diabetes, renal tubular acidosis, and lactose intolerance [30,32,34,45,46].Some patients may also suffer from precocious puberty, arched feet, scoliosis, tendon contracture, and lumbar hyper-lordosis [8,22,23,25,29]. GAN patients often have a characteristic physical appearance with kinky hair, long eyelashes, a high forehead, pale skin, and facial diplegia [26]. Kinky hair and long eyelashes are frequently observed features but not in all cases and are most likely due to abnormal keratin accumulation (Table 2) [32]. Ichthyosis and keratosis pilaris can also occur, although minor [21]. Despite heterogenicity in phenotype within GAN, the relationship between phenotypic differences and the rate of disease progression is yet to be known.
3. Gigaxonin Plays an Important Role in IF Homeostasis
Disorganization of the NF network is a key feature of several neurodegenerative disorders. Likewise, disorganized NFs is prominent in GAN. Though, GAN is unique in that GAN corresponds to a generalized disorganization of the all classes of cytoskeletal IFs [47]. Dysregulated IFs in GAN axons include keratin (class I and II IFs), vimentin, desmin, glial fibrillary acidic protein (GFAP) and peripherin (class III), and NF (class IV) [35,48]. Besides the NF accumulation in axons, aggregates can be seen within peripheral sensorimotor nerves, as well as Schwan cells, endothelial cells, perineurial cells, lens epithelial cells, skin fibroblasts, and muscle fibers [47]. Accumulation also occurs in CNS involving cerebral and cerebellar white matter, middle cerebellar peduncles, brainstem tegmentum, corticospinal tracts, and posterior column, as IFs accumulate in astrocytes, neurons of white matter and spinal tract, supporting white matter abnormalities reported in imaging [39,43]. In support of autonomic nervous system involvement seen with symptoms including vomiting and constipation, IF can also aggregate in the myenteric plexus of the GI tract [39,45,46]. In particular, NFs plays important role in radial growth for achieving rigid cytoskeletal structure and stability of myelinated axons to allow optimal electrical impulse propagation along axons [49]. NF consists of aforementioned sidearms in the form of subunits (NF—light (L), medium (M), and heavy (H) chain, and a-internexin) that allows cross-link to other cellular organelles [49]. Reduction in NFs inhibits axonal radial growth or cross-bridge and interconnection with other non-IF components of the cytoskeleton including microtubules and actin-filament and disrupts synaptic plasticity [49].
In GAN, IF disorganization results from mutations in the GAN gene. The GAN gene encompasses 11 exons located in chromosome 16 and encodes a 65-kDa protein called gigaxonin, which is normally present in cells throughout the body at a low level and strongly expressed in the brain, heart, and skeletal muscle [36]. At least 150 different mutations in the gene have been described. [3], and the disease-causing mutations that lead to the loss of functional gigaxonin or production of abnormal gigaxonin can span the entire coding region of the GAN gene [7,36]. Mutations can reduce mRNA quantity decreasing gigaxonin abundance and destabilizing protein structure by improper folding, impairing its activity and reducing half-lives [35].
Gigaxonin is comprised of an N-terminal BTB (Broad-Complex, Tramtrack, and Bric a brac) domain, a C-terminal Kelch repeat domain, and a BACK domain between the terminus ends and is considered to belong to the BTB/kelch protein family [36,50]. Structural and functional studies demonstrate that they are a substrate-specific adaptor protein for a Cul3-dependent/gigaxonin E3 ubiquitin ligase complex [36,50,51]. Combined with sequence homology, gigaxonin appears to be involved in protein degradation via ubiquitin proteasomal-dependant pathway, implicated in the cytoskeletal network [48,51]. Relevant in the GAN context, a mutation in BTB-Kelch gigaxonin protein leads to IF accumulation. Major subunits of the ligase complex are cullin, RING box protein (Rbx), and an adaptor that directs the substrate near the E2 enzyme, recruited by Rbx [52]. BTB domain of gigaxonin interacts with Rbx1 and directly binds with cullin 3 (Cul3), both a component of E3 ubiquitin ligase complexes, to associate the two to form functional complex [52,53,54,55]. Direct interaction between the BTB domain and Cul3 suggests the interaction between proteasome components [55]. The Kelch domain recruits substrates for polyubiquitination, mediating the addition of ubiquitin chains onto their targets, before their degradation by the proteasome [47,49]. Ubiquitination is a post-translational, enzymatic modification process where ubiquitin-protein binds substrate protein to destine cell fate and modulate cellular function and signaling pathway [7,56]. Subsequently, polyubiquitination, formation of ubiquitin chain, initiates proteolysis and degradation of protein to maintain homeostasis [56]. In particular, gigaxonin control microtubule dynamics as Kelch targets microtubule-associated proteins (MAP), including microtubule-associated protein 1B (MAP1B), tubulin-folding cofactor B (TBCB), and microtubule-associated protein 8 (MAP8), as its binding partner [4]. MAP is involved in many cellular processes including morphogenesis and differentiation, to maintain cytoskeletal integrity [57]. Gigaxonin physically colocalizes with MAP in neurons along the microtubule network and binds directly to MAPs for it to be targeted by the ubiquitin-proteasome system for its degradation. [51,58,59,60]. This interaction is important in the enhancement of microtubule stability, as destabilized microtubules interfere with cytoskeletal balances and aggravate IF aggregation which impairs axonal transport, affecting neuronal function and survival [51,59]. Individually, MAPs can cause neurodegeneration and death when overexpressed, and the survival rate improves when silenced in GAN neurons, as well. Though, functions of gigaxonin focused on MAP have not yet provide a causal link between the loss of functional gigaxonin and IF aggregate formation in patients. For instance, vimentin IF aggregates are not produced upon microtubule clearance in control fibroblasts [7]. Moreover, TBCB that interacts with certain mutated gigaxonin can retain its level of activity [7]. Despite causal microtubule implication in the pathogenesis of GAN cannot be established, gigaxonin plays an important role in controlling microtube dynamics such that GAN mutations impair IF degradation, resulting in an excess IFs in axons and promotes aggregation [35]. In addition, restoration of gigaxonin induces degradation of several IFs [56,61].
Recent evidence also shows gigaxonin-E3 ligase involvement in regulating the autophagy pathway and autophagosome production [7,62]. A third of E3 ligase is involved in the autophagy pathway and mediates ubiquitin-dependent protein destruction by autophagy. Autophagy is a process that delivers cytoplasmic material, such as damaged organelles, ribosomes, and protein aggregates, to the lysosome for degradation [62]. This degradation pathway is important in cell survival and the maintenance of bioenergetic homeostasis. Materials to be degraded need to be surrounded by a phagophore (incomplete autophagosome) which elongates to form a complete autophagosome that can bind with a lysosome. ATG16L1 plays a role in specifying the LC3 lipidation site and thus, is a key determinant for the elongation of the phagophore. As per IFs aggregate, large perinuclear bundles of ATG16L1 can form in some of the neurons under gigaxonin depletion conditions. Alternation in ATG16L1 activities or accumulated ATG16L1 inhibits autophagosome formation by impairing its maturation. This leads to phagophore accumulation and p62 aggregation, which is the main autophagy receptor, due to defective autophagic degradation and diminished autophagosome-lysosome events, impairing autophagic flux. Precisely, GAN neurons can generate autophagosomes but lack the capability of maintaining the production over prolonged induction of autophagy [7,62]. Several studies suggest a role of gigaxonin also in cell metabolism, where nutrient-responsive gigaxonin glycosylation forms the regulatory link between metabolism and IF turnover [63].
4. In Vivo Gene Therapy for GAN Using AAVs
As loss of function mutations in the GAN gene result in GAN, gene replacement therapy to express functional gigaxonin is a potential option for therapeutics [64]. It is expected that the expression of functional gigaxonin will reduce the pathogenic IF aggregates in patients. Gray et al. have established that treatment with a normal copy of human GAN transgene (AAV9/JetT-GAN) restores the normal pattern of IF distribution in fibroblasts obtained from a person with GAN, as well as significantly reduces aggregates of vimentin within 3 days of treatment [64]. Consistent with their in vitro findings, aged GAN knockout (KO) mice exhibited reduced NF aggregates within 4 weeks of treatment with the GAN replacement vector. Additionally, intrathecal (IT) injection of the GAN replacement vector sustained gigaxonin expression in the CNS and PNS of GAN KO mice for up to a year and exhibited conservation of sciatic nerve architecture. Treatment also showed improved rotarod performance in GAN KO mice. However, the improvement in rotarod function was not seen in mice aged 23 months. The authors speculate that the treatment dose may not have been optimal to fully rescue rotarod function. No toxicity from treatment with AAV9/JetT-GAN is reported in vivo.
In this study, the authors also address the concern of causing toxicity due to the overexpression of gigaxonin using the GAN replacement vector [64]. Firstly, a strong promoter to drive the expression of GAN may result in the loss of normal IF cytoskeleton, due to an overexpression of gigaxonin protein. As gigaxonin is expressed at very low levels in humans (~7500 molecules/lymphoblast cell), the authors have used a weak promoter JeT to drive the expression of gigaxonin [65]. From the in vitro and in vivo results, the amount of gigaxonin production driven by the JeT promoter sufficiently reduced the amount of IF and NF aggregates without disrupting the normal network of IF and NFs required for function. Besides, using this construct for the vector keeps the opportunity to increase the dose in human patients to maximize therapeutic benefits as the risk of causing toxicity due to over-expression of gigaxonin protein is not anticipated.
Previously, Gray et al. have also demonstrated that successful GAN gene replacement prevents the formation of IF aggregates in three distinct cell lines (fibroblasts) derived from persons with GAN as well as GAN-null mice [66]. In addition to this study, Wichterle et al. have shown a reduction in IF aggregates after successful GAN gene replacement in induced pluripotent stem cells (iPSCs) derived from three different persons with GAN [61].
Due to the promising results obtained in vitro and in vivo, a Phase I clinical trial to assess the safety of the vector is ongoing (ClinicalTrials.gov Identifier: NCT02362438).
5. Phase I Clinical Trial of GAN
A phase I first-in-human, open-label, non-randomized, single-dose escalation clinical trial to test the safety of gene transfer vector scAAV9/JeT-GAN in persons diagnosed with GAN has been initiated [67]. 21 participants currently enrolled in the trial will receive the treatment intrathecally to target the brain and the spinal cord.
The primary goal of this study is to access the safety of the vector. Assessing disease symptoms upon treatment, examining neuropathology, investigating the presence of inflammatory markers in the cerebrospinal fluid, and analyzing vector shedding are secondary interests of the trial.
6. Concerns about AAV9-Mediated Gene Replacement Therapy for GAN
GAN is caused due to the lack of gigaxonin, a ubiquitously expressed protein. The symptoms of this neurodegenerative disorder manifest as vision loss and optic atrophy as well [61]. In Gray et al.’s study, they observed an accumulation of Ifs (i.e., glial fibrillary acidic protein) in lens epithelial cells of 4-month-old GAN KO mice and human specimens obtained from autopsy [68]. Additionally, in a rat model of GAN, extreme degeneration of the retinal rod and cone photoreceptors were observed. The AAV9-mediated gene replacement therapy for GAN, which is under a phase I clinical trial, does not report on the efficacy of the treatment on photoreceptor cell deterioration. Visual impairment in a person with GAN has not been studied concerning photoreceptor cell loss, however, as vision loss and optic atrophy are symptoms of concern it is important to investigate the efficiency of gene transfer in the human eye [69].
Additionally, the efficiency of AAV9 transduction in Schwann cells is still under investigation [70]. In a study by Gray et al., a low amount of AAV9 was detected in peripheral nerve samples ensheathed by Schwann cells and surrounding endoneurium and perineurium cells. However, they suggested further experiments to ensure that the vector is delivered in Schwann cells particularly as the positive signal detected in their experiments could potentially mean AAV9 was only delivered to the axons or only the endoneurium and perineurium cells [71]. Investigating the efficiency of AAV9 delivery in Schwann cells is of interest as, the GAN KO mouse model has severe IF accumulation of IFs in the cytoplasm of Schwann cells, associated with both myelinated and unmyelinated fibers. Hence, targeting Schwann cells for the treatment of GAN must be prioritized. In preclinical studies for developing AAV9-mediated gene replacement therapy for GAN, GAN KO mice that received treatment showed an increased number of normal-appearing Schwann cells [64].
Another big concern with AAV9-mediated gene replacement therapy for GAN is neurotoxicity. A study by Wilson et al. has shown a neuronal loss in dorsal root ganglion (DRG) in non-human primates upon administration of AAVs [72]. Besides, signs of DRG toxicity were observed in a person with GAN who received gene therapy [73].
For people with GAN, there is currently no approved treatment beyond counseling and symptomatic treatments, such as aquatic and physical therapy, bracing, or painkillers [74]. However, gene therapy for giant axonal neuropathy has shown efficacy in preclinical trials, and a first-in-human phase I clinical trial of intrathecal gene transfer for GAN has been initiated at the National Institutes of Health, NIH (NCT02362438) [64]. However, the effects were unclear, where autopsies of some participants showed signs of dorsal root ganglion (DRG) damage, indicative of AAV neurotoxicity [73].
7. Conclusions
GAN is a rare pediatric disease that has no cure at the moment. However, gene therapy for giant axonal neuropathy has shown efficacy in preclinical trials, and a first-in-human phase I clinical trial of intrathecal gene transfer for GAN has been initiated at the National Institutes of Health, NIH (NCT02362438) [64]. The clinical trial, which is expected to be completed by 2035, will hopefully result in a safe and effective treatment for GAN [67].
Additionally, gene editing and antisense oligonucleotide (ASO) mediated treatment are viable options for treating rare genetic diseases, like GAN, which affect the brain and the spinal cord [75,76,77]. Gene editing tools including zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and CRISPR/Cas systems have been thoroughly studied in pre-clinical trials for the treatment of rare diseases [78,79,80]. However, very few have reached clinical trials for treating rare diseases affecting the CNS [81].
Another therapeutic tool called ASOs holds great potential when it comes to treating neurological diseases [82]. Nusinersen (Spinraza®) is an FDA-approved ASO-mediated drug prescribed for Spinal Muscular Atrophy (SMA), a neuromuscular disorder [83,84]. In a phase III clinical trial, infants treated with nusinersen showed greatly improved motor-milestone response and had a 47% decrease in the risk of fatality and the need for permanent assisted ventilation [85,86]. ASO-mediated treatment for Huntington’s disease (HD), a neurodegenerative disorder, is also under investigation [87]. Upon intrathecal administration, ASOs can be detected in high levels across the CNS and beneficial levels can be maintained with repeated doses [82]. As ASOs have a hopeful future in treating rare neurodegenerative diseases, investigating N-of-1 ASO-mediated treatment for GAN is encouraged [76]. Around 6.6% of people with GAN have mutations at splice donor/acceptor sites [7]. As most ASO-mediated therapies ameliorate mutations in splice sites, this population can particularly benefit from this venture.
With remarkable advances made in the molecular biology field, several strategies to fight rare diseases like GAN are evolving. Although there is no cure for GAN at the moment, with the ongoing clinical trial and several other available strategies to treat neurological maladies, we can be hopeful for the patients and their families.
Author Contributions
S.S. and R.R.R. collected relevant information from the literature and equally contributed to writing the paper. T.Y. provided valuable suggestions, edited the paper, and approved it for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful for the support provided by Muscular Dystrophy Canada, the Friends of Garrett Cumming Research Fund, the HM Toupin Neurological Science Research Fund, Alberta Innovates: Health Solutions (AIHS), Hannah’s Hope Fund, and the Women and Children’s Health Research Institute (WCHRI).
Conflicts of Interest
T.Y. is a founder and shareholder of OligomicsTx, which aims to commercialize antisense oligonucleotide technology.
References
- Kang, J.J.; Liu, I.Y.; Wang, M.B.; Srivatsan, E.S. A Review of Gigaxonin Mutations in Giant Axonal Neuropathy (GAN) and Cancer. Hum. Genet. 2016, 135, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Asbury, A.K.; Gale, M.K.; Cox, S.C.; Baringer, J.R.; Berg, B.O. Giant Axonal Neuropathy? A Unique Case with Segmental Neurofilamentous Masses. Acta Neuropathol. 1972, 20, 237–247. [Google Scholar] [CrossRef]
- Berg, B.O.; Rosenberg, S.H.; Asbury, A.K. Giant Axonal Neuropathy. Pediatrics 1972, 49, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Kerner, B.L.; Roth, L.; Greene, J.P.; Wichterle, H.; Sproule, D.M. Giant Axonal Neuropathy: An Updated Perspective on Its Pathology and Pathogenesis. Muscle Nerve 2014, 50, 467–476. [Google Scholar] [CrossRef]
- Bruno, C.; Bertini, E.; Federico, A.; Tonoli, E.; Lispi, M.L.; Cassandrini, D.; Pedemonte, M.; Santorelli, F.M.; Filocamo, M.; Dotti, M.T.; et al. Clinical and Molecular Findings in Patients with Giant Axonal Neuropathy (GAN). Neurology 2004, 62, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Allen, E.; Ding, J.; Wang, W. Giant Axonal Neuropathy. Cell. Mol. Life Sci. 2007, 64, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Lescouzères, L.; Bomont, P. E3 Ubiquitin Ligases in Neurological Diseases: Focus on Gigaxonin and Autophagy. Front. Physiol. 2020, 11, 1022. [Google Scholar] [CrossRef]
- Fokkema, I.F.A.C.; den Dunnen, J.T.; Taschner, P.E.M. LOVD: Easy Creation of a Locus-Specific Sequence Variation Database Using an “LSDB-in-a-Box” Approach. Hum. Mutat. 2005, 26, 63–68. [Google Scholar] [CrossRef]
- Akagi, M.; Mohri, I.; Iwatani, Y.; Kagitani-Shimono, K.; Okinaga, T.; Sakai, N.; Ozono, K.; Taniike, M. Clinicogenetical Features of a Japanese Patient with Giant Axonal Neuropathy. Brain Dev. 2012, 34, 156–162. [Google Scholar] [CrossRef]
- Abu-Rashid, M.; Mahajnah, M.; Jaber, L.; Kornreich, L.; Bar-On, E.; Basel-Vanagaite, L.; Soffer, D.; Koenig, M.; Straussberg, R. A Novel Mutation in the GAN Gene Causes an Intermediate Form of Giant Axonal Neuropathy in an Arab–Israeli Family. Eur. J. Paediatr. Neurol. 2013, 17, 259–264. [Google Scholar] [CrossRef]
- Bomont, P.; Ioos, C.; Yalcinkaya, C.; Korinthenberg, R.; Vallat, J.; Assami, S.; Munnich, A.; Chabrol, B.; Kurlemann, G.; Tazir, M.; et al. Identification of Seven Novel Mutations in The GAN Gene. Hum. Mutat. 2003, 21, 446. [Google Scholar] [CrossRef] [PubMed]
- Buysse, K.; Vergult, S.; Mussche, S.; Groote, C.C.; Speleman, F.; Menten, B.; Lissens, W.; Van Coster, R. Giant Axonal Neuropathy Caused by Compound Heterozygosity for a Maternally Inherited Microdeletion and a Paternal Mutation within the GAN Gene. Am. J. Med. Genet. Part A 2010, 152A, 2802–2804. [Google Scholar] [CrossRef]
- Edem, P.; Karakaya, M.; Wirth, B.; Okur, T.D.; Yiş, U. Giant Axonal Neuropathy: A Differential Diagnosis of Consideration. Turk. J. Pediatr. 2019, 61, 275. [Google Scholar] [CrossRef] [PubMed]
- Koop, O.; Schirmacher, A.; Nelis, E.; Timmerman, V.; De Jonghe, P.; Ringelstein, B.; Rasic, V.M.; Evrard, P.; Gärtner, J.; Claeys, K.G.; et al. Genotype–Phenotype Analysis in Patients with Giant Axonal Neuropathy (GAN). Neuromuscul. Disord. 2007, 17, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Kuhlenbäumer, G.; Young, P.; Oberwittler, C.; Hünermund, G.; Schirmacher, A.; Domschke, K.; Ringelstein, B.; Stögbauer, F. Giant Axonal Neuropathy (GAN): Case Report and Two Novel Mutations in the Gigaxonin Gene. Neurology 2002, 58, 1273–1276. [Google Scholar] [CrossRef]
- Miyatake, S.; Tada, H.; Moriya, S.; Takanashi, J.; Hirano, Y.; Hayashi, M.; Oya, Y.; Nakashima, M.; Tsurusaki, Y.; Miyake, N.; et al. Atypical Giant Axonal Neuropathy Arising from a Homozygous Mutation by Uniparental Isodisomy. Clin. Genet. 2015, 87, 395–397. [Google Scholar] [CrossRef]
- Mohammad, S.S.; Lau, C.; Burke, C.; McCallum, N.; Robertson, T. Giant Axonal Neuropathy Diagnosed on Skin Biopsy. J. Clin. Neurosci. 2014, 21, 865–867. [Google Scholar] [CrossRef]
- Nalini, A.; Gayathri, N.; Yasha, T.C.; Ravishankar, S.; Urtizberea, A.; Huehne, K.; Rautenstrauss, B. Clinical, Pathological and Molecular Findings in Two Siblings with Giant Axonal Neuropathy (GAN): Report from India. Eur. J. Med. Genet. 2008, 51, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, D.; Wang, Z.; Zhang, W.; Lv, H.; Liu, X.; Meng, L.; Yuan, Y. Heterogeneity of Axonal Pathology in Chinese Patients with Giant Axonal Neuropathy. Muscle Nerve 2014, 50, 200–205. [Google Scholar] [CrossRef]
- Xu, X.; Yang, X.; Su, Z.; Wang, H.; Li, X.; Sun, C.; Wang, W.; Chen, Y.; Zhang, C.; Zhang, H.; et al. Identification of Novel Compound Heterozygous Mutations in the GAN Gene of a Chinese Patient Diagnosed With Giant Axonal Neuropathy. Front. Neurosci. 2020, 14, 85. [Google Scholar] [CrossRef]
- Leung, C.L.; Pang, Y.; Shu, C.; Goryunov, D.; Liem, R.K.H. Alterations in Lipid Metabolism Gene Expression and Abnormal Lipid Accumulation in Fibroblast Explants from Giant Axonal Neuropathy Patients. BMC Genet. 2007, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Tazir, M.; Nouioua, S.; Magy, L.; Huehne, K.; Assami, S.; Urtizberea, A.; Grid, D.; Hamadouche, T.; Rautenstrauss, B.; Vallat, J.-M. Phenotypic Variability in Giant Axonal Neuropathy. Neuromuscul. Disord. 2009, 19, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Normendez-Martínez, M.I.; Monterde-Cruz, L.; Martínez, R.; Marquez-Harper, M.; Esquitin-Garduño, N.; Valdes-Flores, M.; Casas-Avila, L.; de Leon-Suarez, V.P.; Romero-Díaz, V.J.; Hidalgo-Bravo, A. Two Novel Mutations in the GAN Gene Causing Giant Axonal Neuropathy. World J. Pediatr. 2018, 14, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-P.; Zou, L.-P. Clinical and Genetic Studies in a Chinese Family With Giant Axonal Neuropathy. J. Child Neurol. 2009, 24, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Echaniz-Laguna, A.; Cuisset, J.-M.; Guyant-Marechal, L.; Aubourg, P.; Kremer, L.; Baaloul, N.; Verloes, A.; Beladgham, K.; Perrot, J.; Francou, B.; et al. Giant Axonal Neuropathy: A Multicenter Retrospective Study with Genotypic Spectrum Expansion. Neurogenetics 2020, 21, 29–37. [Google Scholar] [CrossRef]
- Demir, E. Giant Axonal Neuropathy: Clinical and Genetic Study in Six Cases. J. Neurol. Neurosurg. Psychiatry 2005, 76, 825–832. [Google Scholar] [CrossRef]
- Aharoni, S.; Barwick, K.E.S.; Straussberg, R.; Harlalka, G.V.; Nevo, Y.; Chioza, B.A.; McEntagart, M.M.; Mimouni-Bloch, A.; Weedon, M.; Crosby, A.H. Novel Homozygous Missense Mutation in GAN Associated with Charcot-Marie-Tooth Disease Type 2 in a Large Consanguineous Family from Israel. BMC Med. Genet. 2016, 17, 82. [Google Scholar] [CrossRef]
- Cai, S.; Lin, J.; Liu, Y.-Q.; Lu, J.-H.; Zhao, C.-B. Giant Axonal Neuropathy with Unusual Neuroimagings Caused by Compound Heterozygous Mutations in GAN Gene. Chin. Med. J. (Engl.) 2018, 131, 2371–2372. [Google Scholar] [CrossRef]
- Garg, M.; Kulkarni, S.D.; Hegde, A.U.; Desai, M.; Sayed, R.J. Giant Axonal Neuropathy: Clinical, Radiological, and Genetic Features. Ann. Indian Acad. Neurol. 2018, 21, 304–308. [Google Scholar] [CrossRef]
- Houlden, H.; Groves, M.; Miedzybrodzka, Z.; Roper, H.; Willis, T.; Winer, J.; Cole, G.; Reilly, M.M. New Mutations, Genotype Phenotype Studies and Manifesting Carriers in Giant Axonal Neuropathy. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1267–1270. [Google Scholar] [CrossRef]
- Xu, M.; Da, Y.; Liu, L.; Wang, F.; Jia, J. Giant Axonal Neuropathy Caused by a Novel Compound Heterozygous Mutation in the Gigaxonin Gene. J. Child Neurol. 2013, 28, 1316–1319. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.A.; Johnson-Kerner, B.L.; Marra, J.D.; LaMarca, N.H.; Sproule, D.M. The Absence of Curly Hair Is Associated with a Milder Phenotype in Giant Axonal Neuropathy. Neuromuscul. Disord. 2014, 24, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Koichihara, R.; Saito, T.; Ishiyama, A.; Komaki, H.; Yuasa, S.; Saito, Y.; Nakagawa, E.; Sugai, K.; Shiihara, T.; Shioya, A.; et al. A Mild Case of Giant Axonal Neuropathy without Central Nervous System Manifestation. Brain Dev. 2016, 38, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Incecik, F.; Herguner, O.M.; Ceylaner, S.; Zorludemir, S.; Altunbasak, S. Giant Axonal Disease: Report of Eight Cases. Brain Dev. 2015, 37, 803–807. [Google Scholar] [CrossRef]
- Boizot, A.; Talmat-Amar, Y.; Morrogh, D.; Kuntz, N.L.; Halbert, C.; Chabrol, B.; Houlden, H.; Stojkovic, T.; Schulman, B.A.; Rautenstrauss, B.; et al. The Instability of the BTB-KELCH Protein Gigaxonin Causes Giant Axonal Neuropathy and Constitutes a New Penetrant and Specific Diagnostic Test. Acta Neuropathol. Commun. 2014, 2, 47. [Google Scholar] [CrossRef]
- Bomont, P.; Cavalier, L.; Blondeau, F.; Ben Hamida, C.; Belal, S.; Tazir, M.; Demir, E.; Topaloglu, H.; Korinthenberg, R.; Tüysüz, B.; et al. The Gene Encoding Gigaxonin, a New Member of the Cytoskeletal BTB/Kelch Repeat Family, Is Mutated in Giant Axonal Neuropathy. Nat. Genet. 2000, 26, 370–374. [Google Scholar] [CrossRef]
- Roth, L.A.; Marra, J.D.; LaMarca, N.H.; Sproule, D.M. Measuring Disease Progression in Giant Axonal Neuropathy. J. Child Neurol. 2015, 30, 741–748. [Google Scholar] [CrossRef]
- Guo, Y.; Su, Q.; Zhu, X.; Wang, J.; Lou, Y.; Miao, P.; Wang, Y.; Zhang, B.; Jin, Y.; Gao, L.; et al. Giant Axonal Neuropathy (GAN) in an 8-year-old Girl Caused by a Homozygous Pathogenic Splicing Variant in GAN Gene. Am. J. Med. Genet. Part A 2022, 188, 836–846. [Google Scholar] [CrossRef]
- Bharucha-Goebel, D.X.; Norato, G.; Saade, D.; Paredes, E.; Biancavilla, V.; Donkervoort, S.; Kaur, R.; Lehky, T.; Fink, M.; Armao, D.; et al. Giant Axonal Neuropathy: Cross-Sectional Analysis of a Large Natural History Cohort. Brain 2021, 144, 3239–3250. [Google Scholar] [CrossRef]
- Kretzschmar, H.A.; Berg, B.O.; Davis, R.L. Giant Axonal Neuropathy. Acta Neuropathol. 1987, 73, 138–144. [Google Scholar] [CrossRef]
- Mohri, I.; Taniike, M.; Yoshikawa, H.; Higashiyama, M.; Itami, S.; Okada, S. A Case of Giant Axonal Neuropathy Showing Focal Aggregation and Hypophosphorylation of Intermediate Filaments. Brain Dev. 1998, 20, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Hou, B.; Tang, H.; Cai, M.; Zheng, W. Investigation of the Effects of Laminin Present in the Basal Lamina of the Peripheral Nervous System on Axon Regeneration and Remyelination Using the Nerve Acellular Scaffold. J. Biomed. Mater. Res. Part A 2020, 108, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Brenner, C.; Speck-Martins, C.E.; Farage, L.; Barker, P.B. 3T MR with Diffusion Tensor Imaging and Single-Voxel Spectroscopy in Giant Axonal Neuropathy. J. Magn. Reson. Imaging 2008, 28, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Majnemer, A.; Rosenblatt, B.; Watters, G.; Andermann, F. Giant Axonal Neuropathy: Central Abnormalities Demonstrated by Evoked Potentials. Ann. Neurol. 1986, 19, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Gambarelli, D.; Hassoun, J.; Pellissier, J.F.; Livet, M.O.; Pinsard, N.; Toga, M. Giant Axonal Neuropathy. Involvement of Peripheral Nerve, Myenteric Plexus and Extra-Neuronal Area. Acta Neuropathol. 1977, 39, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Armao, D.; Bailey, R.M.; Bouldin, T.W.; Kim, Y.; Gray, S.J. Autonomic Nervous System Involvement in the Giant Axonal Neuropathy (GAN) KO Mouse: Implications for Human Disease. Clin. Auton. Res. 2016, 26, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Prineas, J.W.; Ouvrier, R.A.; Wright, R.G.; Walsh, J.C.; McLeod, J.G. Giant Axonal Neuropathy—A Generalized Disorder of Cytoplasmic Microfilament Formation. J. Neuropathol. Exp. Neurol. 1976, 35, 458–470. [Google Scholar] [CrossRef]
- Mahammad, S.; Murthy, S.N.P.; Didonna, A.; Grin, B.; Israeli, E.; Perrot, R.; Bomont, P.; Julien, J.-P.; Kuczmarski, E.; Opal, P.; et al. Giant Axonal Neuropathy–Associated Gigaxonin Mutations Impair Intermediate Filament Protein Degradation. J. Clin. Investig. 2013, 123, 1964–1975. [Google Scholar] [CrossRef]
- Yuan, A.; Rao, M.V.; Veeranna; Nixon, R.A. Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309. [Google Scholar] [CrossRef]
- Mussche, S.; De Paepe, B.; Smet, J.; Devreese, K.; Lissens, W.; Rasic, V.M.; Murnane, M.; Devreese, B.; Van Coster, R. Proteomic Analysis in Giant Axonal Neuropathy: New Insights into Disease Mechanisms. Muscle Nerve 2012, 46, 246–256. [Google Scholar] [CrossRef]
- Wang, W.; Ding, J.; Allen, E.; Zhu, P.; Zhang, L.; Vogel, H.; Yang, Y. Gigaxonin Interacts with Tubulin Folding Cofactor B and Controls Its Degradation through the Ubiquitin-Proteasome Pathway. Curr. Biol. 2005, 15, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.J.; Rush, J.; Gygi, S.P.; Harper, J.W. Dynamics of Cullin-RING Ubiquitin Ligase Network Revealed by Systematic Quantitative Proteomics. Cell 2010, 143, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Kerner, B.L.; Garcia Diaz, A.; Ekins, S.; Wichterle, H. Kelch Domain of Gigaxonin Interacts with Intermediate Filament Proteins Affected in Giant Axonal Neuropathy. PLoS ONE 2015, 10, e0140157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Lo, S.-C.; Sun, Z.; Habib, G.M.; Lieberman, M.W.; Hannink, M. Ubiquitination of Keap1, a BTB-Kelch Substrate Adaptor Protein for Cul3, Targets Keap1 for Degradation by a Proteasome-Independent Pathway. J. Biol. Chem. 2005, 280, 30091–30099. [Google Scholar] [CrossRef]
- Furukawa, M.; He, Y.J.; Borchers, C.; Xiong, Y. Targeting of Protein Ubiquitination by BTB–Cullin 3–Roc1 Ubiquitin Ligases. Nat. Cell Biol. 2003, 5, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, Z.; Yang, Q. The Role of Ubiquitination and Deubiquitination in Cancer Metabolism. Mol. Cancer 2020, 19, 146. [Google Scholar] [CrossRef] [PubMed]
- Pintard, L.; Willems, A.; Peter, M. Cullin-Based Ubiquitin Ligases: Cul3–BTB Complexes Join the Family. EMBO J. 2004, 23, 1681–1687. [Google Scholar] [CrossRef]
- Ding, J.; Liu, J.-J.; Kowal, A.S.; Nardine, T.; Bhattacharya, P.; Lee, A.; Yang, Y. Microtubule-Associated Protein 1B. J. Cell Biol. 2002, 158, 427–433. [Google Scholar] [CrossRef]
- Allen, E.; Ding, J.; Wang, W.; Pramanik, S.; Chou, J.; Yau, V.; Yang, Y. Gigaxonin-Controlled Degradation of MAP1B Light Chain Is Critical to Neuronal Survival. Nature 2005, 438, 224–228. [Google Scholar] [CrossRef]
- Ding, J.; Allen, E.; Wang, W.; Valle, A.; Wu, C.; Nardine, T.; Cui, B.; Yi, J.; Taylor, A.; Jeon, N.L.; et al. Gene Targeting of GAN in Mouse Causes a Toxic Accumulation of Microtubule-Associated Protein 8 and Impaired Retrograde Axonal Transport. Hum. Mol. Genet. 2006, 15, 1451–1463. [Google Scholar] [CrossRef][Green Version]
- Johnson-Kerner, B.L.; Ahmad, F.S.; Diaz, A.G.; Greene, J.P.; Gray, S.J.; Samulski, R.J.; Chung, W.K.; Van Coster, R.; Maertens, P.; Noggle, S.A.; et al. Intermediate Filament Protein Accumulation in Motor Neurons Derived from Giant Axonal Neuropathy IPSCs Rescued by Restoration of Gigaxonin. Hum. Mol. Genet. 2015, 24, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Scrivo, A.; Codogno, P.; Bomont, P. Gigaxonin E3 Ligase Governs ATG16L1 Turnover to Control Autophagosome Production. Nat. Commun. 2019, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Hu, J.; Wu, J.; Huynh, D.T.; Smith, T.J.; Pan, S.; Bisnett, B.J.; Smith, A.B.; Lu, A.; Condon, B.M.; et al. Gigaxonin Glycosylation Regulates Intermediate Filament Turnover and May Impact Giant Axonal Neuropathy Etiology or Treatment. JCI Insight 2020, 5, e127751. [Google Scholar] [CrossRef]
- Bailey, R.M.; Armao, D.; Nagabhushan Kalburgi, S.; Gray, S.J. Development of Intrathecal AAV9 Gene Therapy for Giant Axonal Neuropathy. Mol. Ther. Methods Clin. Dev. 2018, 9, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, D.W.; Yamanaka, K.; Bomont, P. Gigaxonin Controls Vimentin Organization through a Tubulin Chaperone-Independent Pathway. Hum. Mol. Genet. 2009, 18, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Mussche, S.; Devreese, B.; Nagabhushan Kalburgi, S.; Bachaboina, L.; Fox, J.C.; Shih, H.-J.; Van Coster, R.; Samulski, R.J.; Gray, S.J. Restoration of Cytoskeleton Homeostasis After Gigaxonin Gene Transfer for Giant Axonal Neuropathy. Hum. Gene Ther. 2013, 24, 209–219. [Google Scholar] [CrossRef]
- Intrathecal Administration of ScAAV9/JeT-GAN for the Treatment of Giant Axonal Neuropathy. Available online: https://clinicaltrials.gov/ct2/show/NCT02362438 (accessed on 27 August 2022).
- Armao, D.; Bouldin, T.W.; Bailey, R.M.; Hooper, J.E.; Bharucha, D.X.; Gray, S.J. Advancing the Pathologic Phenotype of Giant Axonal Neuropathy: Early Involvement of the Ocular Lens. Orphanet J. Rare Dis. 2019, 14, 27. [Google Scholar] [CrossRef]
- Armao, D.; Bouldin, T.W.; Bailey, R.M.; Gray, S.J. Extensive Rod and Cone Photoreceptor-Cell Degeneration in Rat Models of Giant Axonal Neuropathy: Implications for Gene Therapy of Human Disease. Ophthalmic Genet. 2021, 42, 600–603. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S.; et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol. Ther. 2021, 29, 464–488. [Google Scholar] [CrossRef]
- Bailey, R.M.; Rozenberg, A.; Gray, S.J. Comparison of High-Dose Intracisterna Magna and Lumbar Puncture Intrathecal Delivery of AAV9 in Mice to Treat Neuropathies. Brain Res. 2020, 1739, 146832. [Google Scholar] [CrossRef]
- Hordeaux, J.; Buza, E.L.; Dyer, C.; Goode, T.; Mitchell, T.W.; Richman, L.; Denton, N.; Hinderer, C.; Katz, N.; Schmid, R.; et al. Adeno-Associated Virus-Induced Dorsal Root Ganglion Pathology. Hum. Gene Ther. 2020, 31, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Gene Therapy Community Grapples with Toxicity Issues, as Pipeline Matures. Nat. Rev. Drug Discov. 2021, 20, 804–805. [Google Scholar] [CrossRef]
- Opal, P. GAN-Related Neurodegeneration; University of Washington, Seattle: Seattle, WA, USA, 1993. [Google Scholar]
- Jaklevic, M.C. Advice for N-of-1 Drug Developers. JAMA 2021, 325, 613. [Google Scholar] [CrossRef]
- Synofzik, M.; van Roon-Mom, W.M.C.; Marckmann, G.; van Duyvenvoorde, H.A.; Graessner, H.; Schüle, R.; Aartsma-Rus, A. Preparing N-of-1 Antisense Oligonucleotide Treatments for Rare Neurological Diseases in Europe: Genetic, Regulatory, and Ethical Perspectives. Nucleic Acid Ther. 2022, 32, 83–94. [Google Scholar] [CrossRef]
- Jensen, T.L.; Gøtzsche, C.R.; Woldbye, D.P.D. Current and Future Prospects for Gene Therapy for Rare Genetic Diseases Affecting the Brain and Spinal Cord. Front. Mol. Neurosci. 2021, 14, 695937. [Google Scholar] [CrossRef]
- Croci, S.; Carriero, M.L.; Capitani, K.; Daga, S.; Donati, F.; Papa, F.T.; Frullanti, E.; Lopergolo, D.; Lamacchia, V.; Tita, R.; et al. AAV-Mediated FOXG1 Gene Editing in Human Rett Primary Cells. Eur. J. Hum. Genet. 2020, 28, 1446–1458. [Google Scholar] [CrossRef]
- Zielonka, D.; Mielcarek, M.; Landwehrmeyer, G.B. Update on Huntington’s Disease: Advances in Care and Emerging Therapeutic Options. Parkinsonism Relat. Disord. 2015, 21, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Wild, E.J.; Tabrizi, S.J. Targets for Future Clinical Trials in Huntington’s Disease: What’s in the Pipeline? Mov. Disord. 2014, 29, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Lubroth, P.; Colasante, G.; Lignani, G. In Vivo Genome Editing Therapeutic Approaches for Neurological Disorders: Where Are We in the Translational Pipeline? Front. Neurosci. 2021, 15, 632522. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Krainer, A.R.; Cleveland, D.W. Antisense Oligonucleotide Therapies for Neurodegenerative Diseases. Annu. Rev. Neurosci. 2019, 42, 385–406. [Google Scholar] [CrossRef]
- Aslesh, T.; Yokota, T. Restoring SMN Expression: An Overview of the Therapeutic Developments for the Treatment of Spinal Muscular Atrophy. Cells 2022, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Goodkey, K.; Aslesh, T.; Maruyama, R.; Yokota, T. Nusinersen in the Treatment of Spinal Muscular Atrophy. Methods Mol. Biol. 2018, 1828, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef]
- Li, Q. Nusinersen as a Therapeutic Agent for Spinal Muscular Atrophy. Yonsei Med. J. 2020, 61, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Aslesh, T.; Yokota, T. Development of Antisense Oligonucleotide Gapmers for the Treatment of Huntington’s Disease. Methods Mol. Biol. 2020, 2176, 57–67. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).