The Importance of Managing Modifiable Comorbidities in People with Multiple Sclerosis: A Narrative Review
Abstract
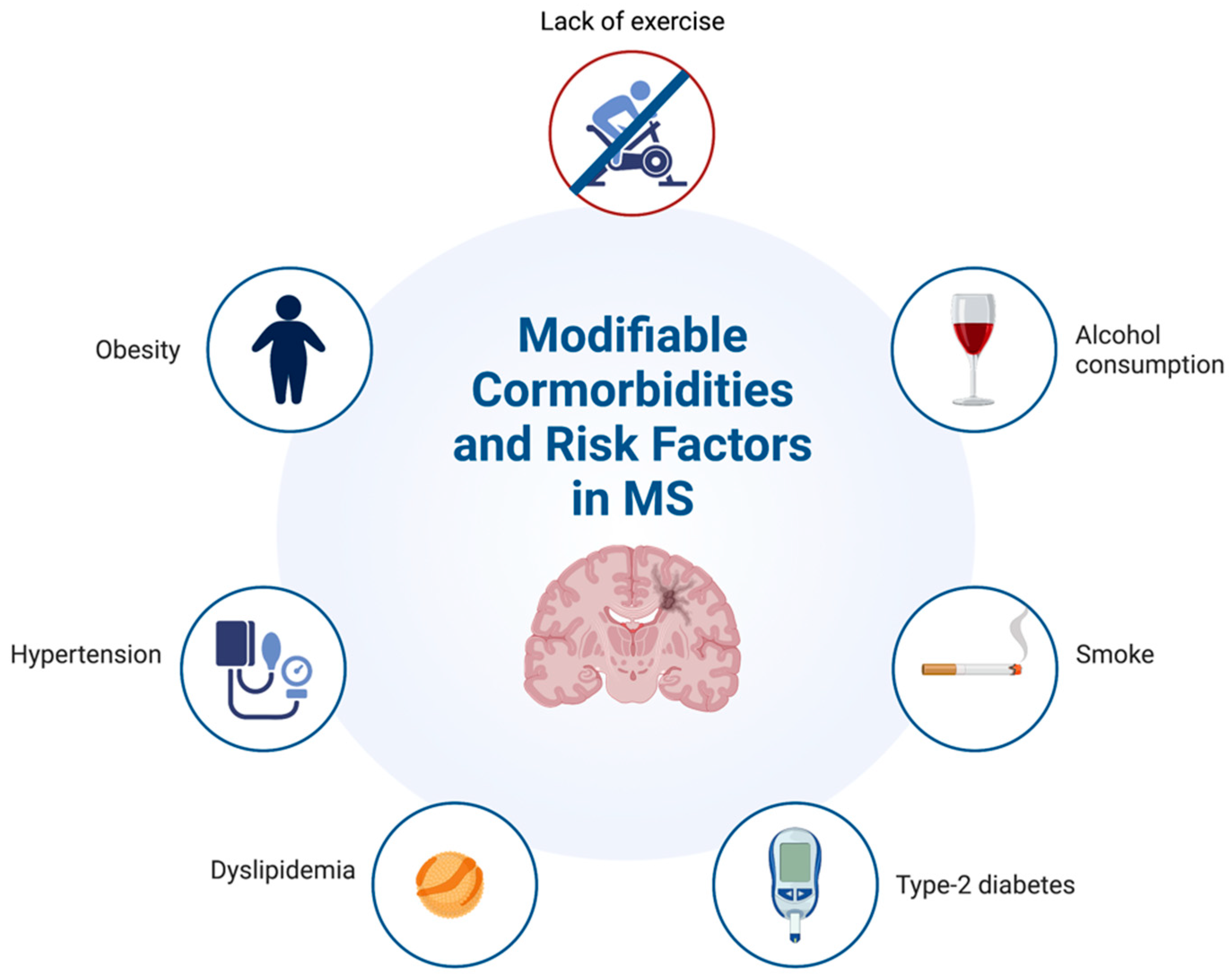
1. Introduction
2. Methods
3. Hypertension
4. Type-2 Diabetes
5. Obesity
6. Smoke
7. Alcohol
8. Dyslipidemia
9. The Aging MS Population and Comorbidities: Implications
Radiological Features of MS and Cerebral Small Vessel Disease (CVSD)
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Naegele, M.; Martin, R. The good and the bad of neuroinflammation in multiple sclerosis. Handb. Clin. Neurol. 2014, 122, 59–87. [Google Scholar] [CrossRef] [PubMed]
- Magyari, M.; Sorensen, P.S. Comorbidity in Multiple Sclerosis. Front. Neurol. 2020, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; Adorini, L.; Rieckmann, P.; Hillert, J.; Kallmann, B.; Comi, G.; Filippi, M. Inflammation in multiple sclerosis: The good, the bad, and the complex. Lancet Neurol. 2002, 1, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Nociti, V.; Romozzi, M. The Role of BDNF in Multiple Sclerosis Neuroinflammation. Int. J. Mol. Sci. 2023, 24, 8447. [Google Scholar] [CrossRef]
- Hohlfeld, R.; Kerschensteiner, M.; Stadelmann, C.; Lassmann, H.; Wekerle, H. The neuroprotective effect of inflammation: Implications for the therapy of multiple sclerosis. J. Neuroimmunol. 2000, 107, 161–166. [Google Scholar] [CrossRef]
- Kalincik, T.; Diouf, I.; Sharmin, S.; Malpas, C.; Spelman, T.; Horakova, D.; Havrdova, E.K.; Trojano, M.; Izquierdo, G.; Lugaresi, A.; et al. Effect of Disease-Modifying Therapy on Disability in Relapsing-Remitting Multiple Sclerosis Over 15 Years. Neurology 2021, 96, e783–e797. [Google Scholar] [CrossRef]
- Amin, M.; Hersh, C.M. Updates and advances in multiple sclerosis neurotherapeutics. Neurodegener. Dis. Manag. 2023, 13, 47–70. [Google Scholar] [CrossRef]
- Valderas, J.M.; Starfield, B.; Sibbald, B.; Salisbury, C.; Roland, M. Defining comorbidity: Implications for understanding health and health services. Ann. Fam. Med. 2009, 7, 357–363. [Google Scholar] [CrossRef]
- Hauer, L.; Perneczky, J.; Sellner, J. A global view of comorbidity in multiple sclerosis: A systematic review with a focus on regional differences, methodology, and clinical implications. J. Neurol. 2021, 268, 4066–4077. [Google Scholar] [CrossRef]
- Marrie, R.A.; Reingold, S.; Cohen, J.; Stuve, O.; Trojano, M.; Sorensen, P.S.; Cutter, G.; Reider, N. The incidence and prevalence of psychiatric disorders in multiple sclerosis: A systematic review. Mult. Scler. J. 2015, 21, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Nociti, V.; Romozzi, M. Multiple Sclerosis and Autoimmune Comorbidities. J. Pers. Med. 2022, 12, 1828. [Google Scholar] [CrossRef] [PubMed]
- Jadidi, E.; Mohammadi, M.; Moradi, T. High risk of cardiovascular diseases after diagnosis of multiple sclerosis. Mult. Scler. 2013, 19, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, T.B.; Berkowitz, A.L.; Samuels, M.A. Cardiovascular Dysfunction in Multiple Sclerosis. Neurologist 2015, 20, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Rudick, R.; Horwitz, R.; Cutter, G.; Tyry, T.; Campagnolo, D.; Vollmer, T. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 2010, 74, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Lo, L.M.P.; Taylor, B.V.; Winzenberg, T.; Palmer, A.J.; Blizzard, L.; van der Mei, I. Change and onset-type differences in the prevalence of comorbidities in people with multiple sclerosis. J. Neurol. 2021, 268, 602–612. [Google Scholar] [CrossRef]
- Kowalec, K.; McKay, K.A.; Patten, S.B.; Fisk, J.D.; Evans, C.; Tremlett, H.; Marrie, R.A. Comorbidity increases the risk of relapse in multiple sclerosis: A prospective study. Neurology 2017, 89, 2455–2461. [Google Scholar] [CrossRef]
- Marrie, R.A. Comorbidity in multiple sclerosis: Implications for patient care. Nat. Rev. Neurol. 2017, 13, 375–382. [Google Scholar] [CrossRef]
- Marrie, R.A.; Horwitz, R.; Cutter, G.; Tyry, T.; Campagnolo, D.; Vollmer, T. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology 2009, 72, 117–124. [Google Scholar] [CrossRef]
- Berrigan, L.I.; Fisk, J.D.; Patten, S.B.; Tremlett, H.; Wolfson, C.; Warren, S.; Fiest, K.M.; McKay, K.A.; Marrie, R.A. Health-related quality of life in multiple sclerosis: Direct and indirect effects of comorbidity. Neurology 2016, 86, 1417–1424. [Google Scholar] [CrossRef]
- Overs, S.; Hughes, C.M.; Haselkorn, J.K.; Turner, A.P. Modifiable comorbidities and disability in multiple sclerosis. Curr. Neurol. Neurosci. Rep. 2012, 12, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Reider, N.; Cohen, J.; Stuve, O.; Trojano, M.; Cutter, G.; Reingold, S.; Sorensen, P.S. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult. Scler. 2015, 21, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Briggs, F.B.S.; Hill, E.; Abboud, H. The prevalence of hypertension in multiple sclerosis based on 37 million electronic health records from the United States. Eur. J. Neurol. 2021, 28, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Briggs, F.B.S.; Thompson, N.R.; Conway, D.S. Prognostic factors of disability in relapsing remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 30, 9–16. [Google Scholar] [CrossRef]
- Edwards, N.C.; Munsell, M.; Menzin, J.; Phillips, A.L. Comorbidity in US patients with multiple sclerosis. Patient Relat. Outcome Meas. 2018, 9, 97–102. [Google Scholar] [CrossRef]
- Saroufim, P.; Zweig, S.A.; Conway, D.S.; Briggs, F.B.S. Cardiovascular conditions in persons with multiple sclerosis, neuromyelitis optica and transverse myelitis. Mult. Scler. Relat. Disord. 2018, 25, 21–25. [Google Scholar] [CrossRef]
- Marrie, R.A.; Kosowan, L.; Singer, A. Management of diabetes and hypertension in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 40, 101987. [Google Scholar] [CrossRef]
- Marrie, R.A.; Patten, S.B.; Tremlett, H.; Wolfson, C.; Warren, S.; Svenson, L.W.; Jette, N.; Fisk, J. Sex differences in comorbidity at diagnosis of multiple sclerosis: A population-based study. Neurology 2016, 86, 1279–1286. [Google Scholar] [CrossRef]
- Lavela, S.L.; Prohaska, T.R.; Furner, S.; Weaver, F.M. Chronic diseases in male veterans with multiple sclerosis. Prev. Chronic Dis. 2012, 9, E55. [Google Scholar] [CrossRef]
- Sternberg, Z.; Leung, C.; Sternberg, D.; Yu, J.; Hojnacki, D. Disease modifying therapies modulate cardiovascular risk factors in patients with multiple sclerosis. Cardiovasc. Ther. 2014, 32, 33–39. [Google Scholar] [CrossRef]
- Allen, N.B.; Lichtman, J.H.; Cohen, H.W.; Fang, J.; Brass, L.M.; Alderman, M.H. Vascular disease among hospitalized multiple sclerosis patients. Neuroepidemiology 2008, 30, 234–238. [Google Scholar] [CrossRef]
- Fleming, S.T.; Blake, R.L., Jr. Patterns of comorbidity in elderly patients with multiple sclerosis. J. Clin. Epidemiol. 1994, 47, 1127–1132. [Google Scholar] [CrossRef]
- Conway, D.S.; Thompson, N.R.; Cohen, J.A. Influence of hypertension, diabetes, hyperlipidemia, and obstructive lung disease on multiple sclerosis disease course. Mult. Scler. 2017, 23, 277–285. [Google Scholar] [CrossRef]
- Chockalingam, A.; Campbell, N.R.; Fodor, J.G. Worldwide epidemic of hypertension. Can. J. Cardiol. 2006, 22, 553–555. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, C.; Rudemiller, N.P.; Abais, J.M.; Mattson, D.L. Inflammation and hypertension: New understandings and potential therapeutic targets. Curr. Hypertens. Rep. 2015, 17, 507. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Yu, B.N.; Leung, S.; Elliott, L.; Caetano, P.; Warren, S.; Wolfson, C.; Patten, S.B.; Svenson, L.W.; Tremlett, H.; et al. Rising prevalence of vascular comorbidities in multiple sclerosis: Validation of administrative definitions for diabetes, hypertension, and hyperlipidemia. Mult. Scler. 2012, 18, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Hussein, W.I.; Reddy, S.S. Prevalence of Diabetes in Patients with Multiple Sclerosis. Diabetes Care 2006, 29, 1984–1985. [Google Scholar] [CrossRef] [PubMed]
- Maric, G.D.; Pekmezovic, T.D.; Mesaros, S.T.; Tamas, O.S.; Ivanovic, J.B.; Martinovic, V.N.; Andabaka, M.M.; Jovanovic, A.L.; Veselinovic, N.D.; Kisic-Tepavcevic, D.B.; et al. The prevalence of comorbidities in patients with multiple sclerosis: Population-based registry data. Neurol. Sci. 2021, 42, 1887–1893. [Google Scholar] [CrossRef]
- Hou, W.H.; Li, C.Y.; Chang, H.H.; Sun, Y.; Tsai, C.C. A population-based cohort study suggests an increased risk of multiple sclerosis incidence in patients with type 2 diabetes mellitus. J. Epidemiol. 2017, 27, 235–241. [Google Scholar] [CrossRef]
- Marrie, R.A.; Patel, R.; Figley, C.R.; Kornelsen, J.; Bolton, J.M.; Graff, L.; Mazerolle, E.L.; Marriott, J.J.; Bernstein, C.N.; Fisk, J.D. Diabetes and anxiety adversely affect cognition in multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 27, 164–170. [Google Scholar] [CrossRef]
- Slawta, J.N.; Wilcox, A.R.; McCubbin, J.A.; Nalle, D.J.; Fox, S.D.; Anderson, G. Health behaviors, body composition, and coronary heart disease risk in women with multiple sclerosis 11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated. Arch. Phys. Med. Rehabil. 2003, 84, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Pilutti, L.A.; McAuley, E.; Motl, R.W. Weight status and disability in multiple sclerosis: An examination of bi-directional associations over a 24-month period. Mult. Scler. Relat. Disord. 2012, 1, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Pilutti, L.A.; Dlugonski, D.; Pula, J.H.; Motl, R.W. Weight status in persons with multiple sclerosis: Implications for mobility outcomes. J. Obes. 2012, 2012, 868256. [Google Scholar] [CrossRef]
- Marrie, R.; Horwitz, R.; Cutter, G.; Tyry, T.; Campagnolo, D.; Vollmer, T. High frequency of adverse health behaviors in multiple sclerosis. Mult. Scler. 2009, 15, 105–113. [Google Scholar] [CrossRef]
- Sioka, C.; Fotopoulos, A.; Georgiou, A.; Papakonstantinou, S.; Pelidou, S.H.; Kyritsis, A.P.; Kalef-Ezra, J.A. Body composition in ambulatory patients with multiple sclerosis. J. Clin. Densitom. 2011, 14, 465–470. [Google Scholar] [CrossRef]
- Langer-Gould, A.; Brara, S.M.; Beaber, B.E.; Koebnick, C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology 2013, 80, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Harroud, A.; Mitchell, R.E.; Richardson, T.G.; Morris, J.A.; Forgetta, V.; Davey Smith, G.; Baranzini, S.E.; Richards, J.B. Childhood obesity and multiple sclerosis: A Mendelian randomization study. Mult. Scler. 2021, 27, 2150–2158. [Google Scholar] [CrossRef]
- Munger, K.L.; Bentzen, J.; Laursen, B.; Stenager, E.; Koch-Henriksen, N.; Sørensen, T.I.; Baker, J.L. Childhood body mass index and multiple sclerosis risk: A long-term cohort study. Mult. Scler. 2013, 19, 1323–1329. [Google Scholar] [CrossRef]
- Hedström, A.K.; Olsson, T.; Alfredsson, L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult. Scler. 2012, 18, 1334–1336. [Google Scholar] [CrossRef]
- Tettey, P.; Simpson, S., Jr.; Taylor, B.; Blizzard, L.; Ponsonby, A.L.; Dwyer, T.; Kostner, K.; van der Mei, I. An adverse lipid profile is associated with disability and progression in disability, in people with MS. Mult. Scler. 2014, 20, 1737–1744. [Google Scholar] [CrossRef]
- Tettey, P.; Simpson, S., Jr.; Taylor, B.; Blizzard, L.; Ponsonby, A.L.; Dwyer, T.; Kostner, K.; van der Mei, I. Adverse lipid profile is not associated with relapse risk in MS: Results from an observational cohort study. J. Neurol. Sci. 2014, 340, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Salter, A.; Tyry, T.; Fox, R.J.; Cutter, G.; Marrie, R.A. Measures of general and abdominal obesity and disability severity in a large population of people with multiple sclerosis. Mult. Scler. 2020, 26, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-García, J.J.; Carrera-Quintanar, L.; López-Roa, R.I.; Márquez-Aguirre, A.L.; Rojas-Mayorquín, A.E.; Ortuño-Sahagún, D. Multiple Sclerosis and Obesity: Possible Roles of Adipokines. Mediat. Inflamm. 2016, 2016, 4036232. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, V.; Procaccini, C.; Calì, G.; Pirozzi, G.; Fontana, S.; Zappacosta, S.; La Cava, A.; Matarese, G. A key role of leptin in the control of regulatory T cell proliferation. Immunity 2007, 26, 241–255. [Google Scholar] [CrossRef]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef]
- Ghadirian, P.; Dadgostar, B.; Azani, R.; Maisonneuve, P. A case-control study of the association between socio-demographic, lifestyle and medical history factors and multiple sclerosis. Can. J. Public Health 2001, 92, 281–285. [Google Scholar] [CrossRef]
- Hernán, M.A.; Jick, S.S.; Logroscino, G.; Olek, M.J.; Ascherio, A.; Jick, H. Cigarette smoking and the progression of multiple sclerosis. Brain 2005, 128, 1461–1465. [Google Scholar] [CrossRef]
- Healy, B.C.; Ali, E.N.; Guttmann, C.R.; Chitnis, T.; Glanz, B.I.; Buckle, G.; Houtchens, M.; Stazzone, L.; Moodie, J.; Berger, A.M.; et al. Smoking and disease progression in multiple sclerosis. Arch. Neurol. 2009, 66, 858–864. [Google Scholar] [CrossRef]
- Hawkes, C.H. Smoking is a risk factor for multiple sclerosis: A metanalysis. Mult. Scler. J. 2007, 13, 610–615. [Google Scholar] [CrossRef]
- Handel, A.E.; Williamson, A.J.; Disanto, G.; Dobson, R.; Giovannoni, G.; Ramagopalan, S.V. Smoking and Multiple Sclerosis: An Updated Meta-Analysis. PLoS ONE 2011, 6, e16149. [Google Scholar] [CrossRef]
- Degelman, M.L.; Herman, K.M. Smoking and multiple sclerosis: A systematic review and meta-analysis using the Bradford Hill criteria for causation. Mult. Scler. Relat. Disord. 2017, 17, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.; Graham, G.D.; Fu, N.; Estrada, E.; Chen, A.Y.; Miake-Lye, I.; Miles, J.N.; Shanman, R.; Shekelle, P.G.; Beroes, J.M.; et al. A systematic review of modifiable risk factors in the progression of multiple sclerosis. Mult. Scler. 2017, 23, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Pittas, F.; Ponsonby, A.L.; van der Mei, I.A.; Taylor, B.V.; Blizzard, L.; Groom, P.; Ukoumunne, O.C.; Dwyer, T. Smoking is associated with progressive disease course and increased progression in clinical disability in a prospective cohort of people with multiple sclerosis. J. Neurol. 2009, 256, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Manouchehrinia, A.; Tench, C.R.; Maxted, J.; Bibani, R.H.; Britton, J.; Constantinescu, C.S. Tobacco smoking and disability progression in multiple sclerosis: United Kingdom cohort study. Brain 2013, 136, 2298–2304. [Google Scholar] [CrossRef]
- Tanasescu, R.; Constantinescu, C.S.; Tench, C.R.; Manouchehrinia, A. Smoking Cessation and the Reduction of Disability Progression in Multiple Sclerosis: A Cohort Study. Nicotine Tob. Res. 2018, 20, 589–595. [Google Scholar] [CrossRef]
- Mitrovic, B.; Parkinson, J.; Merrill, J.E. An in Vitro Model of Oligodendrocyte Destruction by Nitric Oxide and Its Relevance to Multiple Sclerosis. Methods 1996, 10, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Philbrick, D.J.; Hopkins, J.B.; Hill, D.C.; Alexander, J.C.; Thomson, R.G. Effects of prolonged cyanide and thiocyanate feeding in rats. J. Toxicol. Environ. Health 1979, 5, 579–592. [Google Scholar] [CrossRef]
- Garey, K.W.; Neuhauser, M.M.; Robbins, R.A.; Danziger, L.H.; Rubinstein, I. Markers of inflammation in exhaled breath condensate of young healthy smokers. Chest 2004, 125, 22–26. [Google Scholar] [CrossRef]
- Bernard, A.; Ku, J.M.; Vlahos, R.; Miller, A.A. Cigarette smoke extract exacerbates hyperpermeability of cerebral endothelial cells after oxygen glucose deprivation and reoxygenation. Sci. Rep. 2019, 9, 15573. [Google Scholar] [CrossRef]
- Aktan, R.; Ozalevli, S.; Ozakbas, S. Effects of cigarette smoking on respiratory problems and functional levels in multiple sclerosis patients. Mult. Scler. Relat. Disord. 2018, 25, 271–275. [Google Scholar] [CrossRef]
- Quesnel, S.; Feinstein, A. Multiple sclerosis and alcohol: A study of problem drinking. Mult. Scler. 2004, 10, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Massa, J.; O’Reilly, E.J.; Munger, K.L.; Ascherio, A. Caffeine and alcohol intakes have no association with risk of multiple sclerosis. Mult. Scler. 2013, 19, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Qiao, L.; Fang, S.; Ren, Z.; Wu, G.; Zheng, Y.; Yang, B.; Zhao, Y. Alcohol consumption is associated with excessive risk of multiple sclerosis: A meta-analysis observational study. Sao Paulo Med. J. 2022, 140, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Hedström, A.K.; Hillert, J.; Olsson, T.; Alfredsson, L. Alcohol as a modifiable lifestyle factor affecting multiple sclerosis risk. JAMA Neurol. 2014, 71, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Hedström, A.K.; Olsson, T.; Alfredsson, L. The increased risk of multiple sclerosis associated with HLA-DRB1*15:01 and smoking is modified by alcohol consumption. Sci. Rep. 2021, 11, 21237. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.; Søndergaard, H.B.; Bang Oturai, D.; Laursen, J.H.; Gustavsen, S.; Larsen, N.K.; Magyari, M.; Just-Østergaard, E.; Thørner, L.W.; Sellebjerg, F.; et al. Alcohol consumption in adolescence is associated with a lower risk of multiple sclerosis in a Danish cohort. Mult. Scler. 2019, 25, 1572–1579. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Boniface, D. Risk associated behavior in premorbid multiple sclerosis: A case-control study. Mult. Scler. Relat. Disord. 2014, 3, 40–47. [Google Scholar] [CrossRef]
- Pekmezovic, T.; Drulovic, J.; Milenkovic, M.; Jarebinski, M.; Stojsavljevic, N.; Mesaros, S.; Kisic, D.; Kostic, J. Lifestyle factors and multiple sclerosis: A case-control study in Belgrade. Neuroepidemiology 2006, 27, 212–216. [Google Scholar] [CrossRef]
- Pakpoor, J.; Goldacre, R.; Disanto, G.; Giovannoni, G.; Goldacre, M.J. Alcohol Misuse Disorders and Multiple Sclerosis Risk. JAMA Neurol. 2014, 71, 1188–1189. [Google Scholar] [CrossRef]
- Ivashynka, A.; Copetti, M.; Naldi, P.; D’Alfonso, S.; Leone, M.A. The Impact of Lifetime Alcohol and Cigarette Smoking Loads on Multiple Sclerosis Severity. Front. Neurol. 2019, 10, 866. [Google Scholar] [CrossRef]
- D’Hooghe, M.B.; Haentjens, P.; Nagels, G.; De Keyser, J. Alcohol, coffee, fish, smoking and disease progression in multiple sclerosis. Eur. J. Neurol. 2012, 19, 616–624. [Google Scholar] [CrossRef]
- Paz-Ballesteros, W.C.; Monterrubio-Flores, E.A.; de Jesús Flores-Rivera, J.; Corona-Vázquez, T.; Hernández-Girón, C. Cigarette Smoking, Alcohol Consumption and Overweight in Multiple Sclerosis: Disability Progression. Arch. Med. Res. 2017, 48, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Pasala, S.; Barr, T.; Messaoudi, I. Impact of Alcohol Abuse on the Adaptive Immune System. Alcohol Res. 2015, 37, 185–197. [Google Scholar] [PubMed]
- Fahim, M.; Rafiee Zadeh, A.; Shoureshi, P.; Ghadimi, K.; Cheshmavar, M.; Sheikhinia, N.; Afzali, M. Alcohol and multiple sclerosis: An immune system-based review. Int. J. Physiol. Pathophysiol. Pharmacol. 2020, 12, 58–69. [Google Scholar]
- Marrie, R.; Horwitz, R.; Cutter, G.; Tyry, T.; Campagnolo, D.; Vollmer, T. Comorbidity, socioeconomic status and multiple sclerosis. Mult. Scler. 2008, 14, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Weinstock-Guttman, B.; Zivadinov, R.; Mahfooz, N.; Carl, E.; Drake, A.; Schneider, J.; Teter, B.; Hussein, S.; Mehta, B.; Weiskopf, M.; et al. Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J. Neuroinflamm. 2011, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Giubilei, F.; Antonini, G.; Di Legge, S.; Sormani, M.P.; Pantano, P.; Antonini, R.; Sepe-Monti, M.; Caramia, F.; Pozzilli, C. Blood cholesterol and MRI activity in first clinical episode suggestive of multiple sclerosis. Acta Neurol. Scand. 2002, 106, 109–112. [Google Scholar] [CrossRef]
- Weinstock-Guttman, B.; Zivadinov, R.; Horakova, D.; Havrdova, E.; Qu, J.; Shyh, G.; Lakota, E.; O’Connor, K.; Badgett, D.; Tamaño-Blanco, M.; et al. Lipid profiles are associated with lesion formation over 24 months in interferon-β treated patients following the first demyelinating event. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1186–1191. [Google Scholar] [CrossRef]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef]
- Blumenfeld Kan, S.; Staun-Ram, E.; Golan, D.; Miller, A. HDL-cholesterol elevation associated with fingolimod and dimethyl fumarate therapies in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 2055217319882720. [Google Scholar] [CrossRef]
- Weber, M.S.; Youssef, S.; Dunn, S.E.; Prod’homme, T.; Neuhaus, O.; Stuve, O.; Greenwood, J.; Steinman, L.; Zamvil, S.S. Statins in the treatment of central nervous system autoimmune disease. J. Neuroimmunol. 2006, 178, 140–148. [Google Scholar] [CrossRef]
- Lanzillo, R.; Orefice, G.; Quarantelli, M.; Rinaldi, C.; Prinster, A.; Ventrella, G.; Spitaleri, D.; Lus, G.; Vacca, G.; Carotenuto, B.; et al. Atorvastatin combined to interferon to verify the efficacy (ACTIVE) in relapsing-remitting active multiple sclerosis patients: A longitudinal controlled trial of combination therapy. Mult. Scler. 2010, 16, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, T.; Key, L.; Durkalski, V.; Tyor, W.; Corboy, J.; Markovic-Plese, S.; Preiningerova, J.; Rizzo, M.; Singh, I. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet 2004, 363, 1607–1608. [Google Scholar] [CrossRef] [PubMed]
- Chataway, J.; Schuerer, N.; Alsanousi, A.; Chan, D.; MacManus, D.; Hunter, K.; Anderson, V.; Bangham, C.R.; Clegg, S.; Nielsen, C.; et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): A randomised, placebo-controlled, phase 2 trial. Lancet 2014, 383, 2213–2221. [Google Scholar] [CrossRef]
- Sorensen, P.S.; Lycke, J.; Erälinna, J.P.; Edland, A.; Wu, X.; Frederiksen, J.L.; Oturai, A.; Malmeström, C.; Stenager, E.; Sellebjerg, F.; et al. Simvastatin as add-on therapy to interferon β-1a for relapsing-remitting multiple sclerosis (SIMCOMBIN study): A placebo-controlled randomised phase 4 trial. Lancet Neurol. 2011, 10, 691–701. [Google Scholar] [CrossRef]
- Kamm, C.P.; El-Koussy, M.; Humpert, S.; Findling, O.; von Bredow, F.; Burren, Y.; Schwegler, G.; Schött, D.; Donati, F.; Müller, M.; et al. Atorvastatin added to interferon β for relapsing multiple sclerosis: A randomized controlled trial. J. Neurol. 2012, 259, 2401–2413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vaughn, C.B.; Jakimovski, D.; Kavak, K.S.; Ramanathan, M.; Benedict, R.H.B.; Zivadinov, R.; Weinstock-Guttman, B. Epidemiology and treatment of multiple sclerosis in elderly populations. Nat. Rev. Neurol. 2019, 15, 329–342. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013, 12, 483–497. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Li, H.; Xiao, L.; Zhou, Z.; Chen, K.; Gui, L.; Hou, X.; Fan, R.; Chen, K.; et al. Clinical, Radiological and Pathological Characteristics Between Cerebral Small Vessel Disease and Multiple Sclerosis: A Review. Front. Neurol. 2022, 13, 841521. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Schmidt, H.; Haybaeck, J.; Loitfelder, M.; Weis, S.; Cavalieri, M.; Seiler, S.; Enzinger, C.; Ropele, S.; Erkinjuntti, T.; et al. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011, 122, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F. Magnetic resonance signal abnormalities in asymptomatic individuals: Their incidence and functional correlates. Eur. Neurol. 1989, 29, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.M. Lacunes: Small, deep cerebral infarcts. Neurology 1998, 50, 841–841-a. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, W.T., Jr.; Bernick, C.; Manolio, T.A.; Bryan, N.; Jungreis, C.A.; Price, T.R.; Cardiovascular Health Study Collaborative Research Group. Lacunar Infarcts Defined by Magnetic Resonance Imaging of 3660 Elderly People: The Cardiovascular Health Study. Arch. Neurol. 1998, 55, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Raz, E.; Loh, J.P.; Saba, L.; Omari, M.; Herbert, J.; Lui, Y.; Kister, I. Periventricular lesions help differentiate neuromyelitis optica spectrum disorders from multiple sclerosis. Mult. Scler. Int. 2014, 2014, 986923. [Google Scholar] [CrossRef]
- Lv, A.; Zhang, Z.; Fu, Y.; Yan, Y.; Yang, L.; Zhu, W. Dawson’s Fingers in Cerebral Small Vessel Disease. Front. Neurol. 2020, 11, 669. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Mera, R.M.; Costa, A.F.; Silva, P.; Del Brutto, V.J. Dawson Fingers in Older Adults with Cerebral Small Vessel Disease: A Population Study. Eur. Neurol. 2020, 83, 421–425. [Google Scholar] [CrossRef]
- Wattjes, M.P.; Ciccarelli, O.; Reich, D.S.; Banwell, B.; de Stefano, N.; Enzinger, C.; Fazekas, F.; Filippi, M.; Frederiksen, J.; Gasperini, C.; et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021, 20, 653–670. [Google Scholar] [CrossRef]
- Maggi, P.; Sati, P.; Nair, G.; Cortese, I.C.M.; Jacobson, S.; Smith, B.R.; Nath, A.; Ohayon, J.; van Pesch, V.; Perrotta, G.; et al. Paramagnetic Rim Lesions are Specific to Multiple Sclerosis: An International Multicenter 3T MRI Study. Ann. Neurol. 2020, 88, 1034–1042. [Google Scholar] [CrossRef]
- Sinnecker, T.; Clarke, M.A.; Meier, D.; Enzinger, C.; Calabrese, M.; De Stefano, N.; Pitiot, A.; Giorgio, A.; Schoonheim, M.M.; Paul, F.; et al. Evaluation of the Central Vein Sign as a Diagnostic Imaging Biomarker in Multiple Sclerosis. JAMA Neurol. 2019, 76, 1446–1456. [Google Scholar] [CrossRef]
- Maggi, P.; Absinta, M.; Grammatico, M.; Vuolo, L.; Emmi, G.; Carlucci, G.; Spagni, G.; Barilaro, A.; Repice, A.M.; Emmi, L.; et al. Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies. Ann. Neurol. 2018, 83, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Tallantyre, E.C.; Dixon, J.E.; Donaldson, I.; Owens, T.; Morgan, P.S.; Morris, P.G.; Evangelou, N. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology 2011, 76, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Sati, P.; George, I.C.; Shea, C.D.; Gaitán, M.I.; Reich, D.S. FLAIR*: A combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology 2012, 265, 926–932. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nociti, V.; Romozzi, M. The Importance of Managing Modifiable Comorbidities in People with Multiple Sclerosis: A Narrative Review. J. Pers. Med. 2023, 13, 1524. https://doi.org/10.3390/jpm13111524
Nociti V, Romozzi M. The Importance of Managing Modifiable Comorbidities in People with Multiple Sclerosis: A Narrative Review. Journal of Personalized Medicine. 2023; 13(11):1524. https://doi.org/10.3390/jpm13111524
Chicago/Turabian StyleNociti, Viviana, and Marina Romozzi. 2023. "The Importance of Managing Modifiable Comorbidities in People with Multiple Sclerosis: A Narrative Review" Journal of Personalized Medicine 13, no. 11: 1524. https://doi.org/10.3390/jpm13111524
APA StyleNociti, V., & Romozzi, M. (2023). The Importance of Managing Modifiable Comorbidities in People with Multiple Sclerosis: A Narrative Review. Journal of Personalized Medicine, 13(11), 1524. https://doi.org/10.3390/jpm13111524






