Lipedema Research—Quo Vadis?
Abstract
:1. Introduction
1.1. Background
1.2. “Lymph Makes You Fat”—Could This Be Relevant for Lipedema?
1.3. In Search of a Molecular Marker for Lipedema Diagnosis
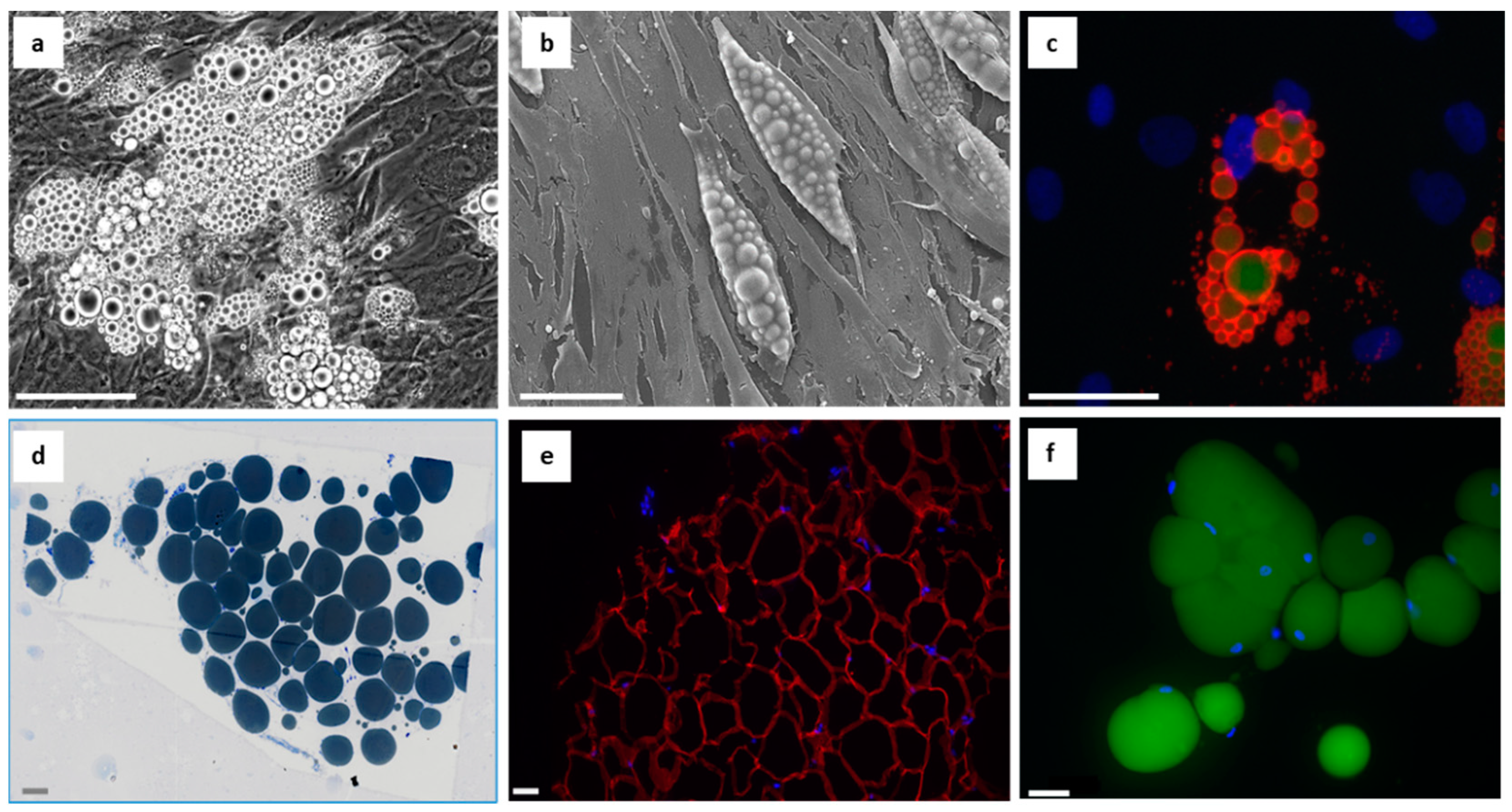
1.4. Focusing on Adipogenesis
1.5. Focusing on Hypertrophy and Hyperplasia
1.6. Chronic Inflammation and Oxidative Stress—Primary or Secondary to Lipedema?
2. Future Considerations
2.1. The Issue of Patients’ Weight and the Dilemma of Assembling a Representative Cohort
2.2. The Dilemma with Edema and Vascular Disturbances/Dysfunctions
2.3. The Dilemma with Age and Interdonor Variability
2.4. The Dilemma with “Controls”
2.5. Is the Current Classification in Light of the Data and Experience Collected from Recent Years Still Sufficient?
3. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herbst, K.L. Rare adipose disorders (RADs) masquerading as obesity. Acta Pharmacol. Sin. 2012, 33, 155–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbst, K.L.; Mirkovskaya, L.; Bharhagava, A.; Chava, Y.; Te, C.H.T. Lipedema Fat and Signs and Symptoms of Illness, Increase with Advancing Stage. Arch. Med. 2015, 7, 1–8. [Google Scholar]
- Okhovat, J.P.; Alavi, A. Lipedema: A Review of the Literature. Int. J. Low. Extrem. Wounds 2015, 14, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Buck, D.W., 2nd; Herbst, K.L. Lipedema: A Relatively Common Disease with Extremely Common Misconceptions. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, Y.S.; Wadeea, R.; Rosas, V.; Herbst, K.L. Lipedema: Friend and foe. Horm. Mol. Biol. Clin. Investig. 2018, 33. [Google Scholar] [CrossRef]
- Buso, G.; Depairon, M.; Tomson, D.; Raffoul, W.; Vettor, R.; Mazzolai, L. Lipedema: A Call to Action! Obesity 2019, 27, 1567–1576. [Google Scholar] [CrossRef]
- Kruppa, P.; Georgiou, I.; Biermann, N.; Prantl, L.; Klein-Weigel, P.; Ghods, M. Lipedema-Pathogenesis, Diagnosis, and Treatment Options. Dtsch. Arztebl. Int. 2020, 117, 396–403. [Google Scholar] [CrossRef]
- Wollina, U. Lipedema—An update. Dermatol. Ther. 2019, 32, e12805. [Google Scholar] [CrossRef]
- Child, A.H.; Gordon, K.D.; Sharpe, P.; Brice, G.; Ostergaard, P.; Jeffery, S.; Mortimer, P.S. Lipedema: An inherited condition. Am. J. Med. Genet. Part A 2010, 152a, 970–976. [Google Scholar] [CrossRef]
- Földi, M.; Földi, E. Földi’s Textbook of Lymphology: For Physicians and Lymphedema Therapists, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 417–427. [Google Scholar]
- Rapprich, S.; Dingler, A.; Podda, M. Liposuction is an effective treatment for lipedema-results of a study with 25 patients. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2011, 9, 33–40. [Google Scholar] [CrossRef]
- Witte, T.; Dadras, M.; Heck, F.C.; Heck, M.; Habermalz, B.; Welss, S.; Lehnhardt, M.; Behr, B. Water-jet-assisted liposuction for the treatment of lipedema: Standardized treatment protocol and results of 63 patients. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Dadras, M.; Mallinger, P.J.; Corterier, C.C.; Theodosiadi, S.; Ghods, M. Liposuction in the Treatment of Lipedema: A Longitudinal Study. Arch. Plast. Surg. 2017, 44, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollina, U.; Heinig, B. Treatment of lipedema by low-volume micro-cannular liposuction in tumescent anesthesia: Results in 111 patients. Dermatol. Ther. 2019, 32, e12820. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Hueppe, M.; Schmeller, W. Long-term benefit of liposuction in patients with lipoedema: A follow-up study after an average of 4 and 8 years. Br. J. Dermatol. 2016, 174, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Schmeller, W.; Hueppe, M.; Meier-Vollrath, I. Tumescent liposuction in lipoedema yields good long-term results. Br. J. Dermatol. 2012, 166, 161–168. [Google Scholar] [CrossRef]
- Sandhofer, M.; Hanke, C.W.; Habbema, L.; Podda, M.; Rapprich, S.; Schmeller, W.; Herbst, K.; Anderhuber, F.; Pilsl, U.; Sattler, G.; et al. Prevention of Progression of Lipedema with Liposuction Using Tumescent Local Anesthesia: Results of an International Consensus Conference. Dermatol. Surg. 2020, 46, 220–228. [Google Scholar] [CrossRef]
- Paolacci, S.; Precone, V.; Acquaviva, F.; Chiurazzi, P.; Fulcheri, E.; Pinelli, M.; Buffelli, F.; Michelini, S.; Herbst, K.L.; Unfer, V.; et al. Genetics of lipedema: New perspectives on genetic research and molecular diagnoses. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5581–5594. [Google Scholar] [CrossRef]
- Naouri, M.; Samimi, M.; Atlan, M.; Perrodeau, E.; Vallin, C.; Zakine, G.; Vaillant, L.; Machet, L. High-resolution cutaneous ultrasonography to differentiate lipoedema from lymphoedema. Br. J. Dermatol. 2010, 163, 296–301. [Google Scholar] [CrossRef]
- Iker, E.; Mayfield, C.K.; Gould, D.J.; Patel, K.M. Characterizing Lower Extremity Lymphedema and Lipedema with Cutaneous Ultrasonography and an Objective Computer-Assisted Measurement of Dermal Echogenicity. Lymphat. Res. Biol. 2019, 17, 525–530. [Google Scholar] [CrossRef]
- Amato, A.C.M.; Saucedo, D.Z.; Santos, K.D.S.; Benitti, D.A. Ultrasound criteria for lipedema diagnosis. Phlebology 2021, 36, 651–658. [Google Scholar] [CrossRef]
- Dietzel, R.; Reisshauer, A.; Jahr, S.; Calafiore, D.; Armbrecht, G. Body composition in lipoedema of the legs using dual-energy X-ray absorptiometry: A case-control study. Br. J. Dermatol. 2015, 173, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Wold, L.E.; Hines, E.A., Jr.; Allen, E.V. Lipedema of the legs; a syndrome characterized by fat legs and edema. Ann. Intern. Med. 1951, 34, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Reich-Schupke, S.; Schmeller, W.; Brauer, W.J.; Cornely, M.E.; Faerber, G.; Ludwig, M.; Lulay, G.; Miller, A.; Rapprich, S.; Richter, D.F.; et al. S1 guidelines: Lipedema. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2017, 15, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.L. Subcutaneous Adipose Tissue Diseases: Dercum Disease, Lipedema, Familial Multiple Lipomatosis, and Madelung Disease. [Updated 2019 December 14]. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK552156/ (accessed on 4 December 2022).
- Duhon, B.H.; Phan, T.T.; Taylor, S.L.; Crescenzi, R.L.; Rutkowski, J.M. Current Mechanistic Understandings of Lymphedema and Lipedema: Tales of Fluid, Fat, and Fibrosis. Int. J. Mol. Sci. 2022, 23, 6621. [Google Scholar] [CrossRef]
- Bertsch, T.; Erbacher, G.; Elwell, R. Lipoedema: A paradigm shift and consensus. J. Wound Care 2020, 29, 1–51. [Google Scholar] [CrossRef]
- Harvey, N.L. The link between lymphatic function and adipose biology. Ann. N. Y. Acad. Sci. 2008, 1131, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Escobedo, N.; Oliver, G. The Lymphatic Vasculature: Its Role in Adipose Metabolism and Obesity. Cell Metab. 2017, 26, 598–609. [Google Scholar] [CrossRef] [Green Version]
- McDonald, D.M. Tighter lymphatic junctions prevent obesity. Science 2018, 361, 551–552. [Google Scholar] [CrossRef]
- Bilancini, S.; Lucchi, M.; Tucci, S.; Eleuteri, P. Functional lymphatic alterations in patients suffering from lipedema. Angiology 1995, 46, 333–339. [Google Scholar] [CrossRef]
- Harwood, C.A.; Bull, R.H.; Evans, J.; Mortimer, P.S. Lymphatic and venous function in lipoedema. Br. J. Dermatol. 1996, 134, 1–6. [Google Scholar] [CrossRef]
- Lohrmann, C.; Foeldi, E.; Langer, M. MR imaging of the lymphatic system in patients with lipedema and lipo-lymphedema. Microvasc. Res. 2009, 77, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Amann-Vesti, B.R.; Franzeck, U.K.; Bollinger, A. Microlymphatic aneurysms in patients with lipedema. Lymphology 2001, 34, 170–175. [Google Scholar] [PubMed]
- Allen, M.; Schwartz, M.; Herbst, K.L. Interstitial Fluid in Lipedema and Control Skin. Womens Health Rep. 2020, 1, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Gil, H.J.; Escobedo, N.; Benito-Martín, A.; Ximénez-Embún, P.; Muñoz, J.; Peinado, H.; Rockson, S.G.; Oliver, G. Platelet factor 4 is a biomarker for lymphatic-promoted disorders. JCI Insight 2020, 5, e135109. [Google Scholar] [CrossRef] [PubMed]
- Duewell, S.; Hagspiel, K.D.; Zuber, J.; von Schulthess, G.K.; Bollinger, A.; Fuchs, W.A. Swollen lower extremity: Role of MR imaging. Radiology 1992, 184, 227–231. [Google Scholar] [CrossRef]
- Birkballe, S.; Jensen, M.R.; Noerregaard, S.; Gottrup, F.; Karlsmark, T. Can tissue dielectric constant measurement aid in differentiating lymphoedema from lipoedema in women with swollen legs? Br. J. Dermatol. 2014, 170, 96–102. [Google Scholar] [CrossRef]
- Rasmussen, J.C.; Aldrich, M.B.; Fife, C.E.; Herbst, K.L.; Sevick-Muraca, E.M. Lymphatic function and anatomy in early stages of lipedema. Obesity 2022, 30, 1391–1400. [Google Scholar] [CrossRef]
- Yoshida, S.; Koshima, I.; Imai, H.; Uchiki, T.; Sasaki, A.; Fujioka, Y.; Nagamatsu, S.; Yokota, K.; Yamashita, S. Lymphovenous Anastomosis for Morbidly Obese Patients with Lymphedema. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2860. [Google Scholar] [CrossRef]
- Szolnoky, G.; Ifeoluwa, A.; Tuczai, M.; Varga, E.; Varga, M.; Dosa-Racz, E.; Kemeny, L. Measurement of capillary fragility: A useful tool to differentiate lipedema from obesity? Lymphology 2017, 50, 203–209. [Google Scholar]
- Szolnoky, G.; Nagy, N.; Kovács, R.K.; Dósa-Rácz, E.; Szabó, A.; Bársony, K.; Balogh, M.; Kemény, L. Complex decongestive physiotherapy decreases capillary fragility in lipedema. Lymphology 2008, 41, 161–166. [Google Scholar]
- Al-Ghadban, S.; Cromer, W.; Allen, M.; Ussery, C.; Badowski, M.; Harris, D.; Herbst, K.L. Dilated Blood and Lymphatic Microvessels, Angiogenesis, Increased Macrophages, and Adipocyte Hypertrophy in Lipedema Thigh Skin and Fat Tissue. J. Obes. 2019, 2019, 8747461. [Google Scholar] [CrossRef]
- Felmerer, G.; Stylianaki, A.; Hollmén, M.; Ströbel, P.; Stepniewski, A.; Wang, A.; Frueh, F.S.; Kim, B.S.; Giovanoli, P.; Lindenblatt, N.; et al. Increased levels of VEGF-C and macrophage infiltration in lipedema patients without changes in lymphatic vascular morphology. Sci. Rep. 2020, 10, 10947. [Google Scholar] [CrossRef] [PubMed]
- Strohmeier, K.; Hofmann, M.; Jacak, J.; Narzt, M.S.; Wahlmueller, M.; Mairhofer, M.; Schaedl, B.; Holnthoner, W.; Barsch, M.; Sandhofer, M.; et al. Multi-Level Analysis of Adipose Tissue Reveals the Relevance of Perivascular Subpopulations and an Increased Endothelial Permeability in Early-Stage Lipedema. Biomedicines 2022, 10, 1163. [Google Scholar] [CrossRef] [PubMed]
- Giannotta, M.; Trani, M.; Dejana, E. VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef] [Green Version]
- Suga, H.; Araki, J.; Aoi, N.; Kato, H.; Higashino, T.; Yoshimura, K. Adipose tissue remodeling in lipedema: Adipocyte death and concurrent regeneration. J. Cutan. Pathol. 2009, 36, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Felmerer, G.; Stylianaki, A.; Hägerling, R.; Wang, A.; Ströbel, P.; Hollmén, M.; Lindenblatt, N.; Gousopoulos, E. Adipose Tissue Hypertrophy, An Aberrant Biochemical Profile and Distinct Gene Expression in Lipedema. J. Surg. Res. 2020, 253, 294–303. [Google Scholar] [CrossRef]
- Wolf, S.; Deuel, J.W.; Hollmén, M.; Felmerer, G.; Kim, B.S.; Vasella, M.; Grünherz, L.; Giovanoli, P.; Lindenblatt, N.; Gousopoulos, E. A Distinct Cytokine Profile and Stromal Vascular Fraction Metabolic Status without Significant Changes in the Lipid Composition Characterizes Lipedema. Int. J. Mol. Sci. 2021, 22, 3313. [Google Scholar] [CrossRef]
- Ishaq, M.; Bandara, N.; Morgan, S.; Nowell, C.; Mehdi, A.M.; Lyu, R.; McCarthy, D.; Anderson, D.; Creek, D.J.; Achen, M.G.; et al. Key signaling networks are dysregulated in patients with the adipose tissue disorder, lipedema. Int. J. Obes. 2022, 46, 502–514. [Google Scholar] [CrossRef]
- Ernst, A.M.; Steiner, M.; Kainz, V.; Tempfer, H.; Spitzer, G.; Plank, T.; Bauer, H.C.; Bresgen, N.; Habenbacher, A.; Bauer, H.; et al. Lipedema: The use of cultured adipocytes for identification of diagnostic markers. PRS, 2022; in press. [Google Scholar]
- Siems, W.; Grune, T.; Voss, P.; Brenke, R. Anti-fibrosclerotic effects of shock wave therapy in lipedema and cellulite. Biofactors 2005, 24, 275–282. [Google Scholar] [CrossRef]
- Priglinger, E.; Wurzer, C.; Steffenhagen, C.; Maier, J.; Hofer, V.; Peterbauer, A.; Nuernberger, S.; Redl, H.; Wolbank, S.; Sandhofer, M. The adipose tissue-derived stromal vascular fraction cells from lipedema patients: Are they different? Cytotherapy 2017, 19, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.T.; von Lukowicz, D.; Lossagk, K.; Hopfner, U.; Kirsch, M.; Moog, P.; Bauer, H.; Machens, H.G.; Schmauss, D. Adipose Stem Cells from Lipedema and Control Adipose Tissue Respond Differently to Adipogenic Stimulation In Vitro. Plast. Reconstr. Surg. 2019, 144, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Diaz, Z.T.; Singer, H.J.; Mert, K.B.; Bunnell, B.A. Increase in Leptin and PPAR-γ Gene Expression in Lipedema Adipocytes Differentiated in vitro from Adipose-Derived Stem Cells. Cells 2020, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ghadban, S.; Pursell, I.A.; Diaz, Z.T.; Herbst, K.L.; Bunnell, B.A. 3D Spheroids Derived from Human Lipedema ASCs Demonstrated Similar Adipogenic Differentiation Potential and ECM Remodeling to Non-Lipedema ASCs In Vitro. Int. J. Mol. Sci. 2020, 21, 8350. [Google Scholar] [CrossRef] [PubMed]
- Priglinger, E.; Strohmeier, K.; Weigl, M.; Lindner, C.; Auer, D.; Gimona, M.; Barsch, M.; Jacak, J.; Redl, H.; Grillari, J.; et al. SVF-derived extracellular vesicles carry characteristic miRNAs in lipedema. Sci. Rep. 2020, 10, 7211. [Google Scholar] [CrossRef] [PubMed]
- Michelini, S.; Chiurazzi, P.; Marino, V.; Dell’Orco, D.; Manara, E.; Baglivo, M.; Fiorentino, A.; Maltese, P.E.; Pinelli, M.; Herbst, K.L.; et al. Aldo-Keto Reductase 1C1 (AKR1C1) as the First Mutated Gene in a Family with Nonsyndromic Primary Lipedema. Int. J. Mol. Sci. 2020, 21, 6264. [Google Scholar] [CrossRef] [PubMed]
- Michelini, S.; Herbst, K.L.; Precone, V.; Manara, E.; Marceddu, G.; Dautaj, A.; Maltese, P.E.; Paolacci, S.; Ceccarini, M.R.; Beccari, T.; et al. A Multi-Gene Panel to Identify Lipedema-Predisposing Genetic Variants by a Next-Generation Sequencing Strategy. J. Pers. Med. 2022, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Baer, P.C. Adipose-Derived Stromal/Stem Cells. Cells 2020, 9, 1997. [Google Scholar] [CrossRef]
- Dykstra, J.A.; Facile, T.; Patrick, R.J.; Francis, K.R.; Milanovich, S.; Weimer, J.M.; Kota, D.J. Concise Review: Fat and Furious: Harnessing the Full Potential of Adipose-Derived Stromal Vascular Fraction. Stem Cells Transl. Med. 2017, 6, 1096–1108. [Google Scholar] [CrossRef]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A. Adipose Tissue-Derived Mesenchymal Stem Cells. Cells 2021, 10, 3433. [Google Scholar] [CrossRef] [PubMed]
- Zohora, F.T.; Aldebs, A.I.; Nosoudi, N.; Singh, S.P.; Ramirez-Vick, J.E. Gene Expression Profiling of Human Adipose Tissue Stem Cells during 2D versus 3D Adipogenesis. Cells Tissues Organs 2019, 208, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Sarantopoulos, C.N.; Banyard, D.A.; Ziegler, M.E.; Sun, B.; Shaterian, A.; Widgerow, A.D. Elucidating the Preadipocyte and Its Role in Adipocyte Formation: A Comprehensive Review. Stem Cell Rev. Rep. 2018, 14, 27–42. [Google Scholar] [CrossRef]
- Hepler, C.; Vishvanath, L.; Gupta, R.K. Sorting out adipocyte precursors and their role in physiology and disease. Genes Dev. 2017, 31, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Jankowski, M.; Dompe, C.; Sibiak, R.; Wąsiatycz, G.; Mozdziak, P.; Jaśkowski, J.M.; Antosik, P.; Kempisty, B.; Dyszkiewicz-Konwińska, M. In Vitro Cultures of Adipose-Derived Stem Cells: An Overview of Methods, Molecular Analyses, and Clinical Applications. Cells 2020, 9, 1783. [Google Scholar] [CrossRef]
- Câmara, D.A.D.; Shibli, J.A.; Müller, E.A.; De-Sá-Junior, P.L.; Porcacchia, A.S.; Blay, A.; Lizier, N.F. Adipose Tissue-Derived Stem Cells: The Biologic Basis and Future Directions for Tissue Engineering. Materials 2020, 13, 3210. [Google Scholar] [CrossRef]
- Danisovic, L.; Oravcova, L.; Krajciova, L.; Varchulova Novakova, Z.; Bohac, M.; Varga, I.; Vojtassak, J. Effect of long-term culture on the biological and morphological characteristics of human adipose tissue-derived stem Cells. J. Physiol. Pharmacol. 2017, 68, 149–158. [Google Scholar]
- Zhang, H.H.; Kumar, S.; Barnett, A.H.; Eggo, M.C. Ceiling culture of mature human adipocytes: Use in studies of adipocyte functions. J. Endocrinol. 2000, 164, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Schmidt, H.; Lai, B.; Ge, K. Transcriptional and Epigenomic Regulation of Adipogenesis. Mol. Cell. Biol. 2019, 39, e00601–e00618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambele, M.A.; Dessels, C.; Durandt, C.; Pepper, M.S. Genome-wide analysis of gene expression during adipogenesis in human adipose-derived stromal cells reveals novel patterns of gene expression during adipocyte differentiation. Stem Cell Res. 2016, 16, 725–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audano, M.; Pedretti, S.; Caruso, D.; Crestani, M.; De Fabiani, E.; Mitro, N. Regulatory mechanisms of the early phase of white adipocyte differentiation: An overview. Cell. Mol. Life Sci. 2022, 79, 139. [Google Scholar] [CrossRef] [PubMed]
- Dufau, J.; Shen, J.X.; Couchet, M.; De Castro Barbosa, T.; Mejhert, N.; Massier, L.; Griseti, E.; Mouisel, E.; Amri, E.Z.; Lauschke, V.M.; et al. In vitro and ex vivo models of adipocytes. Am. J. Physiol. Physiol. 2021, 320, C822–C841. [Google Scholar] [CrossRef]
- Scott, M.A.; Nguyen, V.T.; Levi, B.; James, A.W. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011, 20, 1793–1804. [Google Scholar] [CrossRef]
- Ducharme, N.A.; Bickel, P.E. Lipid droplets in lipogenesis and lipolysis. Endocrinology 2008, 149, 942–949. [Google Scholar] [CrossRef] [Green Version]
- Walther, T.C.; Chung, J.; Farese, R.V., Jr. Lipid Droplet Biogenesis. Annu. Rev. Cell Dev. Biol. 2017, 33, 491–510. [Google Scholar] [CrossRef] [Green Version]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Baganha, F.; Schipper, R.; Hagberg, C.E. Towards better models for studying human adipocytes in vitro. Adipocyte 2022, 11, 413–419. [Google Scholar] [CrossRef]
- Kang, S.; Akerblad, P.; Kiviranta, R.; Gupta, R.K.; Kajimura, S.; Griffin, M.J.; Min, J.; Baron, R.; Rosen, E.D. Regulation of early adipose commitment by Zfp521. PLoS Biol. 2012, 10, e1001433. [Google Scholar] [CrossRef]
- Hepler, C.; Gupta, R.K. The expanding problem of adipose depot remodeling and postnatal adipocyte progenitor recruitment. Mol. Cell. Endocrinol. 2017, 445, 95–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, J.; Zhang, X.; Prabhu, S.; Shahoei, S.H.; Nelson, E.R.; Swanson, K.S.; Anastasio, M.A.; Smith, A.M. 3D microscopy and deep learning reveal the heterogeneity of crown-like structure microenvironments in intact adipose tissue. Sci. Adv. 2021, 7, eabe2480. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.J.; Lee, S.K.; Song, Y.S.; Jang, Y.J.; Park, H.S.; Hong, J.P.; Ko, A.R.; Kim, D.Y.; Kim, J.H.; Lee, Y.J.; et al. IL-34 is associated with obesity, chronic inflammation, and insulin resistance. J. Clin. Endocrinol. Metab. 2014, 99, E1263–E1271. [Google Scholar] [CrossRef] [Green Version]
- Maucher, D.; Schmidt, B.; Schumann, J. Loss of Endothelial Barrier Function in the Inflammatory Setting: Indication for a Cytokine-Mediated Post-Transcriptional Mechanism by Virtue of Upregulation of miRNAs miR-29a-3p, miR-29b-3p, and miR-155-5p. Cells 2021, 10, 2843. [Google Scholar] [CrossRef]
- Alsaffar, H.; Martino, N.; Garrett, J.P.; Adam, A.P. Interleukin-6 promotes a sustained loss of endothelial barrier function via Janus kinase-mediated STAT3 phosphorylation and de novo protein synthesis. Am. J. Physiol. Physiol. 2018, 314, C589–C602. [Google Scholar] [CrossRef] [Green Version]
- Clark, P.R.; Kim, R.K.; Pober, J.S.; Kluger, M.S. Tumor necrosis factor disrupts claudin-5 endothelial tight junction barriers in two distinct NF-κB-dependent phases. PLoS ONE 2015, 10, e0120075. [Google Scholar] [CrossRef] [Green Version]
- Desai, T.R.; Leeper, N.J.; Hynes, K.L.; Gewertz, B.L. Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J. Surg. Res. 2002, 104, 118–123. [Google Scholar] [CrossRef]
- Maruo, N.; Morita, I.; Shirao, M.; Murota, S. IL-6 increases endothelial permeability in vitro. Endocrinology 1992, 131, 710–714. [Google Scholar] [CrossRef]

| METHODS | TISSUE/CELLS | RESULTS | AUTHORS |
|---|---|---|---|
| Immunostaining | Paraffin-embedded adipose tissue Histological sections | Increased infiltration of CD68+ macrophages in lipedema adipose tissue and occurrence of “crown-like structures”; large number of KI67+/CD34+ proliferating cells in lipedema tissue | [47] |
| Histochemical staining Immunostaining | Paraffin-embedded adipose tissue Histological sections | Hypertrophy of adipocytes in adipose tissue from non-obese lipedema donors; increased macrophage density in lipedema skin and fat; increased numbers of dermal blood vessels in lipedema adipose tissue; increased dilatation of capillaries in non-obese lipedema adipose tissue compared to non-obese controls | [43] |
| Immunostaining | Paraffin-embedded adipose tissue Histological sections | Increased M2-macrophage infiltration into lipedema adipose tissue; no morphological changes in lymphatic and blood vasculature; no changes in number, size or percentage coverage of lymphatic vessels or blood vessels in lipedema tissue sections | [44] |
| Histochemical staining Immunostaining | Paraffin-embedded adipose tissue Histological sections | Increased dermal spaces and abnormal vessel phenotype (rounded endothelial cells; perivascular spaces, perivascular immune cell infiltrate) in lipedema specimens compared to controls | [35] |
| Histochemical staining Immunostaining | Paraffin-embedded adipose tissue Histological sections | Increased epidermal thickness in lipedema patients; adipocyte hypertrophy, increased fibrosis and significant increase in CD68+ macrophages in lipedema tissue | [48] |
| Histochemical staining Immunostaining | Paraffin-embedded adipose tissue Histological sections | Significant hypertrophy of lipedema adipocytes | [49] |
| Immunohistochemistry BODIPY staining of droplets | Paraffin-embedded adipose tissue Histological sections | Significantly increased number of CD29/CD34 positive cells in lipedema adipose tissue; enhanced adipogenic potential of lipedema ASCs | [50] |
| Histochemical staining Immunostaining Machine learning analysis | Paraffin-embedded adipose tissue Histological sections SVF, ASCs | No difference in epidermal thickness of thigh tissue between lipedema and control tissue; no signs of fibrosis; no alterations in lymphatic endothelial cells in lipedema adipose tissue; higher number of CD68+ macrophages in CD31+/podoplanin- areas of lipedema tissue; morphological alterations of interendo-thelial junctions between lipedema en-dothelial cells in vitro | [45] |
| Immunostaining Histochemical staining | SVF, ASCs In vitro differentiated adipocytes | Increased occurrence of myofibroblast-like cells in lipedema adipocytes from normal weight and overweight lipedema donors | [51] |
| METHODS | TISSUE/CELLS | RESULTS | AUTHORS |
|---|---|---|---|
| HPLC | Blood samples Plasma | Increased parameters of oxidative stress (plasma MDA and plasma protein carbonyl concentrations) in lipedema patients compared to controls | [52] |
| Immunophenotyping Flow cytometry OilRed O staining | Lipoaspirates SVF/ASCs In vitro differentiated adipocytes | Enhanced SVF cell yield in lipedema preparations with increased CD90 and CD146-positive cells; reduced in vitro differentiation capacity of lipedema ASCs | [53] |
| ELISA OilRedO staining Cell counting | Lipoaspirates SVF/ASCs In vitro differentiated adipocytes | Increased proliferative activity of lipedema ASCs; increased IL-8 levels in supernatants from lipedema ASCs; reduced adipokine and aromatase levels in supernatants from in vitro differentiated lipedema adipocytes; reduced differentiation capacity of lipedema ASCs | [54] |
| Proliferation assay CFU fibroblast assay qPCR OilRedO staining | Lipoaspirates SVF/ASCs (2D cultures) In vitro differentiated adipocytes | Significant increase in CFU potential and higher adipogenic potential of lipedema ASCs; increased expression of leptin and PPARγ in lipedema adipocytes; no change in proliferation rate of lipedema ASCs compared to controls; comparable inflammatory gene expression in lipedema and control ASCs and adipocytes | [55] |
| qPCR OilRedO staining | ASCs spheroids (3D cultures) | No difference in adipogenic gene expression (ADIPOQ, LPL, PPARγ, Glut4) between lipedema and healthy 3D-differentiated adipocytes; upregulation of IL-6 expression in 3D cultures of lipedema ASCs and adipocytes; elevated CFU activity and adipogenic potential of ASCs grown as spheroids | [56] |
| qPCR ELISA | Adipose tissue Serum | Increased levels of VEGF-C in serum from lipedema patients; increased expression of VEGFR-3 in lipedema adipose tissue; significant decrease in VEGF-A and VEGF-D, and Tie-2 expression in lipedema adipose tissue | [44] |
| Gene array of adipose-tissue related genes ELISA | Adipose tissue Serum | Aberrant lipid metabolic profile, increased cholesterol, triglycerides and LDL and ApoB in lipedema serum; no alteration in cytokine profile (IL-6, IL-18, lipocalin-2 and leptin); upregulation of CCND1/cyclinD1 and downregulation of CEBP, CFD, NCOR2, KLF4 in lipedema adipocytes | [48] |
| Analysis of extracellular miRNAs from SVF | Lipoaspirates; conditioned medium from SVF cells; small extracellular vesicles (sEVs) | Identification of lipedema-relevant miRNAs preferentially in sEVs; potential involvement of differentially expressed miRNAs in Notch, Wnt SMAD/TGFß-pathway, oxidative stress and senescence | [57] |
| Mass spectrometry analysis | Blood plasma exosomes (mouse and human) | Increase platelet factor 4 (PF4) levels in circulating exosomes from patients with lipedema | [36] |
| Whole exome sequencing qPCR Molecular modeling | Blood samples (germline DNA) | Discovery of a missense variant in the AKR1C1 gene encoding an aldo-keto reductase involved in progesterone metabolism | [58] |
| Lipidomic analysis (lipid mass spectrometry) Cytokine profiling (Multiplex immunoassay) Mitochondrial stress test | Adipose tissue biopsies Lipoaspirates SVF Serum | Significant increase in IL-11, IL-28A, IL29 expression in lipedema serum; no significant alteration in lipid composition in adipose tissue and serum from lipedema donors; significantly increased oxidative metabolism (enhanced mitochondrial function) of lipedema SVF cells | [49] |
| Transcriptional profiling Lipidomic and metabolomic analyses Functional assays BODIPY staining | Whole adipose tissue biopsies ASCs In vitro differentiated adipocytes | Differential expression of >4400 genes partly involved in cell cycle/cell proliferation and lipid metabolism, in lipedema adipose tissue, >900 changes in lipid composition and >600 differentially altered metabolites in lipedema adipocytes: differential expression of >3400 genes, partly involved in extracellular matrix, cell-cycle/proliferation signaling pathways, in lipedema ASCs; upregulation of the cell cycle regulator Bub1 and enhanced activation of histone H2A in lipedema ASCs; enhanced proliferation and differentiation of lipedema ASCs | [50] |
| qPCR Protein array Endothelial permeability assay | Lipoaspirates Whole adipose tissue (AT) SVF human primary ECs (hECs) SVF-derived sorted EC/PC SVF cell-derived conditioned medium (CM) | Significantly increased ZNF423 in lipedema SVF, EC and PC compared to controls; significant upregulation of aromatase expression in lipedema whole adipose tissue; lipedema SVF cell-induced dysfunction of the vascular endothelial barrier in vitro | [45] |
| RT-PCR, qPCR | Lipoaspirates ASCs In vitro differentiated adipocytes | Significant upregulation of PPARγ, CD36 and FABP4 in differentiated adipocytes from non-obese lipedema donors; reduced adiponectin/leptin ratio in obese but not non-obese lipedema adipocytes | [51] |
| Next-generation sequencing; multi-gene panel | Genomic DNA from peripheral blood | Identification of 21 deleterious variants in genes linked to syndromic fat accumulation (ALDH18A1, GHR) and differential diagnosis (PLIN1, LIPE, PPARγ, POMC, NR0B2, GCKR, NPC1), as well as lipedema candidate genes (RYR1, PPARA) | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ernst, A.M.; Bauer, H.; Bauer, H.-C.; Steiner, M.; Malfertheiner, A.; Lipp, A.-T. Lipedema Research—Quo Vadis? J. Pers. Med. 2023, 13, 98. https://doi.org/10.3390/jpm13010098
Ernst AM, Bauer H, Bauer H-C, Steiner M, Malfertheiner A, Lipp A-T. Lipedema Research—Quo Vadis? Journal of Personalized Medicine. 2023; 13(1):98. https://doi.org/10.3390/jpm13010098
Chicago/Turabian StyleErnst, Anna M., Hannelore Bauer, Hans-Christian Bauer, Marianne Steiner, Anna Malfertheiner, and Anna-Theresa Lipp. 2023. "Lipedema Research—Quo Vadis?" Journal of Personalized Medicine 13, no. 1: 98. https://doi.org/10.3390/jpm13010098
APA StyleErnst, A. M., Bauer, H., Bauer, H. -C., Steiner, M., Malfertheiner, A., & Lipp, A. -T. (2023). Lipedema Research—Quo Vadis? Journal of Personalized Medicine, 13(1), 98. https://doi.org/10.3390/jpm13010098






