Three-Dimensional Customized Imaging Reconstruction for Urological Surgery: Diffusion and Role in Real-Life Practice from an International Survey
Abstract
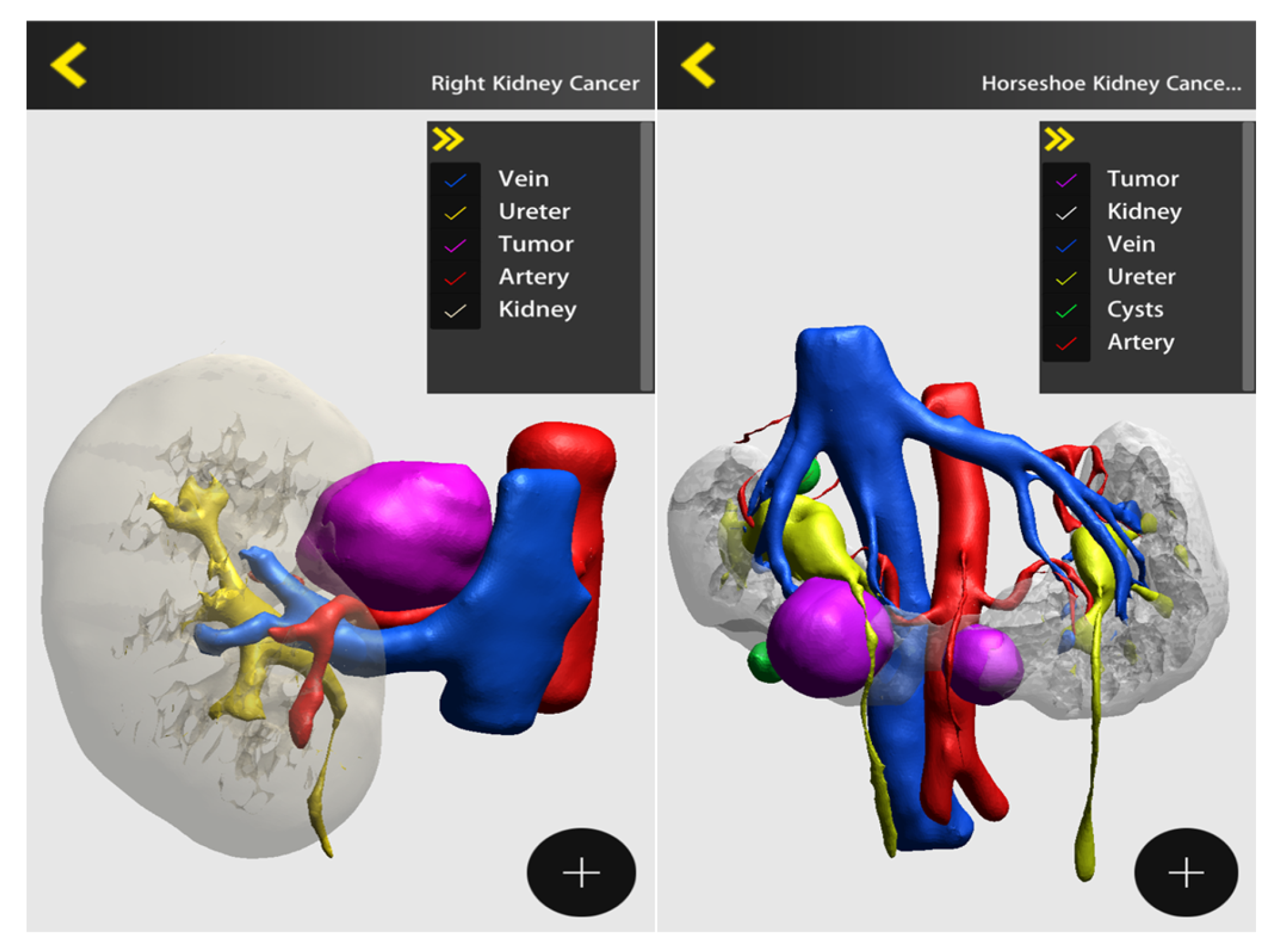
:1. Introduction
2. Materials and Methods
2.1. Survey
2.2. Full Text of the Survey
- Surname, name and affiliation (in case of public acknowledgement)
- Are you working in an Academic or in a Non-Academic institution?
- How old are you?
- How long have you been working as a Urologist?
- Do you mainly practice?
- Are you a trainee?
- How many major laparoscopic/robotic/open surgeries are performed
- Do you mainly perform major laparoscopic/robotic surgery as?
- How often do you use 3D imaging reconstruction in your surgical
- For what sort of laparoscopic/robotic/open surgery do you usually use 3D imaging reconstruction? Specify
- Did you experience 3D imaging systems other than DocDo?
- In which setting do you think that 3D imaging reconstruction is more useful?
- How many times do you show your patients 3D images to explain the intervention?
- Do you feel 3D findings may change surgical pre-planning?
- Kidney surgery: do you feel 3D imaging is more useful to assess: nephrometric score based on 3D; pedicle and vascular anatomy; The volume of the remaining kidney; other comment)
- Prostate surgery: Do you feel that 3D imaging carries advantages over the 2D for the assessment of: Localization of the main tumor; lesion proximity to the capsule; other
- How much do you believe 3D imaging use is able to improve your surgical outcomes? (5-Likert scale)
- How can the 3D imaging reconstruction software be improved?
2.3. Timeframe
2.4. Participants Selection
2.5. Statistics
3. Results
3.1. Demographics
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. Cornford P.EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update—Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Vis, A.N.; van den Bergh, R.C.N.; van der Poel, H.G.; Mottrie, A.; Stricker, P.D.; Graefen, M.; Patel, V.; Rocco, B.; Lissenberg-Witte, B.; van Leeuwen, P.J. Selection of patients for nerve sparing surgery in robot-assisted radical prostatectomy. BJUI Compass 2021, 3, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.C.; Schonlau, M.; Litwin, M.S.; Lai, J.; Saigal, C.S. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer 2008, 112, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Elkin, E.B.; Levey, A.S.; Jang, T.L.; Russo, P. Par- tial Nephrectomy Versus Radical Nephrectomy in Patients with Small Renal Tumors—Is There a Difference in Mortality and Cardiovascular Outcomes? J. Urol. 2009, 181, 55–62. [Google Scholar] [CrossRef]
- Leow, J.J.; Heah, N.H.; Chang, S.L.; Chong, Y.L.; Png, K.S. Out-comes of Robotic versus Laparoscopic Partial Nephrectomy: An Updated Meta-Analysis of 4,919 Patients. J. Urol. 2016, 196, 1371–1377. [Google Scholar] [CrossRef]
- Azhar, R.A. The influence of 3D renal reconstruction on surgical planning for complex renal tumors: An interactive case-based survey. Int. Braz. J. Urol. 2023, 49, 372–382. [Google Scholar] [CrossRef]
- Sempels, M.; Ben Chehida, M.A.; Meunier, P.; Waltregny, D. Open and Laparoscopic Partial Nephrectomy: Comparison and Validation of Preoperative Scoring Systems, Including PADUA, RENAL, ABC Nephrometric Scores and Perinephric Fat Evaluation with Mayo Adhesive Probability Score. Res. Rep. Urol. 2021, 13, 509–517. [Google Scholar] [CrossRef]
- Xiao, Y.; Shan, Z.J.; Yang, J.F.; Len, J.J.; Yu, Y.H.; Yang, M.L. Nephrometric scoring system: Recent advances and outlooks. Urol. Oncol. 2023, 41, 15–26. [Google Scholar] [CrossRef]
- Partin, A.W.; Dmochowski, R.R.; Kavoussi, L.R.; Peters, C.A.; Wein, A.J. (Eds.) Chapter 57: Campbell-Walsh-Wein Urology, 12th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Thakker, P.U.; O’Rourke, T., Jr.; Hemal, A.K. Technologic advances in robot-assisted nephron sparing surgery: A narrative review. Transl. Androl. Urol. 2023, 12, 1184–1198. [Google Scholar] [CrossRef]
- Kang, S.K.; Zhang, A.; Pandharipande, P.V.; Chandarana, H.; Braithwaite, R.S.; Littenberg, B. DWI for Renal Mass Characterization: Systematic Review and Meta-Analysis of Diagnostic Test Performance. Am. J. Roentgenol. 2015, 205, 317–324. [Google Scholar] [CrossRef]
- Shirk, J.D.; Thiel, D.D.; Wallen, E.M.; Linehan, J.M.; White, W.M.; Badani, K.K.; Porter, J.R. Effect of 3-Dimensional Virtual Reality Models for Surgical Planning of Robotic-Assisted Partial Nephrectomy on Surgical Outcomes: A Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e1911598. [Google Scholar] [CrossRef] [PubMed]
- Piramide, F.; Kowalewski, K.F.; Cacciamani, G.; Rivero Belenchon, I.; Taratkin, M.; Carbonara, U.; Marchioni, M.; De Groote, R.; Knipper, S.; Pecoraro, A. Three-dimensional Model-assisted Minimally Invasive Partial Nephrectomy: A Systematic Review with Meta-analysis of Comparative Studies. Eur. Urol. Oncol. 2022, 5, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, E.; Amparore, D.; Fiori, C.; Manfredi, M.; Ivano, M.; Di Dio, M.; Niculescu, G.; Piramide, F.; Cattaneo, G.; Piazzolla, P.; et al. 3D imaging applications for robotic urologic surgery: An ESUT YAUWP review. World J. Urol. 2020, 38, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, E.; Amparore, D.; Volpi, G.; Piramide, F.; De Cillis, S.; Piana, A.; Alessio, P.; Verri, P.; Piscitello, S.; Carbonaro, B.; et al. Percutaneous puncture during PCNL: New perspective for the future with virtual imaging guidance. World J. Urol. 2022, 40, 639–650. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Okhunov, Z.; Meneses, A.D.; Rodriguez-Socarras, M.E.; Rivas, J.G.; Porpiglia, F.; Liatsikos, E.; Veneziano, D. Impact of Three-dimensional Printing in Urology: State of the Art and Future Perspectives. A Systematic Review by ESUT-YAUWP Group. Eur. Urol. 2019, 76, 209–221. [Google Scholar] [CrossRef]
- Porpiglia, F.; Bertolo, R.; Checcucci, E.; Amparore, D.; Autorino, R.; Dasgupta, P.; Wiklund, P.; Tewari, A.; Liatsikos, E.; Fiori, C.; et al. Development and validation of 3D printed virtual models for robot-assisted radical prostatectomy and partial nephrectomy: Urologists’ and patients’ perception. World J. Urol. 2018, 36, 201–207. [Google Scholar] [CrossRef]
- Eysenbach, G. Improving the quality of Web surveys: The Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J. Med. Internet Res. 2004, 6, e34. [Google Scholar] [CrossRef]
- Puliatti, S.; Eissa, A.; Checcucci, E.; Piazza, P.; Amato, M.; Ferretti, S.; Scarcella, S.; Rivas, J.G.; Taratkin, M.; Marenco, J.; et al. New imaging technologies for robotic kidney cancer surgery. Asian J. Urol. 2022, 9, 253–262. [Google Scholar] [CrossRef]
- Moldovanu, C.G.; Lebovici, A.; Buruian, M.M. A systematic review of the clinical value and applications of three-dimensional virtual reconstructions in renal tumors. Med. Pharm. Rep. 2022, 95, 11–23. [Google Scholar] [CrossRef]
- Dwivedi, D.K.; Chatzinoff, Y.; Zhang, Y.; Yuan, Q.; Fulkerson, M.; Chopra, R.; Brugarolas, J.; Cadeddu, J.A.; Kapur, P.; Pedrosa, I. Development of a Patient-specific Tumor Mold Using Magnetic Resonance Imaging and 3-Dimensional Printing Technology for Targeted Tissue Procurement and Radiomics Analysis of Renal Masses. Urology 2018, 112, 209–214. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; Wu, G.; Wang, T.; Wang, Y.; Zhao, H.; Zhou, Z.; Wu, J. The role of three-dimensional reconstruction in laparoscopic partial nephrectomy for complex renal tumors. World J. Surg. Oncol. 2019, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Komai, Y.; Sakai, Y.; Gotohda, N.; Kobayashi, T.; Kawakami, S.; Saito, N. A novel 3-dimensional image analysis system for case-specific kidney anatomy and surgical simulation to facilitate clampless partial nephrectomy. Urology 2014, 83, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Amparore, D.; Checcucci, E.; Autorino, R.; Manfredi, M.; Iannizzi, G.; Fiori, C.; ESUT Research Group. Current Use of Three-dimensional Model Technology in Urology: A Road Map for Personalised Surgical Planning. Eur. Urol. Focus 2018, 4, 652–656. [Google Scholar] [CrossRef]
- Paparidis, S.; Spartalis, E.; Mavrigiannaki, E.; Ferakis, N.; Stravodimos, K.; Tsourouflis, G.; Dimitroulis, D.; Nikiteas, N.I. Record and Appraisal of Endophytic Tumor Localization Techniques in Minimally Invasive Kidney-Sparing Procedures. A Systematic Review. Urol. J. 2022, 19, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Li, J.; Li, M.; Ye, M.; Pei, X.; Li, F.; Zhu, S.; Weiqin, H.; Zhou, X.; Xie, Y. Three-Dimensional Physical Model-Assisted Planning and Navigation for Laparoscopic Partial Nephrectomy in Patients with Endophytic Renal Tumors. Sci. Rep. 2018, 12, 582. [Google Scholar] [CrossRef]
- Komai, Y.; Sugimoto, M.; Gotohda, N.; Matsubara, N.; Kobayashi, T.; Sakai, Y.; Shiga, Y.; Saito, N. Patient-specific 3-dimensional Printed Kidney Designed for “4D” Surgical Navigation: A Novel Aid to Facilitate Minimally Invasive Off-clamp Partial Nephrectomy in Complex Tumor Cases. Urology 2016, 91, 226–233. [Google Scholar] [CrossRef]
- Lasser, M.S.; Doscher, M.; Keehn, A.; Chernyak, V.; Garfein, E.; Ghavamian, R. Virtual surgical planning: A novel aid to robot-assisted laparoscopic partial nephrectomy. J. Endourol. 2012, 26, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qi, L.; Yuan, P.; Zu, X.; Chen, W.; Cao, Z.; Li, Y.; Wang, L. Application of Three-Dimensional Visualization Technology in Laparoscopic Partial Nephrectomy of Renal Tumor: A Comparative Study. J. Laparoendosc. Adv. Surg. Tech. A 2017, 27, 516–523. [Google Scholar] [CrossRef]
- Alyaev, Y.G.; Sirota, E.S.; Bezrukov, E.A.; Fiev, D.N.; Bukatov, M.D.; Letunovskii, A.V.; Byadretdinov, I.S. Application of 3D soft print models of the kidney for treatment of patients with localized cancer of the kidney (a pilot study). Urologiia 2017, 6, 12–19. [Google Scholar]
- Bertolo, R.; Autorino, R.; Fiori, C.; Amparore, D.; Checcucci, E.; Mottrie, A.; Porter, J.; Haber, G.P.; Derweesh, I.; Porpiglia, F. Expanding the Indications of Robotic Partial Nephrectomy for Highly Complex Renal Tumors: Urologists’ Perception of the Impact of Hyperaccuracy Three-Dimensional Reconstruction. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 233–239. [Google Scholar] [CrossRef]
- Bianchi, L.; Schiavina, R.; Bortolani, B.; Cercenelli, L.; Gaudiano, C.; Carpani, G.; Rustici, A.; Droghetti, M.; Mottaran, A.; Boschi, S.; et al. Interpreting nephrometry scores with three-dimensional virtual modelling for better planning of robotic partial nephrectomy and predicting complications. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 836.e1–836.e9. [Google Scholar] [CrossRef] [PubMed]
- Rocco, B.; Sighinolfi, M.C.; Dourado Menezes, A.; Eissa, A.; Inzillo, R.; Sandri, M.; Puliatti, S.; Turri, F.; Ciarlariello, S.; Amato, M.; et al. Three-dimensional virtual reconstruction with DocDo, a novel interactive tool to score renal mass complexity. BJU Int. 2020, 125, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Amparore, D.; Checcucci, E.; Manfredi, M.; Stura, I.; Migliaretti, G.; Autorino, R.; Ficarra, V.; Fiori, C. Three-dimensional virtual imaging of renal tumours: A new tool to improve the accuracy of nephrometry scores. BJU Int. 2019, 124, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Campos, T.J.F.L.; de VFilho, F.E.; Rocha, M.F.H. Assessment of the complexity of renal tumors by nephrometry (R.E.N.A.L. score) with CT and MRI images versus 3D reconstruction model images. Int. Braz. J. Urol. 2021, 47, 896–901. [Google Scholar] [CrossRef]
- Esperto, F.; Prata, F.; Autrán-Gómez, A.M.; Rivas, J.G.; Socarras, M.; Marchioni, M.; Albisinni, S.; Cataldo, R.; Scarpa, R.M.; Papalia, R. New Technologies for Kidney Surgery Planning 3D, Impression, Augmented Reality 3D, Reconstruction: Current Realities and Expectations. Curr. Urol. Rep. 2021, 22, 35. [Google Scholar] [CrossRef]
- Maddox, M.M.; Feibus, A.; Liu, J.; Wang, J.; Thomas, R.; Silberstein, J.L. 3D-printed soft-tissue physical models of renal malignancies for individualized surgical simulation: A feasibility study. J. Robot Surg. 2018, 12, 27–33. [Google Scholar] [CrossRef]
- Kyung, Y.S.; Kim, N.; Jeong, I.G.; Hong, J.H.; Kim, C.S. Application of 3-D printed kidney model in partial nephrectomy for predicting surgical outcomes: A feasibility study. Clin. Genitourin. Cancer 2019, 17, e878–e884. [Google Scholar] [CrossRef]
- Fan, G.; Meng, Y.; Zhu, S.; Ye, M.; Li, M.; Li, F.; Ye, Y.; Liu, Z.; Weiqin, H.; Xie, Y. Three-dimensional printing for laparoscopic partial nephrectomy in patients with renal tumours. J. Int. Med. Res. 2019, 47, 4324–4332. [Google Scholar] [CrossRef]
- Kwon Kim, J.; Ryu, H.; Kim, M.; Kwon, E.K.; Lee, H.; Joon Park, S.; Byun, S.S. Personalised three-dimensional printed transparent kidney model for robot-assisted partial nephrectomy in patients with complex renal tumours (R.E.N.A.L. nephrometry score≥7): A prospective case-matched study. BJU Int. 2021, 127, 567–574. [Google Scholar] [CrossRef]
- Wake, N.; Rosenkrantz, A.B.; Huang, R.; Park, K.U.; Wysock, J.S.; Taneja, S.S.; Huang, W.C.; Sodickson, D.K.; Chandarana, H. Patient-specific 3D printed and augmented reality kidney and prostate cancer models: Impact on patient education. 3D Print Med. 2019, 5, 4. [Google Scholar] [CrossRef]
- Silberstein, J.L.; Maddox, M.M.; Dorsey, P.; Feibus, A.; Thomas, R.; Lee, B.R. Physical models of renal malignancies using standard cross-sectional imaging and 3-dimensional printers: A pilot study. Urology 2014, 84, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Teishima, J.; Takayama, Y.; Iwaguro, S.; Hayashi, T.; Inoue, S.; Hieda, K.; Shinmei, S.; Kato, R.; Mita, K.; Matsubara, A. Usefulness of personalized three-dimensional printed model on the satisfaction of preoperative education for patients undergoing robot-assisted partial nephrectomy and their families. Int. Urol. Nephrol. 2018, 50, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, H.; Wang, X.; Yao, H.; Wang, D.; Sun, F.; Bao, X.; Zhou, Z.; Wang, J.; Wu, J. The role of three-dimensional model in preoperative communication before partial nephrectomy and postoperative management. Asia Pac. J. Oncol. Nurs. 2023, 10, 100222. [Google Scholar] [CrossRef]
- de Rooij, M.; Hamoen, E.H.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-Analysis. Eur. Urol. 2016, 70, 233–245. [Google Scholar] [CrossRef]
- Wang, S.; Frisbie, J.; Keepers, Z.; Bolten, Z.; Hevaganinge, A.; Boctor, E.; Leonard, S.; Tokuda, J.; Krieger, A.; Siddiqui, M.M. The Use of Three-dimensional Visualization Techniques for Prostate Procedures: A Systematic Review. Eur. Urol. Focus 2021, 7, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.M.; Rais-Bahrami, S.; Turkbey, B.; George, A.K.; Rothwax, J.; Shakir, N.; Okoro, C.; Raskolnikov, D.; Parnes, H.L.; Linehan, W.M.; et al. Comparison of MR/ ultrasound fusion–guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015, 313, 390–397. [Google Scholar] [CrossRef]
- Makary, J.; van Diepen, D.C.; Arianayagam, R.; McClintock, G.; Fallot, J.; Leslie, S.; Thanigasalam, R. The evolution of image guidance in robotic-assisted laparoscopic prostatectomy (RALP): A glimpse into the future. J. Robot. Surg. 2022, 16, 765–774. [Google Scholar] [CrossRef]
- Lupulescu, C.; Sun, Z. A Systematic Review of the Clinical Value and Applications of Three-Dimensional Printing in Renal Surgery. J. Clin. Med. 2019, 8, 990. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sighinolfi, M.C.; Menezes, A.D.; Patel, V.; Moschovas, M.; Assumma, S.; Calcagnile, T.; Panio, E.; Sangalli, M.; Turri, F.; Sarchi, L.; et al. Three-Dimensional Customized Imaging Reconstruction for Urological Surgery: Diffusion and Role in Real-Life Practice from an International Survey. J. Pers. Med. 2023, 13, 1435. https://doi.org/10.3390/jpm13101435
Sighinolfi MC, Menezes AD, Patel V, Moschovas M, Assumma S, Calcagnile T, Panio E, Sangalli M, Turri F, Sarchi L, et al. Three-Dimensional Customized Imaging Reconstruction for Urological Surgery: Diffusion and Role in Real-Life Practice from an International Survey. Journal of Personalized Medicine. 2023; 13(10):1435. https://doi.org/10.3390/jpm13101435
Chicago/Turabian StyleSighinolfi, Maria Chiara, Aurus Dourado Menezes, Vipul Patel, Marcio Moschovas, Simone Assumma, Tommaso Calcagnile, Enrico Panio, Mattia Sangalli, Filippo Turri, Luca Sarchi, and et al. 2023. "Three-Dimensional Customized Imaging Reconstruction for Urological Surgery: Diffusion and Role in Real-Life Practice from an International Survey" Journal of Personalized Medicine 13, no. 10: 1435. https://doi.org/10.3390/jpm13101435
APA StyleSighinolfi, M. C., Menezes, A. D., Patel, V., Moschovas, M., Assumma, S., Calcagnile, T., Panio, E., Sangalli, M., Turri, F., Sarchi, L., Micali, S., Varca, V., Annino, F., Leonardo, C., Bozzini, G., Cacciamani, G., Gregori, A., Morini, E., Terzoni, S., ... Rocco, B. (2023). Three-Dimensional Customized Imaging Reconstruction for Urological Surgery: Diffusion and Role in Real-Life Practice from an International Survey. Journal of Personalized Medicine, 13(10), 1435. https://doi.org/10.3390/jpm13101435






