The Role of Nodes and Nodal Assessment in Diagnosis, Treatment and Prediction in ER+, Node-Positive Breast Cancer
Abstract
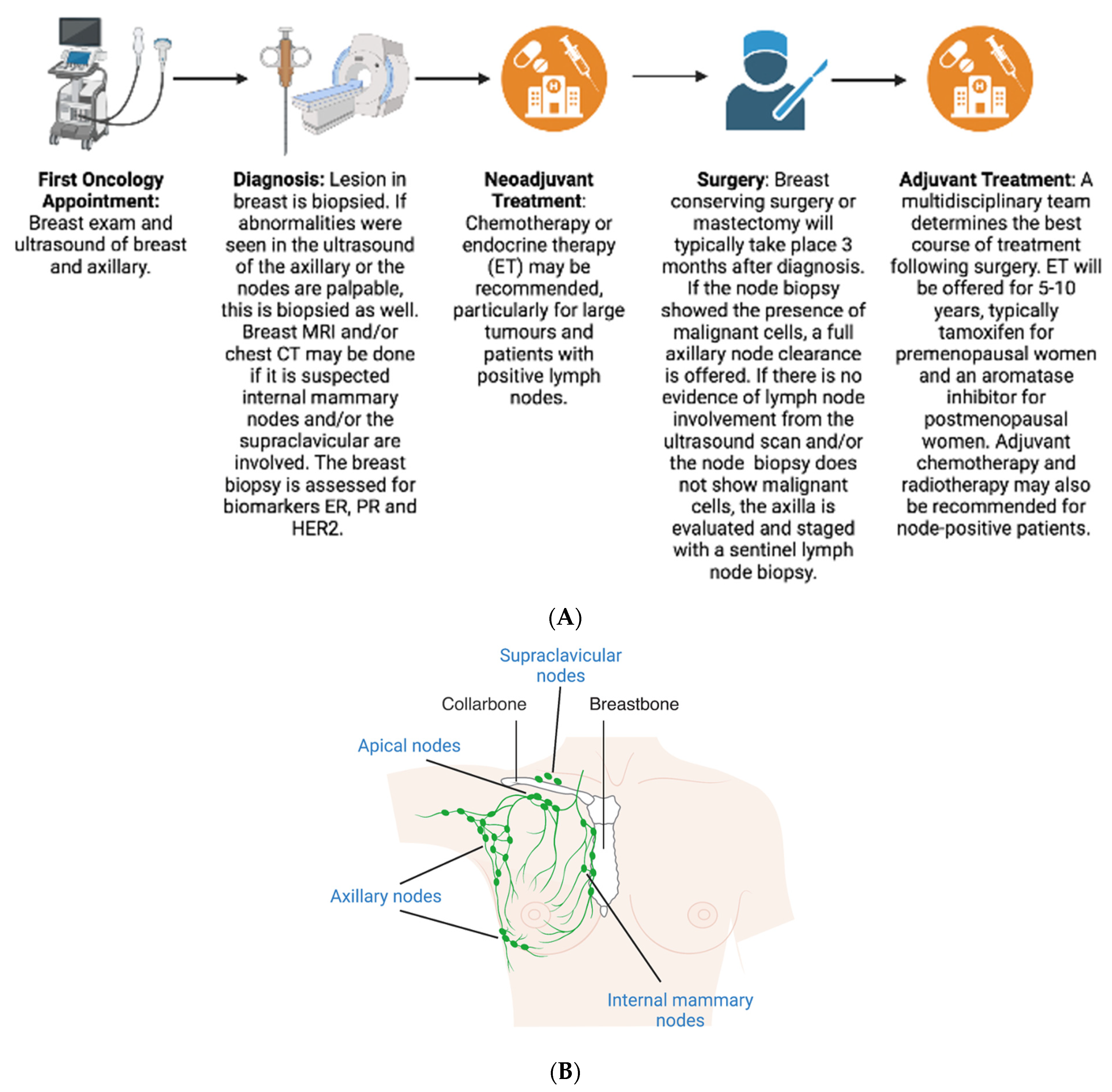
:1. Background
2. Diagnosis
3. Pathological Assessment
4. Treatment
4.1. Neoadjuvant
4.2. Surgery
4.3. Adjuvant
5. Prognosis
6. Prediction
6.1. Predict
6.2. Oncotype DX
6.3. MammaPrint
6.4. PAM50 (Prosigna)
6.5. EndoPredict
6.6. Breast Cancer Index
6.7. IHC4
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NIH National Cancer Institute. Cancer Stat Facts: Female Breast Cancer. 2022. Available online: http://seer.cancer.gov/statfacts/html/breast.html#survival (accessed on 20 March 2023).
- Cancer Research UK. Breast Cancer Statistics. 2018. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer (accessed on 18 March 2023).
- World Health Organization. Breast Cancer. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 18 March 2023).
- Kohler, B.A.; Sherman, R.L.; Howlader, N.; Jemal, A.; Ryerson, A.B.; Henry, K.A.; Boscoe, F.P.; Cronin, K.A.; Lake, A.; Noone, A.M.; et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl. Cancer Inst. 2015, 107, djv048. [Google Scholar]
- Konecny, G.; Pauletti, G.; Pegram, M.; Untch, M.; Dandekar, S.; Aguilar, Z.; Wilson, C.; Rong, H.-M.; Bauerfeind, I.; Felber, M.; et al. Quantitative Association Between HER-2/neu and Steroid Hormone Receptors in Hormone Receptor-Positive Primary Breast Cancer. JNCI J. Natl. Cancer Inst. 2003, 95, 142–153. [Google Scholar] [PubMed]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.G.; Cronin, K.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst. 2014, 106, dju055. [Google Scholar] [PubMed]
- Kennecke, H.F.; Olivotto, I.A.; Speers, C.; Norris, B.; Chia, S.K.; Bryce, C.; Gelmon, K.A. Late risk of relapse and mortality among postmenopausal women with estrogen responsive early breast cancer after 5 years of tamoxifen. Ann. Oncol. 2007, 18, 45–51. [Google Scholar]
- NIH National Cancer Institute. Sentinel Lymph Node Biopsy Fact Sheet. 2019. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/staging/sentinel-node-biopsy-fact-sheet (accessed on 20 March 2023).
- Chang, J.M.; Leung, J.W.T.; Moy, L.; Ha, S.M.; Moon, W.K. Axillary nodal evaluation in breast cancer: State of the art. Radiology 2020, 295, 500–515. [Google Scholar] [PubMed]
- National Institute for Health Care Excellence. Early and Locally Advanced Breast Cancer: Diagnosis and Management [NG101]; NICE [NG101]2018; National Institute for Health Care Excellence: London, UK, 2018. [Google Scholar]
- Dixon, J.M.; Cartlidge, C.W.J. Twenty-five years of change in the management of the axilla in breast cancer. Breast J. 2020, 26, 22–26. [Google Scholar] [PubMed]
- Veronesi, U.; Paganelli, G.; Viale, G.; Luini, A.; Zurrida, S.; Galimberti, V.I.M.; Veronesi, P.; Robertson, C.; Maisonneuve, P.; Renne, G. A Randomized Comparison of Sentinel-Node Biopsy with Routine Axillary Dissection in Breast Cancer. N. Engl. J. Med. 2003, 349, 546–553. [Google Scholar]
- Magnoni, F.; Galimberti, V.; Corso, G.; Intra, M.; Sacchini, V.; Veronesi, P. Axillary surgery in breast cancer: An updated historical perspective. Semin. Oncol. 2020, 47, 341–352. [Google Scholar]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA J. Am. Med. Assoc. 2017, 318, 918–926. [Google Scholar]
- Dixon, J.M.; Grewar, J.; Twelves, D.; Graham, A.; Martinez-Perez, C.; Turnbull, A. Factors affecting the number of sentinel lymph nodes removed in patients having surgery for breast cancer. Breast Cancer Res. Treat. 2020, 184, 335–343. [Google Scholar]
- Chagpar, A.; Martin III, R.C.; Chao, C.; Wong, S.L.; Edwards, M.J.; Tuttle, T.; McMasters, K.M. Validation of Subareolar and Periareolar Injection Techniques for Breast Sentinel Lymph Node Biopsy. Arch. Surg. 2004, 139, 614–620. [Google Scholar]
- Bonneau, C.; Bendifallah, S.; Reyal, F.; Rossi, L.; Rouzier, R. Association of the number of sentinel lymph nodes harvested with survival in breast cancer. Eur. J. Surg. Oncol. (EJSO) 2015, 41, 52–58. [Google Scholar]
- Choosing, W. Don’t Routinely Use Sentinel Node Biopsy in Clinically Node Negative Women ≥ 70 Years of Age with Early Stage Hormone Receptor Positive, HER2 Negative Invasive Breast Cancer. 2021. Available online: https://www.choosingwisely.org/clinician-lists/sso-sentinel-node-biopsy-in-node-negative-women-70-and-over/ (accessed on 21 March 2023).
- Matar, R.; Barrio, A.V.; Sevilimedu, V.; Le, T.; Heerdt, A.; Morrow, M.; Tadros, A. Can We Successfully De-Escalate Axillary Surgery in Women Aged ≥ 70 Years with Ductal Carcinoma in Situ or Early-Stage Breast Cancer Undergoing Mastectomy? Ann. Surg. Oncol. 2022, 29, 2263–2272. [Google Scholar]
- Chagpar, A.B.; McMasters, K.M.; Edwards, M.J. Can Sentinel Node Biopsy Be Avoided in Some Elderly Breast Cancer Patients? Ann. Surg. 2009, 249, 455–460. [Google Scholar] [PubMed]
- Hieken, T.J. Sentinel Lymph Node & Axillary Lymph Node Procedures for Breast Cancer at Mayo Clinic. 2022. Available online: https://www.mayoclinic.org/sentinel-lymph-node-axillary-lymph-node-procedures-for-breast-cancer-at-mayo-clinic/vid-20535475 (accessed on 21 March 2023).
- Lyman, G.H.; Temin, S.; Edge, S.B.; Newman, L.A.; Turner, R.R.; Weaver, D.L.; Benson, A.B.; Bosserman, L.D.; Burstein, H.J.; Cody, H.; et al. Sentinel Lymph Node Biopsy for Patients with Early-Stage Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2014, 32, 1365–1383. [Google Scholar] [PubMed]
- Nasser, I.A.; Lee, A.K.; Bosari, S.; Saganich, R.; Heatley, G.; Silverman, M.L. Occult axillary lymph node metastases in “node-negative” breast carcinoma. Hum. Pathol. 1993, 24, 950–957. [Google Scholar] [PubMed]
- Huvos, A.G.; Hutter, R.V.; Berg, J.W. Significance of axillary macrometastases and micrometastases in mammary cancer. Ann. Surg. 1971, 173, 44–46. [Google Scholar] [PubMed]
- Fisher, E.R.; Palekar, A.; Rockette, H.; Redmond, C.; Fisher, B. Pathologic findings from the National Surgical Adjuvant Breast Project (Protocol No. 4). V. Significance of axillary nodal micro- and macrometastases. Cancer 1978, 42, 2032–2038. [Google Scholar] [PubMed]
- Apple, S.K. Sentinel Lymph Node in Breast Cancer: Review Article from a Pathologist’s Point of View. J. Pathol. Transl. Med. 2016, 50, 83–95. [Google Scholar]
- Balla, A.; Weaver, D.L. Pathologic Evaluation of Lymph Nodes in Breast Cancer: Contemporary Approaches and Clinical Implications. Surg. Pathol. Clin. 2022, 15, 15–27. [Google Scholar]
- Dumitru, D.; Ghanakumar, S.; Provenzano, E.; Benson, J.R. A Prospective Study Evaluating the Accuracy of Indocyanine Green (ICG) Fluorescence Compared with Radioisotope for Sentinel Lymph Node (SLN) Detection in Early Breast Cancer. Ann. Surg. Oncol. 2022, 29, 3014–3020. [Google Scholar] [PubMed]
- Zhao, D.; Xu, M.; Yang, S.; Ma, H.; Li, H.; Wu, R.; He, Y.; Wang, S.; Liang, X. Specific diagnosis of lymph node micrometastasis in breast cancer by targeting activatable near-infrared fluorescence imaging. Biomaterials 2022, 282, 121388. [Google Scholar]
- Houvenaeghel, G.; de Nonneville, A.; Cohen, M.; Chopin, N.; Coutant, C.; Reyal, F.; Mazouni, C.; Gimbergues, P.; Azuar, A.S.; Chauvet, M.P.; et al. Lack of prognostic impact of sentinel node micro-metastases in endocrine receptor-positive early breast cancer: Results from a large multicenter cohort. ESMO Open 2021, 6, 100151. [Google Scholar]
- Merfeld, E.C.; Burr, A.R.; Brickson, C.; Neuman, H.B.; Anderson, B.M. De-escalating Locoregional Therapy for Axillary Micrometastases in Breast Cancer: How Much is Too Much? Clin. Breast Cancer 2022, 22, 336–342. [Google Scholar] [PubMed]
- American Cancer Society. Lymph Node Surgery for Breast Cancer. 2023. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/surgery-for-breast-cancer/lymph-node-surgery-for-breast-cancer.html (accessed on 17 March 2023).
- Giuliano, A.E.; Edge, S.B.; Hortobagyi, G.N. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann. Surg. Oncol. 2018, 25, 1783–1785. [Google Scholar] [PubMed]
- Nemoto, T.; Vana, J.; Bedwani, R.N.; Baker, H.W.; McGregor, F.H.; Murphy, G.P. Management and survival of female breast cancer: Results of a national survey by the American college of surgeons. Cancer 1980, 45, 2917–2924. [Google Scholar] [PubMed]
- Fisher, B.; Bauer, M.; Wickerham, D.L.; Redmond, C.K.; Fisher, E.R.; Cruz, A.B.; Foster, R.; Gardner, B.; Lerner, H.; Margolese, R.; et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 1983, 52, 1551–1557. [Google Scholar]
- Carter, C.L.; Allen, C.; Henson, D.E. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989, 63, 181–187. [Google Scholar] [PubMed]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar]
- Tonellotto, F.; Bergmann, A.; de Souza Abrahão, K.; de Aguiar, S.S.; Bello, M.A.; Thuler, L.C.S. Impact of Number of Positive Lymph Nodes and Lymph Node Ratio on Survival of Women with Node-Positive Breast Cancer. Eur. J. Breast Health 2019, 15, 76–84. [Google Scholar]
- Soran, A.; Ozmen, T.; Salamat, A.; Soybir, G.; Johnson, R. Lymph Node Ratio (LNR): Predicting Prognosis after Neoadjuvant Chemotherapy (NAC) in Breast Cancer Patients. Eur. J. Breast Health 2019, 15, 249–255. [Google Scholar]
- Ai, X.; Liao, X.; Wang, M.; Hu, Y.; Li, J.; Zhang, Y.; Tang, P.; Jiang, J. Prognostic Value of Lymph Node Ratio in Breast Cancer Patients with Adequate Pathologic Evidence after Neoadjuvant Chemotherapy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e922420-1. [Google Scholar]
- Sakin, A.; Aldemir, M.N. Lymph Node Ratio Predicts Long-Term Survival in Lymph Node-Positive Breast Cancer. Eur. J. Breast Health 2020, 16, 270–275. [Google Scholar] [PubMed]
- Nicolini, A.; Ferrari, P.; Duffy, M.J. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin. Cancer Biol. 2018, 52, 56–73. [Google Scholar]
- Zardavas, D.; Irrthum, A.; Swanton, C.; Piccart, M. Clinical management of breast cancer heterogeneity. Nat. Rev. Clin. Oncol. 2015, 12, 381–394. [Google Scholar] [PubMed]
- Martínez-Pérez, C.; Turnbull, A.K.; Dixon, J.M. The evolving role of receptors as predictive biomarkers for metastatic breast cancer. Expert Rev. Anticancer Ther. 2019, 19, 121–138. [Google Scholar] [PubMed]
- Aitken, S.J.; Thomas, J.S.; Langdon, S.P.; Harrison, D.J.; Faratian, D. Quantitative analysis of changes in, E.R.; PR and HER2 expression in primary breast cancer and paired nodal metastases. Ann. Oncol. 2010, 21, 1254–1261. [Google Scholar] [PubMed]
- Barrio, A.V.; Montagna, G.; Mamtani, A.; Sevilimedu, V.; Edelweiss, M.; Capko, D.; Cody Iii, H.S.; El-Tamer, M.; Gemignani, M.L.; Heerdt, A.; et al. Nodal Recurrence in Patients with Node-Positive Breast Cancer Treated with Sentinel Node Biopsy Alone after Neoadjuvant Chemotherapy—A Rare Event. JAMA Oncol. 2021, 7, 1851–1855. [Google Scholar]
- Cao, L.; Sugumar, K.; Keller, E.; Li, P.; Rock, L.; Simpson, A.; Freyvogel, M.; Montero, A.J.; Shenk, R.; Miller, M.E. Neoadjuvant Endocrine Therapy as an Alternative to Neoadjuvant Chemotherapy among Hormone Receptor-Positive Breast Cancer Patients: Pathologic and Surgical Outcomes. Ann Surg Oncol 2021, 28, 5730–5741. [Google Scholar]
- Stafford, A.; Williams, A.; Edmiston, K.; Cocilovo, C.; Cohen, R.; Bruce, S.; Yoon-Flannery, K.; De La Cruz, L. Axillary Response in Patients Undergoing Neoadjuvant Endocrine Treatment for Node-Positive Breast Cancer: Systematic Literature Review and NCDB Analysis. Ann. Surg. Oncol. 2020, 27, 4669–4677. [Google Scholar] [PubMed]
- Solá, M.; Alberro, J.A.; Fraile, M.; Santesteban, P.; Ramos, M.; Fabregas, R.; Moral, A.; Ballester, B.; Vidal, S. Complete Axillary Lymph Node Dissection Versus Clinical Follow-up in Breast Cancer Patients with Sentinel Node Micrometastasis: Final Results from the Multicenter Clinical Trial AATRM 048/13/2000. Ann. Surg. Oncol. 2013, 20, 120–127. [Google Scholar] [PubMed]
- Zhang, J.; Wang, C. Axillary radiotherapy: An alternative treatment option for adjuvant axillary management of breast cancer. Sci. Rep. 2016, 6, 26304. [Google Scholar] [PubMed]
- Li, L.; Chang, B.; Jiang, X.; Fan, X.; Li, Y.; Li, T.; Wu, S.; Zhang, J.; Kariminia, S.; Li, Q. Clinical outcomes comparison of 10 years versus 5 years of adjuvant endocrine therapy in patients with early breast cancer. BMC Cancer 2018, 18, 977. [Google Scholar]
- Lei, J.T.; Anurag, M.; Haricharan, S.; Gou, X.; Ellis, M.J. Endocrine therapy resistance: New insights. Breast 2019, 48 (Suppl. S1), S26–S30. [Google Scholar] [PubMed]
- Anurag, M.; Ellis, M.J.; Haricharan, S. DNA damage repair defects as a new class of endocrine treatment resistance driver. Oncotarget 2018, 9, 36252. [Google Scholar] [PubMed]
- Wu, X.; Baig, A.; Kasymjanova, G.; Kafi, K.; Holcroft, C.; Mekouar, H.; Carbonneau, A.; Bahoric, B.; Sultanem, K.; Muanza, T. Pattern of Local Recurrence and Distant Metastasis in Breast Cancer By Molecular Subtype. Cureus 2016, 8, e924. [Google Scholar]
- Jung, S.U.; Sohn, G.; Kim, J.; Chung, I.Y.; Lee, J.W.; Kim, H.J.; Ko, B.S.; Son, B.H.; Ahn, S.H.; Yang, S.W.; et al. Survival outcome of adjuvant endocrine therapy alone for patients with lymph node-positive, hormone-responsive, HER2-negative breast cancer. Asian J. Surg. 2019, 42, 914–921. [Google Scholar]
- Malam, Y.; Rabie, M.; Geropantas, K.; Alexander, S.; Pain, S.; Youssef, M. The impact of Oncotype DX testing on adjuvant chemotherapy decision making in 1–3 node positive breast cancer. Cancer Rep. 2022, 5, e1546. [Google Scholar]
- Goldvaser, H.; Amir, E. Role of Bisphosphonates in Breast Cancer Therapy. Curr. Treat. Options Oncol. 2019, 20, 26. [Google Scholar]
- Matikas, A.; Foukakis, T.; Swain, S.; Bergh, J. Avoiding over- and undertreatment in patients with resected node-positive breast cancer with the use of gene expression signatures: Are we there yet? Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1044–1050. [Google Scholar]
- Williams, J. Sentinel Node Biopsy: Breast Cancer Lymph Node Surgery. The True South Through My Eyes—HK Edgerton 2018. Available online: https://www.youtube.com/watch?v=_02F3PnhrFc (accessed on 21 March 2023).
- Wishart, G.C.; Azzato, E.M.; Greenberg, D.C.; Rashbass, J.; Kearins, O.; Lawrence, G.; Caldas, C.; Pharoah, P.D. PREDICT: A new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res. 2010, 12, R1. [Google Scholar]
- UK National Health Service. Predict Tool v2.2 2020. Available online: https://breast.predict.nhs.uk/tool (accessed on 16 February 2023).
- Cao, L.; Stabellini, N.; Towe, C.W.; Miller, M.E.; Shenk, R.; Amin, A.L.; Montero, A.J. BPI22-014: Independent Validation of the PREDICT Prognostication Tool in U.S. Breast Cancer Patients Using the National Cancer Database (NCDB). J. Natl. Compr. Cancer Netw. 2022, 20, BPI22-014. [Google Scholar]
- Candido dos Reis, F.J.; Wishart, G.C.; Dicks, E.M.; Greenberg, D.; Rashbass, J.; Schmidt, M.K.; van den Broek, A.J.; Ellis, I.O.; Green, A.; Rakha, E.; et al. An updated PREDICT breast cancer prognostication and treatment benefit prediction model with independent validation. Breast Cancer Res. 2017, 19, 58. [Google Scholar] [PubMed]
- Gray, E.; Marti, J.; Brewster, D.H.; Wyatt, J.C.; Hall, P.S. Independent validation of the PREDICT breast cancer prognosis prediction tool in 45,789 patients using Scottish Cancer Registry data. Br. J. Cancer 2018, 119, 808–814. [Google Scholar]
- Ahles, T.A.; Saykin, A.J. Breast Cancer Chemotherapy-Related Cognitive Dysfunction. Clin. Breast Cancer 2002, 3, S84–S90. [Google Scholar] [PubMed]
- Kim, G.M.; Kim, S.; Park, H.S.; Kim, J.Y.; Nam, S.; Park, S.; Kim, S.I.; Kim, D.Y.; Sohn, J. Chemotherapy-induced irreversible alopecia in early breast cancer patients. Breast Cancer Res. Treat. 2017, 163, 527–533. [Google Scholar]
- Di Nardo, P.; Lisanti, C.; Garutti, M.; Buriolla, S.; Alberti, M.; Mazzeo, R.; Puglisi, F. Chemotherapy in patients with early breast cancer: Clinical overview and management of long-term side effects. Expert Opin. Drug Saf. 2022, 21, 1341–1355. [Google Scholar]
- Paik, S.; Shak, S.; Tang, G.; Kim, C.; Baker, J.; Cronin, M.; Baehner, F.L.; Walker, M.G.; Watson, D.; Park, T.; et al. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. N. Engl. J. Med. 2004, 351, 2817–2826. [Google Scholar]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E.; Dees, E.C.; Goetz, M.P.; Olson, J.A.; et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar]
- Albain, K.S.; Barlow, W.E.; Shak, S.; Hortobagyi, G.N.; Livingston, R.B.; Yeh, I.T.; Ravdin, P.; Bugarini, R.; Baehner, F.L.; Davidson, N.E.; et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010, 11, 55–65. [Google Scholar]
- Kalinsky, K.; Barlow, W.E.; Gralow, J.R.; Meric-Bernstam, F.; Albain, K.S.; Hayes, D.F.; Lin, N.U.; Perez, E.A.; Goldstein, L.J.; Chia, S.K.L.; et al. 21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. N. Engl. J. Med. 2021, 385, 2336–2347. [Google Scholar] [PubMed]
- Roberts, M.C.; Miller, D.P.; Shak, S.; Petkov, V.I. Breast cancer-specific survival in patients with lymph node-positive hormone receptor-positive invasive breast cancer and Oncotype DX Recurrence Score results in the SEER database. Breast Cancer Res. Treat. 2017, 163, 303–310. [Google Scholar]
- Berdunov, V.; Millen, S.; Paramore, A.; Hall, P.; Perren, T.; Brown, R.; Griffin, J.; Reynia, S.; Fryer, N.; Longworth, L. Cost-effectiveness analysis of the Oncotype DX Breast Recurrence Score test in node-positive early breast cancer. J. Med. Econ. 2022, 25, 591–604. [Google Scholar] [PubMed]
- Yordanova, M.; Hassan, S. The Role of the 21-Gene Recurrence Score® Assay in Hormone Receptor-Positive, Node-Positive Breast Cancer: The Canadian Experience. Curr. Oncol. 2022, 29, 2008–2020. [Google Scholar] [PubMed]
- van ‘t Veer, L.J.; Dai, H.; van de Vijver, M.J.; He, Y.D.; Hart, A.A.M.; Mao, M.; Peterse, H.L.; van der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530–536. [Google Scholar] [PubMed]
- van de Vijver, M.J.; He, Y.D.; van ‘t Veer, L.J.; Dai, H.; Hart, A.A.M.; Voskuil, D.W.; Schreiber, G.J.; Peterse, J.L.; Roberts, C.; Marton, M.J.; et al. A Gene-Expression Signature as a Predictor of Survival in Breast Cancer. N. Engl. J. Med. 2002, 347, 1999–2009. [Google Scholar] [PubMed]
- Drukker, C.A.; Bueno-De-Mesquita, J.M.; Retèl, V.P.; Van Harten, W.H.; Van Tinteren, H.; Wesseling, J.; Roumen, R.M.H.; Knauer, M.; Van ‘t Veer, L.J.; Sonke, G.S.; et al. A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int. J. Cancer 2013, 133, 929–936. [Google Scholar]
- Cardoso, F.; van’t Veer, L.J.; Bogaerts, J.; Slaets, L.; Viale, G.; Delaloge, S.; Pierga, J.-Y.; Brain, E.; Causeret, S.; DeLorenzi, M.; et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N. Engl. J. Med. 2016, 375, 717–729. [Google Scholar]
- Knauer, M.; Mook, S.; Rutgers, E.J.T.; Bender, R.A.; Hauptmann, M.; van de Vijver, M.J.; Koornstra, R.H.T.; Bueno-de-Mesquita, J.M.; Linn, S.C.; van ’t Veer, L.J. The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res. Treat. 2010, 120, 655–661. [Google Scholar]
- National Institute for Health Care Excellence. Tumour Profiling Tests to Guide Adjuvant Chemotherapy Decisions in Early Breast Cancer [DG34]; NICE [DG34]2018; National Institute for Health Care Excellence: London, UK, 2018. [Google Scholar]
- Bernard, P.S.; Parker, J.S.; Mullins, M.; Cheung, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar]
- Nielsen, T.O.; Parker, J.S.; Leung, S.; Voduc, D.; Ebbert, M.; Vickery, T.; Davies, S.R.; Snider, J.; Stijleman, I.J.; Reed, J.; et al. A Comparison of PAM50 Intrinsic Subtyping with Immunohistochemistry and Clinical Prognostic Factors in Tamoxifen-Treated Estrogen Receptor–Positive Breast Cancer. Clin. Cancer Res. 2010, 16, 5222–5232. [Google Scholar]
- Wallden, B.; Storhoff, J.; Nielsen, T.; Dowidar, N.; Schaper, C.; Ferree, S.; Liu, S.; Leung, S.; Geiss, G.; Snider, J.; et al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med. Genom. 2015, 8, 54. [Google Scholar]
- Liu, S.; Chapman, J.A.W.; Burnell, M.J.; Levine, M.N.; Pritchard, K.I.; Whelan, T.J.; Rugo, H.S.; Albain, K.S.; Perez, E.A.; Virk, S.; et al. Prognostic and predictive investigation of PAM50 intrinsic subtypes in the NCIC CTG MA.21 phase III chemotherapy trial. Breast Cancer Res. Treat. 2015, 149, 439–448. [Google Scholar] [PubMed]
- Liu, M.C.; Pitcher, B.N.; Mardis, E.R.; Davies, S.R.; Friedman, P.N.; Snider, J.E.; Vickery, T.L.; Reed, J.P.; Deschryver, K.; Singh, B.; et al. PAM50 gene signatures and breast cancer prognosis with adjuvant anthracycline-and taxane-based chemotherapy: Correlative analysis of C9741 (alliance). NPJ Breast Cancer 2016, 2, 15023. [Google Scholar] [PubMed]
- Ohnstad, H.O.; Borgen, E.; Falk, R.S.; Lien, T.G.; Aaserud, M.; Sveli, M.A.T.; Kyte, J.A.; Kristensen, V.N.; Geitvik, G.A.; Schlichting, E.; et al. Prognostic value of PAM50 and risk of recurrence score in patients with early-stage breast cancer with long-term follow-up. Breast Cancer Res. 2017, 19, 120. [Google Scholar] [PubMed]
- Prat, A.; Lluch, A.; Turnbull, A.K.; Dunbier, A.K.; Calvo, L.; Albanell, J.; de la Haba-Rodríguez, J.; Arcusa, A.; Chacón, J.I.; Sánchez-Rovira, P.; et al. A PAM50-Based Chemoendocrine Score for Hormone Receptor–Positive Breast Cancer with an Intermediate Risk of Relapse. Clin. Cancer Res. 2017, 23, 3035–3044. [Google Scholar]
- Pu, M.; Messer, K.; Davies, S.R.; Vickery, T.L.; Pittman, E.; Parker, B.A.; Ellis, M.J.; Flatt, S.W.; Marinac, C.R.; Nelson, S.H.; et al. Research-based PAM50 signature and long-term breast cancer survival. Breast Cancer Res. Treat. 2020, 179, 197–206. [Google Scholar]
- Stein, R.C.; Makris, A.; MacPherson, I.R.; Hughes-Davies, L.; Marshall, A.; Dotchin, G.; Cameron, D.A.; Kiely, B.E.; Tsang, J.; Naume, B.; et al. Optima: Optimal personalised treatment of early breast cancer using multi-parameter analysis, an international randomized trial of tumor gene expression test-directed chemotherapy treatment in a largely node-positive population. J. Clin. Oncol. 2021, 39 (Suppl. S15), TPS599. [Google Scholar]
- OPTIMA Trial Team. OPTIMA Breast Study. 2019. Available online: https://www.optimabreaststudy.com/ (accessed on 24 August 2023).
- Filipits, M.; Rudas, M.; Jakesz, R.; Dubsky, P.; Fitzal, F.; Singer, C.F.; Dietze, O.; Greil, R.; Jelen, A.; Sevelda, P.; et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin. Cancer Res. 2011, 17, 6012–6020. [Google Scholar]
- Dubsky, P.; Brase, J.C.; Jakesz, R.; Rudas, M.; Singer, C.F.; Greil, R.; Dietze, O.; Luisser, I.; Klug, E.; Sedivy, R.; et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br. J. Cancer 2013, 109, 2959–2964. [Google Scholar]
- Constantinidou, A.; Marcou, Y.; Toss, M.S.; Simmons, T.; Bernhisel, R.; Hughes, E.; Probst, B.; Meek, S.; Kakouri, E.; Georgiou, G.; et al. Clinical Validation of EndoPredict in Pre-Menopausal Women with ER-Positive, HER2-Negative Primary Breast Cancer. Clin. Cancer Res. 2022, 28, 4435–4443. [Google Scholar]
- Martin, M.; Brase, J.C.; Ruiz, A.; Prat, A.; Kronenwett, R.; Calvo, L.; Petry, C.; Bernard, P.S.; Ruiz-Borrego, M.; Weber, K.E.; et al. Prognostic ability of EndoPredict compared to research-based versions of the PAM50 risk of recurrence (ROR) scores in node-positive, estrogen receptor-positive, and HER2-negative breast cancer. A GEICAM/9906 sub-study. Breast Cancer Res. Treat. 2016, 156, 81–89. [Google Scholar]
- Almstedt, K.; Mendoza, S.; Otto, M.; Battista, M.J.; Steetskamp, J.; Heimes, A.S.; Krajnak, S.; Poplawski, A.; Gerhold-Ay, A.; Hasenburg, A.; et al. EndoPredict® in early hormone receptor-positive, HER2-negative breast cancer. Breast Cancer Res. Treat. 2020, 182, 137–146. [Google Scholar] [PubMed]
- Bartlett, J.M.S.; Sgroi, D.C.; Treuner, K.; Zhang, Y.; Ahmed, I.; Piper, T.; Salunga, R.; Brachtel, E.F.; Pirrie, S.J.; Schnabel, C.A.; et al. Breast Cancer Index and prediction of benefit from extended endocrine therapy in breast cancer patients treated in the Adjuvant Tamoxifen—To Offer More? (aTTom) trial. Ann. Oncol. 2019, 30, 1776–1783. [Google Scholar] [PubMed]
- Noordhoek, I.; Treuner, K.; Putter, H.; Zhang, Y.; Wong, J.; Meershoek-Klein Kranenbarg, E.; Kranenbarg, E.; Duijm-de Carpentier, M.; van de Velde, C.J.H.; Schnabel, C.A.; et al. Breast Cancer Index Predicts Extended Endocrine Benefit to Individualize Selection of Patients with HR+ Early-stage Breast Cancer for 10 Years of Endocrine Therapy. Clin. Cancer Res. 2021, 27, 311–319. [Google Scholar] [PubMed]
- Liefers, G.-J.; Noordhoek, I.; Zhang, Y.; Sgroi, D.C.; Putter, H.; Treuner, K.; Wong, J.; Meershoek-Klein Kranenbarg, E.; Duijm-de Carpentier, M.; van de Velde, C.; et al. An optimized Breast Cancer Index node-positive (BCIN+) prognostic model for late distant recurrence in patients with hormone receptor positive (HR+) node positive breast cancer. Ann. Oncol. 2021, 32, S23–S24. [Google Scholar]
- Shah, S.; Shaing, C.; Khatib, J.; Lodrigues, W.; Dreadin-Pulliam, J.; Anderson, B.B.; Unni, N.; Farr, D.; Li, H.-C.; Sadeghi, N.; et al. The Utility of Breast Cancer Index (BCI) Over Clinical Prognostic Tools for Predicting the Need for Extended Endocrine Therapy: A Safety Net Hospital Experience. Clin. Breast Cancer 2022, 22, 823–827. [Google Scholar]
- Cuzick, J.; Dowsett, M.; Pineda, S.; Wale, C.; Salter, J.; Quinn, E.; Zabaglo, L.; Mallon, E.; Green, A.R.; Ellis, I.O.; et al. Prognostic Value of a Combined Estrogen Receptor, Progesterone Receptor, Ki-67, and Human Epidermal Growth Factor Receptor 2 Immunohistochemical Score and Comparison with the Genomic Health Recurrence Score in Early Breast Cancer. J. Clin. Oncol. 2011, 29, 4273–4278. [Google Scholar]
- Yeo, B.; Zabaglo, L.; Hills, M.; Dodson, A.; Smith, I.; Dowsett, M. Clinical utility of the IHC4+C score in oestrogen receptor-positive early breast cancer: A prospective decision impact study. Br. J. Cancer 2015, 113, 390–395. [Google Scholar]
- Cheang, M.C.U.; Bliss, J.M.; Viale, G.; Speirs, V.; Palmieri, C.; Shaaban, A.; Lønning, P.E.; Morden, J.; Porta, N.; Jassem, J.; et al. Evaluation of applying IHC4 as a prognostic model in the translational study of Intergroup Exemestane Study (IES): PathIES. Breast Cancer Res. Treat. 2018, 168, 169–178. [Google Scholar]
- Abubakar, M.; Figueroa, J.; Ali, H.R.; Blows, F.; Lissowska, J.; Caldas, C.; Easton, D.F.; Sherman, M.E.; Garcia-Closas, M.; Dowsett, M.; et al. Combined quantitative measures of ER, PR, HER2, and KI67 provide more prognostic information than categorical combinations in luminal breast cancer. Mod. Pathol. 2019, 32, 1244–1256. [Google Scholar] [PubMed]
- Harel, N.; Cheema, S.; Williams, D.; Ireland-Jenkin, K.; Fancourt, T.; Dodson, A.; Yeo, B. The IHC4+C score: An affordable and reproducible non-molecular decision-aid in hormone receptor-positive breast cancer. Does it still hold value for patients in 2020? Asia-Pac. J. Clin. Oncol. 2021, 17, 368–376. [Google Scholar]
- Liu, M.; Tang, S.-X.; Tsang, J.Y.S.; Shi, Y.-J.; Ni, Y.-B.; Law, B.K.B.; Tse, G.M.K. Core needle biopsy as an alternative to whole section in IHC4 score assessment for breast cancer prognostication. J. Clin. Pathol. 2018, 71, 1084. [Google Scholar] [PubMed]
- Jin, L.; Chen, K.; Tan, C.; Li, J.; Luo, J.; Yang, Y.; Li, Y.; Li, S.; Zhu, L.; Hu, Y.; et al. Prognostic Value of Modified IHC4 Score in Patients with Estrogen Receptor-Positive Metastatic Breast Cancer. Oncologist 2020, 25, e1170–e1180. [Google Scholar] [PubMed]
- Yao, Z.-X.; Lu, L.-J.; Wang, R.-J.; Jin, L.-B.; Liu, S.-C.; Li, H.-Y.; Ren, G.-S.; Wu, K.-N.; Wang, D.-L.; Kong, L.-Q. Discordance and clinical significance of ER, PR, and HER2 status between primary breast cancer and synchronous axillary lymph node metastasis. Med. Oncol. 2014, 31, 798. [Google Scholar]
- Georgescu, R.; Boros, M.; Moncea, D.; Bauer, O.; Coros, M.-F.; Oprea, A.; Moldovan, C.; Podoleanu, C.; Stolnicu, S. Discordance Rate in Estrogen Receptor, Progesterone Receptor, HER2 Status, and Ki67 Index Between Primary Unifocal and Multiple Homogenous Breast Carcinomas and Synchronous Axillary Lymph Node Metastases Have an Impact on Therapeutic Decision. Appl. Immunohistochem. Mol. Morphol. AIMM 2018, 26, 533–538. [Google Scholar]
- Porter, C.; Rockey, M.L.; Grauer, D.; Henry, D.W.; Sharma, P.; Fan, F.; Khan, Q.J. Prognostic marker discordance between breast and axilla in patients with early breast cancer. J. Clin. Oncol. 2015, 33 (Suppl. S15), e11618. [Google Scholar]
- Xi, X.; Huang, X.-W.; Yuan, H.-Z.; He, C.; Ni, J.; Yang, F.-L. Biomarker heterogeneity between primary breast cancer and synchronous axillary lymph node metastases. Oncol. Lett. 2020, 20, 273. [Google Scholar]
- Mannell, A.; Nel, C.E.; Smilg, J.S.; Haberfield, J.; Nietz, S.; Candy, G.P. A prospective study of receptor profiles in breast cancer and the ipsilateral axillary lymph node metastases measured simultaneously in treatment naïve cases. S. Afr. J. Surg. 2020, 58, 86–90. [Google Scholar]
- Weydandt, L.; Nel, I.; Kreklau, A.; Horn, L.C.; Aktas, B. Heterogeneity between Core Needle Biopsy and Synchronous Axillary Lymph Node Metastases in Early Breast Cancer Patients-A Comparison of HER2, Estrogen and Progesterone Receptor Expression Profiles during Primary Treatment Regime. Cancers 2022, 14, 1863. [Google Scholar]
- Kao, J.Y.; Tsai, J.H.; Wu, T.Y.; Wang, C.K.; Kuo, Y.L. Receptor discordance and phenotype change in metastatic breast cancer. Asian J. Surg. 2021, 44, 192–198. [Google Scholar]
- Prat, A.; Brase, J.C.; Cheng, Y.; Nuciforo, P.; Pare, L.; Pascual, T.; Martinez, D.; Galvan, P.; Vidal, M.; Adamo, B.; et al. Everolimus plus Exemestane for Hormone Receptor-Positive Advanced Breast Cancer: A PAM50 Intrinsic Subtype Analysis of BOLERO-2. Oncologist 2019, 24, 893–900. [Google Scholar] [PubMed]
- Andre, F.; Ismaila, N.; Allison, K.H.; Barlow, W.E.; Collyar, D.E.; Damodaran, S.; Henry, N.L.; Jhaveri, K.; Kalinsky, K.; Kuderer, N.M.; et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 1816–1837. [Google Scholar] [PubMed]
- Saghatchian, M.; Mook, S.; Pruneri, G.; Viale, G.; Glas, A.M.; Guerin, S.; Cardoso, F.; Piccart, M.; Tursz, T.; Delaloge, S.; et al. Additional prognostic value of the 70-gene signature (MammaPrint®) among breast cancer patients with 4–9 positive lymph nodes. Breast 2013, 22, 682–690. [Google Scholar]
- Li, A.; Keck, J.M.; Parmar, S.; Patterson, J.; Labrie, M.; Creason, A.L.; Johnson, B.E.; Downey, M.; Thomas, G.; Beadling, C.; et al. Characterizing advanced breast cancer heterogeneity and treatment resistance through serial biopsies and comprehensive analytics. NPJ Precis. Oncol. 2021, 5, 28. [Google Scholar]
- Turnbull, A.K.; Arthur, L.M.; Renshaw, L.; Larionov, A.A.; Kay, C.; Dunbier, A.K.; Thomas, J.S.; Dowsett, M.; Sims, A.H.; Michael Dixon, J. Accurate Prediction and Validation of Response to Endocrine Therapy in Breast Cancer. J. Clin. Oncol. 2015, 33, 2270–2278. [Google Scholar] [PubMed]
- Aushev, V.N.; Lee, E.; Zhu, J.; Gopalakrishnan, K.; Li, Q.; Teitelbaum, S.L.; Wetmur, J.; Degli Esposti, D.; Hernandez-Vargas, H.; Herceg, Z.; et al. Novel Predictors of Breast Cancer Survival Derived from miRNA Activity Analysis. Clin. Cancer Res. 2018, 24, 581–591. [Google Scholar]
- Loughman, T.; Barron, S.; Wang, C.-J.A.; Dynoodt, P.; Fender, B.; Lopez-Ruiz, C.; Stapleton, S.; Fabre, A.; Quinn, C.; Nodin, B.; et al. Analytical Validation of a Novel 6-Gene Signature for Prediction of Distant Recurrence in Estrogen Receptor-Positive, HER2-Negative, Early-Stage Breast Cancer. Clin. Chem. 2022, 68, 837–847. [Google Scholar]
- Srour, M.K.; Qu, Y.; Deng, N.; Carlson, K.; Mirocha, J.; Gao, B.; Dadmanesh, F.; Cui, X.; Giuliano, A.E. Gene expression comparison between primary estrogen receptor-positive and triple-negative breast cancer with paired axillary lymph node metastasis. Breast J. 2021, 27, 432–440. [Google Scholar]
| Tumour | ||
| TX | Primary tumour can’t be assessed | |
| T0 | No sign of a primary tumour | |
| Tis | Carcincoma in situ (DCIS or Paget’s disease) | |
| T1 | Tumour ≤ 20 mm. There are 4 subcategories of T1: | |
| T1mi | Tumour ≤ 1mm | |
| T1a | Tumour > 1 mm but not >5 mm | |
| T1b | Tumour > 5 mm but <10 mm | |
| T1c | Tumour > 10 mm but not >20 mm | |
| T2 | Tumour > 20 mm but not >50 mm | |
| T3 | Tumour > 50 mm | |
| T4 | There are 4 subcategories of T4: | |
| T4a | Tumour has spread into the chest wall | |
| T4b | Tumour has spread into the skin | |
| T4c | Tumour has spread into both the chest wall and the skin | |
| T4d | Inflammatory breast cancer | |
| Node | ||
| NX | Lymph nodes can’t be assessed | |
| N0 | No cancer in the lymph nodes or only clusters of cancer cells < 0.2 mm | |
| N1 | Cancer is described as one of the following: | |
| N1mi | Cancer has spread to the axillary nodes and is >0.2 mm but not >2 mm | |
| N1a | Cancer has spread to 1–3 axillary nodes and is >2 mm | |
| N1b | Cancer has spread to internal mammary nodes, is >0.2 mm and found by SLNB | |
| N1c | Both N1a and N1b are true | |
| N2 | Cancer has spread to 4–9 axillary nodes or cancer has enlarged the internal mammary lymph nodes | |
| N2a | Cancer has spread to 4–9 axillary nodes and is >2 mm | |
| N2b | Cancer has spread to mammary nodes and the cancer was found by imaging tests | |
| N3 | ||
| N3a | Cancer has spread to ≥ 10 axillary nodes and the cancer in at least one is >2 mm or the cancer has spread to the infraclavicular nodes | |
| N3b | Cancer has spread to 1–9 axillary nodes, the cancer in at least one of the lymph nodes is >2 mm and the cancer is found on imaging tests. Alternatively, the cancer has spread to 4–9 axillary nodes, cancer in at least one of the nodes is >2 mm, cancer has also spread to the internal mammary nodes and the cancer is >0.2 mm and found by SLNB | |
| N3c | Cancer has spread to the supraclavicular nodes with at least one area of cancer spread > 2 mm | |
| Metastasis | ||
| M0 | No sign the cancer has spread to other parts of the body | |
| M1 | Cancer has spread to other parts of the body, most often the bones, lungs, liver or brain | |
| Name of Test | Provider | Number of Genes/Biomarkers in Test | Type of Test | Sample Required | Patient Group | Accurate with Positive Nodes? | Score | Risk Groups | Predictions | Prognostic Information |
|---|---|---|---|---|---|---|---|---|---|---|
| Oncotype DX | Exact Sciences | 21 | RT-qPCR | RNA from FFPE tumour tissue | Pre and post menopausal women with early HR+/HER2- breast cancer | Yes (1–3 positive nodes) | Recurrence Score (RS) 0–100 | RS score < 18 is low risk, RS 18–30 intermediate risk & RS > 31 high risk | Benefit from adjuvant chemotherapy | Risk of distant recurrence after 10 years |
| MammaPrint | Agendia | 70 | Microarray | RNA from FFPE tumour tissue | Pre and post menopausal women with early breast cancer smaller than 5cm | Yes (1–3 positive nodes) | MammaPrint score −1 to +1 | Genomic low risk (score of more than 0) or genomic high risk (score of 0 or less) | Benefit from adjuvant chemotherapy | Risk of distant recurrence after 10 years |
| PAM50 (Prosigna) | Veracyte | 50 | mRNA counting on nCounter Digital Analyser (NanoString) | RNA from FFPE tumour tissue | Postmenopausal women with early HR+/HER2- breast cancer who have been on ET for 5 years | Yes (1–3 positive nodes) | Risk of recurrence (ROR) score 0–100 | Node-negative: low risk 0 to 40, intermediate risk 41 to 60 or high risk 61 to 100 Node-positive (up to 3): low risk 0 to 15, intermediate risk 16 to 40, or high risk 41 to 100 | Benefit from adjuvant chemotherapy | Risk of distant recurrence after 10 years |
| EndoPredict (EP)/EPclin | Myriad Genetics | 12 | RT-qPCR | RNA from FFPE tumour tissue | Postmenopausal women with early HR+/HER2 breast cancer who have been on ET for 5 years | Yes (1–3 positive nodes) | EP score | EP score of 0 to <5 low risk, EP 5 to 15 high risk; EPclin < 3.3 low risk, EPclin ≥ 3.3 high risk | Benefit from adjuvant chemotherapy | Risk of distant and late recurrence after 10 and 15 years |
| Breast Cancer Index (BCI) | Biotheranostics | 11 | RT-qPCR | FFPE tumour tissue | Pre and post menopausal women with early HR+/HER2- breast cancer who have been on ET for 5 years | Yes (1–3 positive nodes) | BCI Score (0–10) | Low risk: BCI < 5; intermediate risk: BCI 5–6.4; high risk: BCI > 6.4 | Benefit from extended ET | Risk of distant recurrence after 10 years |
| IHC4/IHC4+C | Not yet available | 4 | IHC staining + alorithm | FFPE tumour tissue | Postmenopausal women with early HR+/HER2- breast cancer who have been on ET for 5 years | Yes (1–3 positive nodes) | IHC4+C score | IHC4+C: low risk < 10%; intermediate risk 10–20%; high risk > 20% | Benefit from adjuvant chemotherapy | Risk of distant recurrence after 10 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kay, C.; Martinez-Perez, C.; Dixon, J.M.; Turnbull, A.K. The Role of Nodes and Nodal Assessment in Diagnosis, Treatment and Prediction in ER+, Node-Positive Breast Cancer. J. Pers. Med. 2023, 13, 1476. https://doi.org/10.3390/jpm13101476
Kay C, Martinez-Perez C, Dixon JM, Turnbull AK. The Role of Nodes and Nodal Assessment in Diagnosis, Treatment and Prediction in ER+, Node-Positive Breast Cancer. Journal of Personalized Medicine. 2023; 13(10):1476. https://doi.org/10.3390/jpm13101476
Chicago/Turabian StyleKay, Charlene, Carlos Martinez-Perez, J. Michael Dixon, and Arran K. Turnbull. 2023. "The Role of Nodes and Nodal Assessment in Diagnosis, Treatment and Prediction in ER+, Node-Positive Breast Cancer" Journal of Personalized Medicine 13, no. 10: 1476. https://doi.org/10.3390/jpm13101476
APA StyleKay, C., Martinez-Perez, C., Dixon, J. M., & Turnbull, A. K. (2023). The Role of Nodes and Nodal Assessment in Diagnosis, Treatment and Prediction in ER+, Node-Positive Breast Cancer. Journal of Personalized Medicine, 13(10), 1476. https://doi.org/10.3390/jpm13101476








