Ratings of the Effectiveness of 13 Therapeutic Diets for Autism Spectrum Disorder: Results of a National Survey
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Demographics
3.2. Medical History
3.3. Diets in General
3.4. Benefits and Adverse Effects of the Different Therapeutic Diets
3.5. Frequency of Therapeutic Diet Usage
3.6. Symptom Improvements from Diets
3.6.1. Average Symptom Improvements from All Diets
3.6.2. Symptom Improvements of the Different Therapeutic Diets
3.7. Comparison of Diets with Nutraceuticals and Medications
3.8. Correlations between Strictly Following Diet, Advice Received, and Overall Benefit of Diet
3.9. Change in Autism Severity between Participants Who Used Diet vs. Those Who Did Not
4. Discussion
4.1. Diet Surveys
4.2. Therapeutic Diets
4.2.1. Healthy Diet
4.2.2. Feingold Diet
4.2.3. Gluten and Casein Free Diets
4.2.4. Low Sugar Diet
4.2.5. Soy-Free, Corn-Free and Food Avoidance Diets
Soy-Free Diet
Corn-Free Diet
Food Avoidance, Based on IgG/IgE Testing
Food Avoidance Diet, Based on Observation
4.2.6. Grain-Free and Carbohydrate Limiting Diets
Ketogenic Diet
Specific Carbohydrate Diet
Paleo
4.3. Personalized Nutrition for a Heterogeneous Condition
4.4. Performance of Diets vs. Nutraceuticals and Medications
4.5. Correlations of Strict Adherence to Diet and Dietary Benefit
4.6. Change in Autism Severity with Therapeutic Diets
4.7. Diet Therapy Is Cost Effective
4.8. ANRC Autism Treatment Rater App
4.9. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maenner, M.J. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill. Summ. 2021, 70, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Buescher, A.V.S.; Cidav, Z.; Knapp, M.; Mandell, D.S. Costs of Autism Spectrum Disorders in the United Kingdom and the United States. JAMA Pediatr. 2014, 168, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Bryson, S.E.; Rogers, S.J.; Fombonne, E. Autism Spectrum Disorders: Early Detection, Intervention, Education, and Psychopharmacological Management. Can. J. Psychiatry 2003, 48, 506–516. [Google Scholar] [CrossRef]
- Famitafreshi, H.; Karimian, M. Overview of the Recent Advances in Pathophysiology and Treatment for Autism. CNS Neurol. Disord. Drug Targets 2018, 17, 590–594. [Google Scholar] [CrossRef]
- Kawicka, A.; Regulska-Ilow, B. How Nutritional Status, Diet and Dietary Supplements Can Affect Autism. A Review. Rocz. Panstw. Zakl. Hig. 2013, 64, 1–12. [Google Scholar]
- Madra, M.; Ringel, R.; Margolis, K.G. Gastrointestinal Issues and Autism Spectrum Disorder. Psychiatr. Clin. N. Am. 2021, 44, 69–81. [Google Scholar] [CrossRef]
- Doreswamy, S.; Bashir, A.; Guarecuco, J.E.; Lahori, S.; Baig, A.; Narra, L.R.; Patel, P.; Heindl, S.E. Effects of Diet, Nutrition, and Exercise in Children With Autism and Autism Spectrum Disorder: A Literature Review. Cureus 2020, 12, e12222. [Google Scholar] [CrossRef]
- Hopf, K.P.; Madren, E.; Santianni, K.A. Use and Perceived Effectiveness of Complementary and Alternative Medicine to Treat and Manage the Symptoms of Autism in Children: A Survey of Parents in a Community Population. J. Altern. Complement. Med. 2016, 22, 25–32. [Google Scholar] [CrossRef]
- Şenel, H.G. Parents’ Views and Experiences About Complementary and Alternative Medicine Treatments for Their Children with Autistic Spectrum Disorder. J. Autism Dev. Disord. 2010, 40, 494–503. [Google Scholar] [CrossRef]
- Winburn, E.; Charlton, J.; McConachie, H.; McColl, E.; Parr, J.; O’Hare, A.; Baird, G.; Gringras, P.; Wilson, D.C.; Adamson, A.; et al. Parents’ and Child Health Professionals’ Attitudes Towards Dietary Interventions for Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2014, 44, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-L.; Lin, C.-Y.; Chen, C.-L.; Wang, C.-M.; Wong, M.-K. The Effects of a Gluten and Casein-Free Diet in Children with Autism: A Case Report. Chang Gung Med. J. 2009, 32, 459–465. [Google Scholar]
- Herbert, M.R.; Buckley, J.A. Autism and Dietary Therapy: Case Report and Review of the Literature. J. Child. Neurol. 2013, 28, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Molina-López, J.; Leiva-García, B.; Planells, E.; Planells, P. Food Selectivity, Nutritional Inadequacies, and Mealtime Behavioral Problems in Children with Autism Spectrum Disorder Compared to Neurotypical Children. Int. J. Eat. Disord. 2021, 54, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Figuerola, P.; Canals, J.; Fernández-Cao, J.C.; Arija Val, V. Differences in Food Consumption and Nutritional Intake between Children with Autism Spectrum Disorders and Typically Developing Children: A Meta-Analysis. Autism 2019, 23, 1079–1095. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Nutritional and Metabolic Status of Children with Autism vs. Neurotypical Children, and the Association with Autism Severity. Nutr. Metab. 2011, 8, 34. [Google Scholar] [CrossRef]
- Morton, J.T.; Jin, D.-M.; Mills, R.H.; Shao, Y.; Rahman, G.; McDonald, D.; Berding, K.; Needham, B.D.; Zurita, M.F.; David, M.; et al. Multi-Omic Analysis along the Gut-Brain Axis Points to a Functional Architecture of Autism. bioRxiv 2022. [Google Scholar] [CrossRef]
- Iovene, M.R.; Bombace, F.; Maresca, R.; Sapone, A.; Iardino, P.; Picardi, A.; Marotta, R.; Schiraldi, C.; Siniscalco, D.; Serra, N.; et al. Intestinal Dysbiosis and Yeast Isolation in Stool of Subjects with Autism Spectrum Disorders. Mycopathologia 2017, 182, 349–363. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Otašević, S.; Momčilović, S.; Petrović, M.; Radulović, O.; Stojanović, N.M.; Arsić-Arsenijević, V. The Dietary Modification and Treatment of Intestinal Candida Overgrowth—A Pilot Study. J. Mycol. Med. 2018, 28, 623–627. [Google Scholar] [CrossRef]
- Horn, J.; Mayer, D.E.; Chen, S.; Mayer, E.A. Role of Diet and Its Effects on the Gut Microbiome in the Pathophysiology of Mental Disorders. Transl. Psychiatry 2022, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Soltys, K.; Kemenyova, P.; Karhanek, M.; Babinska, K. The Influence of Food Intake Specificity in Children with Autism on Gut Microbiota. Int. J. Mol. Sci. 2020, 21, 2797. [Google Scholar] [CrossRef]
- Wolter, M.; Grant, E.T.; Boudaud, M.; Steimle, A.; Pereira, G.V.; Martens, E.C.; Desai, M.S. Leveraging Diet to Engineer the Gut Microbiome. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Giulivi, C.; Zhang, Y.-F.; Omanska-Klusek, A.; Ross-Inta, C.; Wong, S.; Hertz-Picciotto, I.; Tassone, F.; Pessah, I.N. Mitochondrial Dysfunction in Autism. JAMA 2010, 304, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Melnyk, S.; Jernigan, S.; Cleves, M.A.; Halsted, C.H.; Wong, D.H.; Cutler, P.; Bock, K.; Boris, M.; Bradstreet, J.J.; et al. Metabolic Endophenotype and Related Genotypes Are Associated with Oxidative Stress in Children with Autism. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2006, 141B, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, F.; Adams, J.B.; Audhya, T.; Hahn, J. Multivariate Analysis of Metabolomic and Nutritional Profiles among Children with Autism Spectrum Disorder. J. Pers. Med. 2022, 12, 923. [Google Scholar] [CrossRef]
- Konstantynowicz, J.; Porowski, T.; Zoch-Zwierz, W.; Wasilewska, J.; Kadziela-Olech, H.; Kulak, W.; Owens, S.C.; Piotrowska-Jastrzebska, J.; Kaczmarski, M. A Potential Pathogenic Role of Oxalate in Autism. Eur. J. Paediatr. Neurol. 2012, 16, 485–491. [Google Scholar] [CrossRef]
- Cade, R.; Privette, M.; Fregly, M.; Rowland, N.; Sun, Z.; Zele, V.; Wagemaker, H.; Edelstein, C. Autism and Schizophrenia: Intestinal Disorders. Nutr. Neurosci. 2000, 3, 57–72. [Google Scholar] [CrossRef]
- Kushak, R.I.; Lauwers, G.Y.; Winter, H.S.; Buie, T.M. Intestinal Disaccharidase Activity in Patients with Autism: Effect of Age, Gender, and Intestinal Inflammation. Autism 2011, 15, 285–294. [Google Scholar] [CrossRef]
- Horvath, K.; Perman, J.A. Autistic Disorder and Gastrointestinal Disease. Curr. Opin. Pediatr. 2002, 14, 583–587. [Google Scholar] [CrossRef]
- Horvath, K.; Papadimitriou, J.C.; Rabsztyn, A.; Drachenberg, C.; Tildon, J.T. Gastrointestinal Abnormalities in Children with Autistic Disorder. J. Pediatr. 1999, 135, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.L.; Hornig, M.; Buie, T.; Bauman, M.L.; Cho Paik, M.; Wick, I.; Bennett, A.; Jabado, O.; Hirschberg, D.L.; Lipkin, W.I. Impaired Carbohydrate Digestion and Transport and Mucosal Dysbiosis in the Intestines of Children with Autism and Gastrointestinal Disturbances. PLoS ONE 2011, 6, e24585. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, K.L.; Knivsberg, A.M. Can the Pathophysiology of Autism Be Explained by the Nature of the Discovered Urine Peptides? Nutr. Neurosci. 2003, 6, 19–28. [Google Scholar] [CrossRef]
- Quan, L.; Xu, X.; Cui, Y.; Han, H.; Hendren, R.L.; Zhao, L.; You, X. A Systematic Review and Meta-Analysis of the Benefits of a Gluten-Free Diet and/or Casein-Free Diet for Children with Autism Spectrum Disorder. Nutr. Rev. 2022, 80, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.W.Y.; Corley, M.J.; Pang, A.; Arakaki, G.; Abbott, L.; Nishimoto, M.; Miyamoto, R.; Lee, E.; Yamamoto, S.; Maunakea, A.K.; et al. A Modified Ketogenic Gluten-Free Diet with MCT Improves Behavior in Children with Autism Spectrum Disorder. Physiol. Behav. 2018, 188, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Chen, L.; She, D.; Chung, Y.; Ge, L.; Han, L. Ketogenic Diet for Epilepsy: An Overview of Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2022, 76, 1234–1244. [Google Scholar] [CrossRef]
- Boutros, N.N.; Lajiness-O’Neill, R.; Zillgitt, A.; Richard, A.E.; Bowyer, S.M. EEG Changes Associated with Autistic Spectrum Disorders. Neuropsychiatr. Electrophysiol. 2015, 1, 3. [Google Scholar] [CrossRef]
- El-Rashidy, O.; El-Baz, F.; El-Gendy, Y.; Khalaf, R.; Reda, D.; Saad, K. Ketogenic Diet versus Gluten Free Casein Free Diet in Autistic Children: A Case-Control Study. Metab. Brain Dis. 2017, 32, 1935–1941. [Google Scholar] [CrossRef]
- Ābele, S.; Meija, L.; Folkmanis, V.; Tzivian, L. Specific Carbohydrate Diet (SCD/GAPS) and Dietary Supplements for Children with Autistic Spectrum Disorder. Proc. Latv. Acad. Sci. 2021, 75, 417–425. [Google Scholar] [CrossRef]
- Feingold, B.F. Hyperkinesis and Learning Disabilities Linked to Artificial Food Flavors and Colors. Am. J. Nurs. 1975, 75, 797–803. [Google Scholar] [CrossRef]
- Vita, A.A.; Zwickey, H.; Bradley, R. Associations between Food-Specific IgG Antibodies and Intestinal Permeability Biomarkers. Front. Nutr. 2022, 9, 962093. [Google Scholar] [CrossRef] [PubMed]
- Fidler Mis, N.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A.; et al. Sugar in Infants, Children and Adolescents: A Position Paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal Flora and Gastrointestinal Status in Children with Autism—Comparisons to Typical Children and Correlation with Autism Severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J.; Jóźwik-Pruska, J. Nutritional Strategies and Personalized Diet in Autism-Choice or Necessity? Trends Food Sci. Technol. 2016, 49, 45–50. [Google Scholar] [CrossRef]
- Mandecka, A.; Regulska-Ilow, B. The importance of nutritional management and education in the treatment of autism. Rocz. Panstw. Zakl. Hig. 2022, 73, 247–258. [Google Scholar] [CrossRef]
- Cekici, H.; Sanlier, N. Current Nutritional Approaches in Managing Autism Spectrum Disorder: A Review. Nutr. Neurosci. 2019, 22, 145–155. [Google Scholar] [CrossRef]
- Ristori, M.V.; Quagliariello, A.; Reddel, S.; Ianiro, G.; Vicari, S.; Gasbarrini, A.; Putignani, L. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients 2019, 11, 2812. [Google Scholar] [CrossRef]
- Hartman, R.E.; Patel, D. Dietary Approaches to the Management of Autism Spectrum Disorders. Adv. Neurobiol. 2020, 24, 547–571. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; Geis, E.; Gehn, E.; Fimbres, V.; Pollard, E.L.; Mitchell, J.; Ingram, J.; Hellmers, R.; Laake, D.; et al. Comprehensive Nutritional and Dietary Intervention for Autism Spectrum Disorder-A Randomized, Controlled 12-Month Trial. Nutrients 2018, 10, 369. [Google Scholar] [CrossRef]
- Rimland, B.; Edelson, S. Parent Ratings of Behavioral Effects of Biomedical Interventions. In Autism Research Institute Newsletter; ARI Publication: San Diego, CA, USA, 2009; Volume 34. [Google Scholar]
- Adams, J.B.; Bhargava, A.; Coleman, D.M.; Frye, R.E.; Rossignol, D.A. Ratings of the Effectiveness of Nutraceuticals for Autism Spectrum Disorders: Results of a National Survey. J. Pers. Med. 2021, 11, 878. [Google Scholar] [CrossRef]
- Coleman, D.M.; Adams, J.B.; Anderson, A.L.; Frye, R.E. Rating of the Effectiveness of 26 Psychiatric and Seizure Medications for Autism Spectrum Disorder: Results of a National Survey. J. Child. Adolesc. Psychopharmacol. 2019, 29, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, J.; Chen, X.; Fu, J.; Wang, X.; Pu, L.; Gu, C.; Cai, C. Efficacy and Safety of Diet Therapies in Children With Autism Spectrum Disorder: A Systematic Literature Review and Meta-Analysis. Front. Neurol. 2022, 13, 844117. [Google Scholar] [CrossRef] [PubMed]
- Viscidi, E.W.; Triche, E.W.; Pescosolido, M.F.; McLean, R.L.; Joseph, R.M.; Spence, S.J.; Morrow, E.M. Clinical Characteristics of Children with Autism Spectrum Disorder and Co-Occurring Epilepsy. PLoS ONE 2013, 8, e67797. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, X.; Jin, Y.; Zhan, X.; Cao, M.; Guo, X.; Liu, S.; Ou, X.; Gu, T.; Jing, J.; et al. Association between Dietary Quality and Executive Functions in School-Aged Children with Autism Spectrum Disorder. Front. Nutr. 2022, 9, 940246. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Donovan, S.M. Diet Can Impact Microbiota Composition in Children With Autism Spectrum Disorder. Front. Neurosci. 2018, 12, 515. [Google Scholar] [CrossRef]
- Mayes, S.D.; Zickgraf, H. Atypical Eating Behaviors in Children and Adolescents with Autism, ADHD, Other Disorders, and Typical Development. Res. Autism Spectr. Disord. 2019, 64, 76–83. [Google Scholar] [CrossRef]
- Chistol, L.T.; Bandini, L.G.; Must, A.; Phillips, S.; Cermak, S.A.; Curtin, C. Sensory Sensitivity and Food Selectivity in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 583–591. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Fang, H.; Chen, Y.; Weng, J.; Zhai, M.; Xiao, T.; Ke, X. Study on Aberrant Eating Behaviors, Food Intolerance, and Stereotyped Behaviors in Autism Spectrum Disorder. Front. Psychiatry 2020, 11, 493695. [Google Scholar] [CrossRef]
- Raithel, M.; Baenkler, H.W.; Naegel, A.; Buchwald, F.; Schultis, H.W.; Backhaus, B.; Kimpel, S.; Koch, H.; Mach, K.; Hahn, E.G.; et al. Significance of Salicylate Intolerance in Diseases of the Lower Gastrointestinal Tract. J. Physiol. Pharmacol. 2005, 56 (Suppl. S5), 89–102. [Google Scholar]
- Kęszycka, P.K.; Lange, E.; Gajewska, D. Effectiveness of Personalized Low Salicylate Diet in the Management of Salicylates Hypersensitive Patients: Interventional Study. Nutrients 2021, 13, 991. [Google Scholar] [CrossRef]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food Additives and Hyperactive Behaviour in 3-Year-Old and 8/9-Year-Old Children in the Community: A Randomised, Double-Blinded, Placebo-Controlled Trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Alberti, A.; Pirrone, P.; Elia, M.; Waring, R.H.; Romano, C. Sulphation Deficit in “Low-Functioning” Autistic Children: A Pilot Study. Biol. Psychiatry 1999, 46, 420–424. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Itokazu, N. Innate Immunity Associated with Inflammatory Responses and Cytokine Production against Common Dietary Proteins in Patients with Autism Spectrum Disorder. Neuropsychobiology 2002, 46, 76–84. [Google Scholar] [CrossRef]
- Knivsberg, A.M.; Reichelt, K.L.; Høien, T.; Nødland, M. A Randomised, Controlled Study of Dietary Intervention in Autistic Syndromes. Nutr. Neurosci. 2002, 5, 251–261. [Google Scholar] [CrossRef]
- Whiteley, P.; Haracopos, D.; Knivsberg, A.-M.; Reichelt, K.L.; Parlar, S.; Jacobsen, J.; Seim, A.; Pedersen, L.; Schondel, M.; Shattock, P. The ScanBrit Randomised, Controlled, Single-Blind Study of a Gluten- and Casein-Free Dietary Intervention for Children with Autism Spectrum Disorders. Nutr. Neurosci. 2010, 13, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Ghalichi, F.; Ghaemmaghami, J.; Malek, A.; Ostadrahimi, A. Effect of Gluten Free Diet on Gastrointestinal and Behavioral Indices for Children with Autism Spectrum Disorders: A Randomized Clinical Trial. World J. Pediatr. 2016, 12, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Piwowarczyk, A.; Horvath, A.; Pisula, E.; Kawa, R.; Szajewska, H. Gluten-Free Diet in Children with Autism Spectrum Disorders: A Randomized, Controlled, Single-Blinded Trial. J. Autism Dev. Disord. 2020, 50, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Karagözlü, S.; Dalgıç, B.; İşeri, E. The Relationship of Severity of Autism with Gastrointestinal Symptoms and Serum Zonulin Levels in Autistic Children. J. Autism Dev. Disord. 2022, 52, 623–629. [Google Scholar] [CrossRef]
- Lucarelli, S.; Frediani, T.; Zingoni, A.M.; Ferruzzi, F.; Giardini, O.; Quintieri, F.; Barbato, M.; D’Eufemia, P.; Cardi, E. Food Allergy and Infantile Autism. Panminerva Med. 1995, 37, 137–141. [Google Scholar] [PubMed]
- Marí-Bauset, S.; Llopis-González, A.; Zazpe, I.; Marí-Sanchis, A.; Suárez-Varela, M.M. Nutritional Impact of a Gluten-Free Casein-Free Diet in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 673–684. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Effect of a Vitamin/Mineral Supplement on Children and Adults with Autism. BMC Pediatr. 2011, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Markus, C.R.; Rogers, P.J. Effects of High and Low Sucrose-Containing Beverages on Blood Glucose and Hypoglycemic-like Symptoms. Physiol. Behav. 2020, 222, 112916. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive Intake of Sugar: An Accomplice of Inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef]
- Kuhlow, D.; Zarse, K.; Voigt, A.; Schulz, T.J.; Petzke, K.J.; Schomburg, L.; Pfeiffer, A.F.H.; Ristow, M. Opposing Effects of Dietary Sugar and Saturated Fat on Cardiovascular Risk Factors and Glucose Metabolism in Mitochondrially Impaired Mice. Eur. J. Nutr. 2010, 49, 417–427. [Google Scholar] [CrossRef]
- Tan, S.; Pan, N.; Xu, X.; Li, H.; Lin, L.; Chen, J.; Jin, C.; Pan, S.; Jing, J.; Li, X. The Association between Sugar-Sweetened Beverages and Milk Intake with Emotional and Behavioral Problems in Children with Autism Spectrum Disorder. Front. Nutr. 2022, 9, 927212. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Chen, H.; Chen, X.; Sun, Y.; Xie, Y.; Deng, M.; Hesketh, T.; Wang, X.; Chen, J. Sugar-Sweetened Beverages, Artificially Sweetened Beverages and Natural Juices and Risk of Inflammatory Bowel Disease: A Cohort Study of 121,490 Participants. Aliment. Pharmacol. Ther. 2022, 56, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, B.; Wang, J.; Zhang, Z.; Chen, O. Global Prevalence of Autism Spectrum Disorder and Its Gastrointestinal Symptoms: A Systematic Review and Meta-Analysis. Front. Psychiatry 2022, 13, 963102. [Google Scholar] [CrossRef]
- Ohinata, K.; Agui, S.; Yoshikawa, M. Soymorphins, Novel μ Opioid Peptides Derived from Soy β-Conglycinin β-Subunit, Have Anxiolytic Activities. Biosci. Biotechnol. Biochem. 2007, 71, 2618–2621. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Geng, L.; Ruby, A.; Reddy, C.; Zimmerman-Bier, B. Evaluation of an Association between Gastrointestinal Symptoms and Cytokine Production against Common Dietary Proteins in Children with Autism Spectrum Disorders. J. Pediatr. 2005, 146, 605–610. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Geng, L.; Ruby, A.; Zimmerman-Bier, B. Dysregulated Innate Immune Responses in Young Children with Autism Spectrum Disorders: Their Relationship to Gastrointestinal Symptoms and Dietary Intervention. Neuropsychobiology 2005, 51, 77–85. [Google Scholar] [CrossRef]
- Westmark, C.J. Soy Infant Formula and Seizures in Children with Autism: A Retrospective Study. PLoS ONE 2014, 9, e80488. [Google Scholar] [CrossRef] [PubMed]
- Samsel, A.; Seneff, S. Glyphosate, Pathways to Modern Diseases II: Celiac Sprue and Gluten Intolerance. Interdiscip. Toxicol. 2013, 6, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A Review on Metabolism, Toxicity, Occurrence in Food, Occupational Exposure, and Detoxification Methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- De Magistris, L.; Picardi, A.; Siniscalco, D.; Riccio, M.P.; Sapone, A.; Cariello, R.; Abbadessa, S.; Medici, N.; Lammers, K.M.; Schiraldi, C.; et al. Antibodies against Food Antigens in Patients with Autistic Spectrum Disorders. BioMed Res. Int. 2013, 2013, 729349. [Google Scholar] [CrossRef]
- Pizzo, F.; Collotta, A.D.; Di Nora, A.; Costanza, G.; Ruggieri, M.; Falsaperla, R. Ketogenic Diet in Pediatric Seizures: A Randomized Controlled Trial Review and Meta-Analysis. Expert. Rev. Neurother. 2022, 22, 169–177. [Google Scholar] [CrossRef]
- Qu, C.; Keijer, J.; Adjobo-Hermans, M.J.W.; van de Wal, M.; Schirris, T.; van Karnebeek, C.; Pan, Y.; Koopman, W.J.H. The Ketogenic Diet as a Therapeutic Intervention Strategy in Mitochondrial Disease. Int. J. Biochem. Cell Biol. 2021, 138, 106050. [Google Scholar] [CrossRef]
- Mu, C.; Corley, M.J.; Lee, R.W.Y.; Wong, M.; Pang, A.; Arakaki, G.; Miyamoto, R.; Rho, J.M.; Mickiewicz, B.; Dowlatabadi, R.; et al. Metabolic Framework for the Improvement of Autism Spectrum Disorders by a Modified Ketogenic Diet: A Pilot Study. J. Proteome Res. 2020, 19, 382–390. [Google Scholar] [CrossRef]
- Haas, S.V.; Haas, M.P. The Treatment of Celiac Disease with the Specific Carbohydrate Diet; Report on 191 Additional Cases. Am. J. Gastroenterol. 1955, 23, 344–360. [Google Scholar]
- Barnhill, K.; Devlin, M.; Moreno, H.T.; Potts, A.; Richardson, W.; Schutte, C.; Hewitson, L. Brief Report: Implementation of a Specific Carbohydrate Diet for a Child with Autism Spectrum Disorder and Fragile X Syndrome. J. Autism Dev. Disord. 2020, 50, 1800–1808. [Google Scholar] [CrossRef]
- Canitano, R.; Vivanti, G. Tics and Tourette Syndrome in Autism Spectrum Disorders. Autism 2007, 11, 19–28. [Google Scholar] [CrossRef]
- Mesleh, A.G.; Abdulla, S.A.; El-Agnaf, O. Paving the Way toward Personalized Medicine: Current Advances and Challenges in Multi-OMICS Approach in Autism Spectrum Disorder for Biomarkers Discovery and Patient Stratification. J. Pers. Med. 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.; Ball, L.; Hiddink, G.J. Nutrition in Medical Education: A Systematic Review. Lancet Planet. Health 2019, 3, e379–e389. [Google Scholar] [CrossRef] [PubMed]
- Antoniazzi, L.; Arroyo-Olivares, R.; Bittencourt, M.S.; Tada, M.T.; Lima, I.; Jannes, C.E.; Krieger, J.E.; Pereira, A.C.; Quintana-Navarro, G.; Muñiz-Grijalvo, O.; et al. Adherence to a Mediterranean Diet, Dyslipidemia and Inflammation in Familial Hypercholesterolemia. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2014–2022. [Google Scholar] [CrossRef] [PubMed]




| Improved Symptoms | Adverse Effects |
|---|---|
| General benefit (no one particular symptom) Aggression/agitation Anxiety Attention Cognition (ability to think) Constipation Depression Diarrhea Eczema/skin problem Health (fewer illnesses and/or less severe illnesses) Hyperactivity Irritability Language/communication Lethargy (easily tired) OCD Reflux/vomiting Seizures Self-injury Sensory sensitivity Sleep (falling asleep) Sleep (staying asleep) Social interaction and understanding Stimming/perseveration/desire for sameness Tics/abnormal movements Other (with a write-in option) | General worsening (no one specific symptom) Aggression/agitation Anxiety Bedwetting/bladder control Behavior problems Decreased cognition (difficulty thinking/remembering) Depression Dizziness/unsteadiness Dry mouth Fatigue/drowsiness Gastrointestinal problems Headache/migraine Irritability Liver/kidney problem Loss of appetite Nausea Rash Seizures Self-injurious Sleep problems Stimming/perseveration/desire for sameness Tics/abnormal movements Weight gain Weight loss Other (with a write-in option) |
| N | % | |
|---|---|---|
| Number of participants | 818 | |
| Partipants that used dietary intervention | 486 | |
| Partipants that did not used dietary intervention | 332 | |
| Age of individual with ASD | ||
| Under 3 years | 21 | 2.6% |
| 3–5 years | 131 | 16% |
| 6–9 years | 181 | 22% |
| 10–12 years | 124 | 15% |
| 13–15 years | 98 | 12% |
| 16–18 years | 60 | 7.3% |
| 19–21 years | 46 | 5.6% |
| 22–30 years | 80 | 9.8% |
| 31–40 years | 30 | 3.7% |
| 41–50 years | 23 | 2.8% |
| 51–60 years | 15 | 1.8% |
| Over 60 years | 8 | 1.0% |
| Gender | ||
| Male | 610 | 75% |
| Female | 203 | 25% |
| Other | 3 | 0.4% |
| Survey filled out by | ||
| Primary caregiver | 711 | 87% |
| High functioning individual with autism, no guardian | 58 | 7.1% |
| Completed by individual with autism, has guardian | 17 | 2.1% |
| Other | 32 | 3.9% |
| Diagnosis | ||
| Autism | 349 | 43% |
| Autism Spectrum Disorder (this is less severe than a diagnosis of Autism) | 199 | 24% |
| Pervasive Developmental Disorder—Not Otherwise Specified (PDDNOS) | 47 | 5.7% |
| High-Functioning Autism | 97 | 12% |
| Asperger’s Syndrome | 119 | 15% |
| Other | 7 | 0.9% |
| Developmental history | ||
| Normal development, followed by major regression | 169 | 21% |
| Normal development, followed by a plateau in development that lasted for several months or longer | 186 | 23% |
| Normal development, followed by a major regression and a plateau lasting several months or longer | 101 | 12% |
| Abnormal development from early infancy, with no major regression or plateau in development | 266 | 33% |
| Other | 91 | 11% |
| Severity at age 3 | ||
| No autistic symptoms | 31 | 3.8% |
| Nearly normal, with only very mild symptoms | 146 | 18% |
| Mild autism | 180 | 22% |
| Moderate autism | 300 | 37% |
| Severe autism | 152 | 19% |
| Severity currently | ||
| No autistic symptoms | 4 | 0.5% |
| Nearly normal, with only very mild symptoms | 123 | 15% |
| Mild autism | 254 | 31% |
| Moderate autism | 309 | 38% |
| Severe autism | 122 | 15% |
| Rounds of oral antibiotics from 0–36 months of age | ||
| Mean/Average | 7 | |
| 1st Quartile | 1 | |
| Median | 3 | |
| 3rd Quartile | 6 | |
| 0 rounds | 101 | 14% |
| 1 round | 135 | 19% |
| 2 rounds | 92 | 11% |
| 3 rounds | 111 | 16% |
| 4 rounds | 41 | 5.8% |
| 5 rounds | 48 | 6.8% |
| 6 rounds | 41 | 5.8% |
| 7 rounds | 15 | 2.1% |
| 8 rounds | 13 | 1.8% |
| 9 rounds | 6 | 0.8% |
| 10–14 rounds | 36 | 5.0% |
| 15–19 rounds | 13 | 1.8% |
| 20–24 rounds | 10 | 1.4% |
| 25–29 rounds | 4 | 0.5% |
| 30+ rounds | 45 | 6.1% |
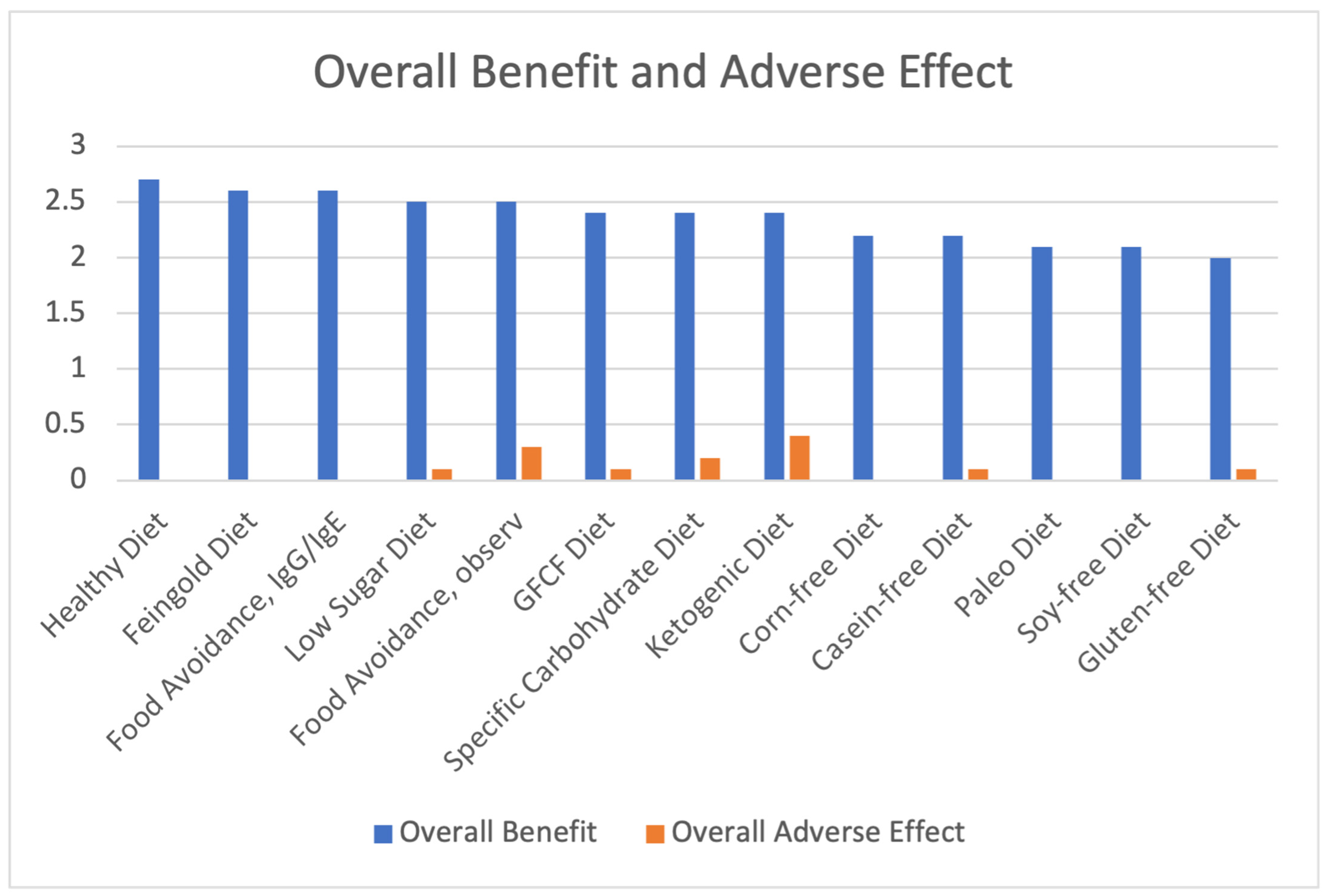
| Diet | Overall Benefit | Overall Adverse Effect | Net Benefit |
|---|---|---|---|
| Healthy Diet | 2.7 | 0 | 2.7 |
| Feingold Diet | 2.6 | 0 | 2.6 |
| Food Avoidance, IgG/IgE | 2.6 | 0 | 2.6 |
| Low Sugar Diet | 2.5 | 0.1 | 2.4 |
| GFCF Diet | 2.4 | 0.1 | 2.3 |
| Food Avoidance, observation | 2.5 | 0.3 | 2.2 |
| Corn-free Diet | 2.2 | 0 | 2.2 |
| Specific Carbohydrate Diet | 2.4 | 0.2 | 2.2 |
| Casein-Free Diet | 2.2 | 0.1 | 2.1 |
| Soy-Free Diet | 2.1 | 0 | 2.1 |
| Paleo Diet | 2.1 | 0 | 2.1 |
| Ketogenic Diet | 2.4 | 0.4 | 2.0 |
| Gluten-free Diet | 2.0 | 0.1 | 1.9 |
| Average of All Diets | 2.36 | 0.10 | 2.26 |
| Diet | Respondents That Used Diet |
|---|---|
| GFCF Diet | 221 |
| Healthy Diet | 179 |
| Casein-Free Diet | 134 |
| Gluten-Free Diet | 114 |
| Low Sugar Diet | 104 |
| Food Avoidance, observation | 82 |
| Feingold Diet | 74 |
| Soy-Free Diet | 62 |
| Food Avoidance, IgG/IgE | 54 |
| Corn-Free Diet | 46 |
| Specific Carbohydrate Diet | 37 |
| Ketogenic Diet | 21 |
| Paleo Diet | 21 |
| Symptom Improvement | Top Diets |
|---|---|
| Aggression |
|
| Anxiety |
|
| Attention |
|
| Cognition |
|
| Constipation |
|
| Depression |
|
| Diarrhea |
|
| Eczema/skin problems |
|
| Health |
|
| Hyperactivity |
|
| Irritability |
|
| Language/Communication |
|
| Lethargy |
|
| OCD |
|
| Reflux/Vomiting |
|
| Seizures |
|
| Self-injury |
|
| Sensory sensitivity |
|
| Sleep (falling) |
|
| Sleep (staying) |
|
| Social Interaction and Understanding |
|
| Stimming/Perseveration/Desire for Sameness |
|
| Tics |
|
| Therapeutic Diets | Top Symptom Improvement (% of Participants with Improvement) |
|---|---|
| Healthy Diet | General benefit 67% Health 24% Constipation 17% Attention 12% Cognition 12% Irritability 12% Anxiety 11% Hyperactivity 11% Diarrhea 10% Sleep (falling) 10% |
| Feingold Diet | Hyperactivity 45% General benefit 41% Irritability 38% Attention 37% Aggression/Agitation 34% Anxiety 28% Cognition 28% Sensory sensitivity 22% Health 19% Sleep (falling) 19% Sleep (staying) 15% Constipation 14% Language/Communication 14% Social Interaction and Understanding 12% Eczema/Skin problem 11% Stimming/Perseveration/Desire for Sameness 11% |
| Food Avoidance Diet, Based on IgG/IgE Testing | General benefit 43% Eczema/Skin problem 22% Health 15% Reflux/vomiting 13% Sensory sensitivity 13% Anxiety 11% Attention 11% Cognition 11% Constipation 11% Hyperactivity 11% Irritability 11% |
| Low Sugar Diet | Hyperactivity 43% General benefit 39% Attention 25% Aggression/Agitation 23% Irritability 23% Health 20% Anxiety 18% Cognition 18% Sensory sensitivity 11% Sleep (falling) 11% Language/Communication 10% Sleep (staying) 10% |
| GFCF Diet | General benefit 39% Attention 31% Cognition 29% Language/Communication 25% Diarrhea 22% Hyperactivity 22% Social Interaction and Understanding 22% Eczema/Skin problem 20% Health 20% Irritability 20% Constipation 19% Sensory sensitivity 19% Stimming/Perseveration/Desire for Sameness 19% Aggression/Agitation 17% Anxiety 15% Sleep (falling) 11% Sleep (staying) 10% |
| Food Avoidance Diet, Based on Observation | General benefit 32% Health 24% Irritability 23% Attention 22% Eczema/Skin problem 22% Aggression/Agitation 21% Diarrhea 21% Cognition 18% Hyperactivity 18% Constipation 17% Sensory sensitivity 17% Anxiety 16% Reflux/vomiting 16% Stimming/Perseveration/Desire for Sameness 13% Language/Communication 12% Depression 11% Sleep (falling) 11% Sleep (staying) 11% Social Interaction and Understanding 11% Lethargy (easily tired) 10% |
| Corn-Free Diet | General benefit 44% Diarrhea 26% Constipation 22% Aggression/Agitation 17% Anxiety 11% Cognition 11% Eczema/Skin problem 11% Hyperactivity 11% |
| Specific Carbohydrate Diet | General benefit 57% Attention 24% Cognition 22% Anxiety 19% Diarrhea 19% Health 16% Irritability 16% Language/Communication 14% Social Interaction and Understanding 14% Stimming/Perseveration/Desire for Sameness 14% Aggression/Agitation 11% Hyperactivity 11% Sensory sensitivity 11% |
| Casein-Free Diet | General benefit 28% Cognition 17% Attention 16% Constipation 16% Aggression/Agitation 14% Diarrhea 14% Eczema/Skin problem 13% Health 13% Language/Communication 13% Hyperactivity 11% Anxiety 10% Irritability 10% Social Interaction and Understanding 10% |
| Soy-Free Diet | General benefit 45% Diarrhea 16% Health 11% Aggression/Agitation 10% Irritability 10% |
| Paleo Diet | General benefit 57% Attention 19% Constipation 19% Irritability 19% Anxiety 14% Cognition 14% Diarrhea 14% Health 14% Sensory sensitivity 14% Aggression/Agitation 10% Depression 10% Eczema/Skin problem 10% Hyperactivity 10% Language/Communication 10% Lethargy 10% OCD 10% Reflux/vomiting 10% Self-injury 10% Sleep (falling) 10% Sleep (staying) 10% Social Interaction and Understanding 10% Stimming/Perseveration/Desire for Sameness 10% Tics/abnormal movements 10% |
| Ketogenic Diet | Attention 43% General benefit 38% Cognition 38% Anxiety 33% Irritability 29% Language/Communication 29% Social Interaction and Understanding 29% Constipation 24% Lethargy 19% Sleep (falling) 19% Seizures 19% Aggression/Agitation 19% Health 19% Stimming/Perseveration/Desire for Sameness 19% Depression 14% Diarrhea 14% Eczema/Skin problem 14% Hyperactivity 14% Sleep (staying) 14% OCD 10% Reflux/vomiting 10% Sensory sensitivity 10% Self-injury 10% |
| Gluten-Free Diet | General benefit 36% Attention 18% Cognition 18% Diarrhea 18% Irritability 16% Constipation 15% Language/Communication 15% Aggression/Agitation 12% Health 12% Anxiety 11% Eczema/Skin problem 11% Social Interaction and Understanding 11% Stimming/Perseveration/Desire for Sameness 11% Hyperactivity 10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthews, J.S.; Adams, J.B. Ratings of the Effectiveness of 13 Therapeutic Diets for Autism Spectrum Disorder: Results of a National Survey. J. Pers. Med. 2023, 13, 1448. https://doi.org/10.3390/jpm13101448
Matthews JS, Adams JB. Ratings of the Effectiveness of 13 Therapeutic Diets for Autism Spectrum Disorder: Results of a National Survey. Journal of Personalized Medicine. 2023; 13(10):1448. https://doi.org/10.3390/jpm13101448
Chicago/Turabian StyleMatthews, Julie S., and James B. Adams. 2023. "Ratings of the Effectiveness of 13 Therapeutic Diets for Autism Spectrum Disorder: Results of a National Survey" Journal of Personalized Medicine 13, no. 10: 1448. https://doi.org/10.3390/jpm13101448
APA StyleMatthews, J. S., & Adams, J. B. (2023). Ratings of the Effectiveness of 13 Therapeutic Diets for Autism Spectrum Disorder: Results of a National Survey. Journal of Personalized Medicine, 13(10), 1448. https://doi.org/10.3390/jpm13101448






